©The Author(s) 2025.
World J Gastroenterol. Aug 21, 2025; 31(31): 109605
Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.109605
Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.109605
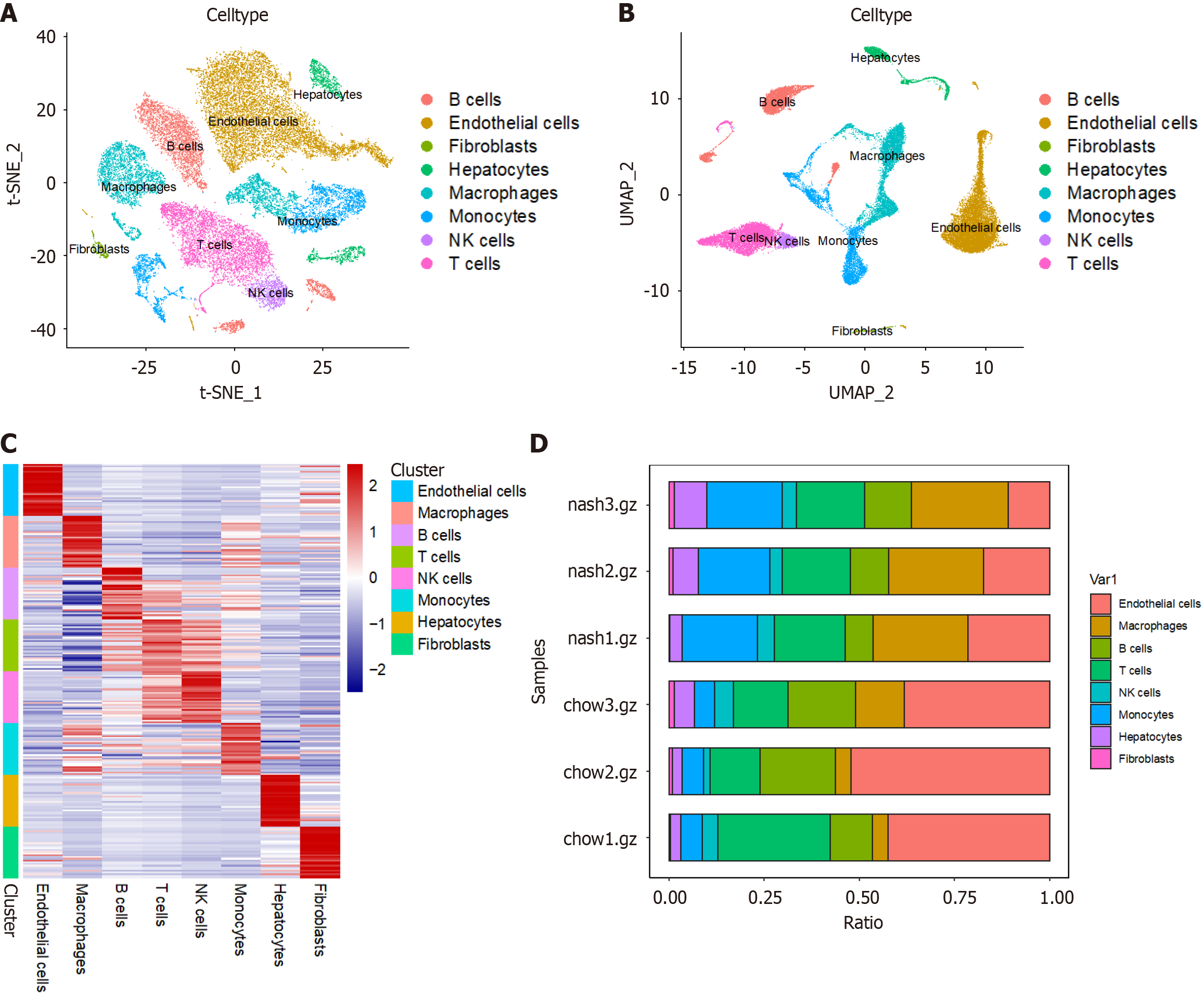
Figure 1 Single-cell atlas of liver tissue from two groups of mice.
A: T-distributed stochastic neighbor embedding plot of single cells from liver tissue of two groups of mice; B: Uniform manifold approximation plot of single cells from liver tissue of two groups of mice; C: Heatmap of characteristic genes of various cell types in liver tissue; D: Cell proportion plot in each sample. t-SNE: T-distributed stochastic neighbor embedding; UMAP: Uniform manifold approximation; NK: Nature killer.
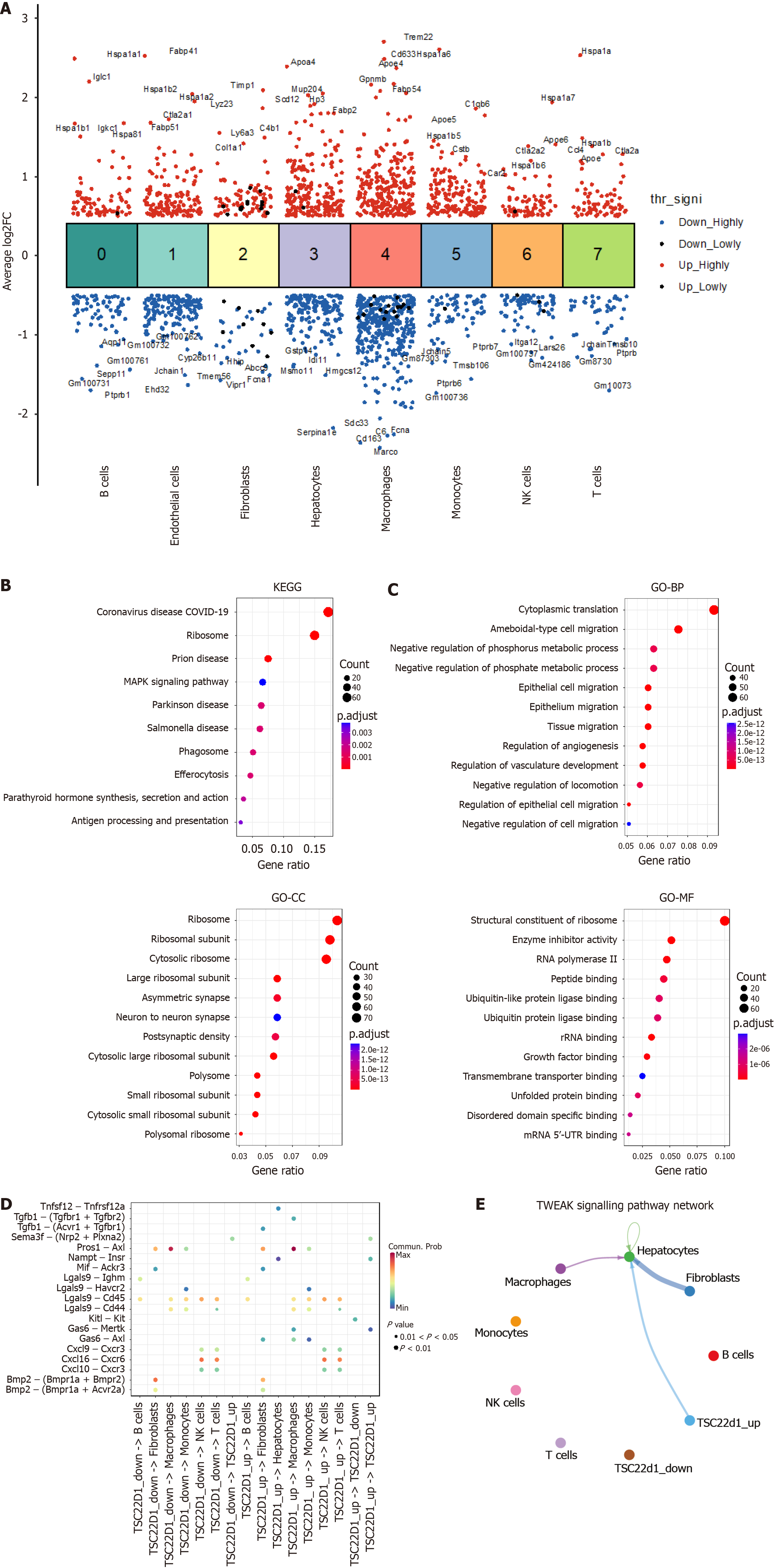
Figure 2 Cell communication analysis in nonalcoholic fatty liver disease.
A: Differential gene expression analysis showing up- and down-regulated genes across all eight clusters. An adjusted P value < 0.01 is indicated in red, while an adjusted P value ≥ 0.01 is indicated in black; B: Kyoto Encyclopedia of Genes and Genomes enrichment analysis of genetic variations in hepatic sinusoidal endothelial cells; C: Gene Ontology enrichment analysis of genetic variations in hepatic sinusoidal endothelial cells; D: Analysis of TSC22D1 expression levels in hepatic sinusoidal endothelial cells and the strength of communication with other cell types; E: Different cells interact via the tumor necrosis factor-like weak inducer of apoptosis fibroblast growth factor-inducible 14 signaling pathway. NK: Nature killer; FC: Fold change; COVID-19: Corona virus disease 2019; MAPK: Mitogen-activated protein kinase; KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology; BP: Biological process; CC: Cellular component; MF: Molecular function; 5’-UTR: 5’ untranslated region; mRNA: Message RNA.
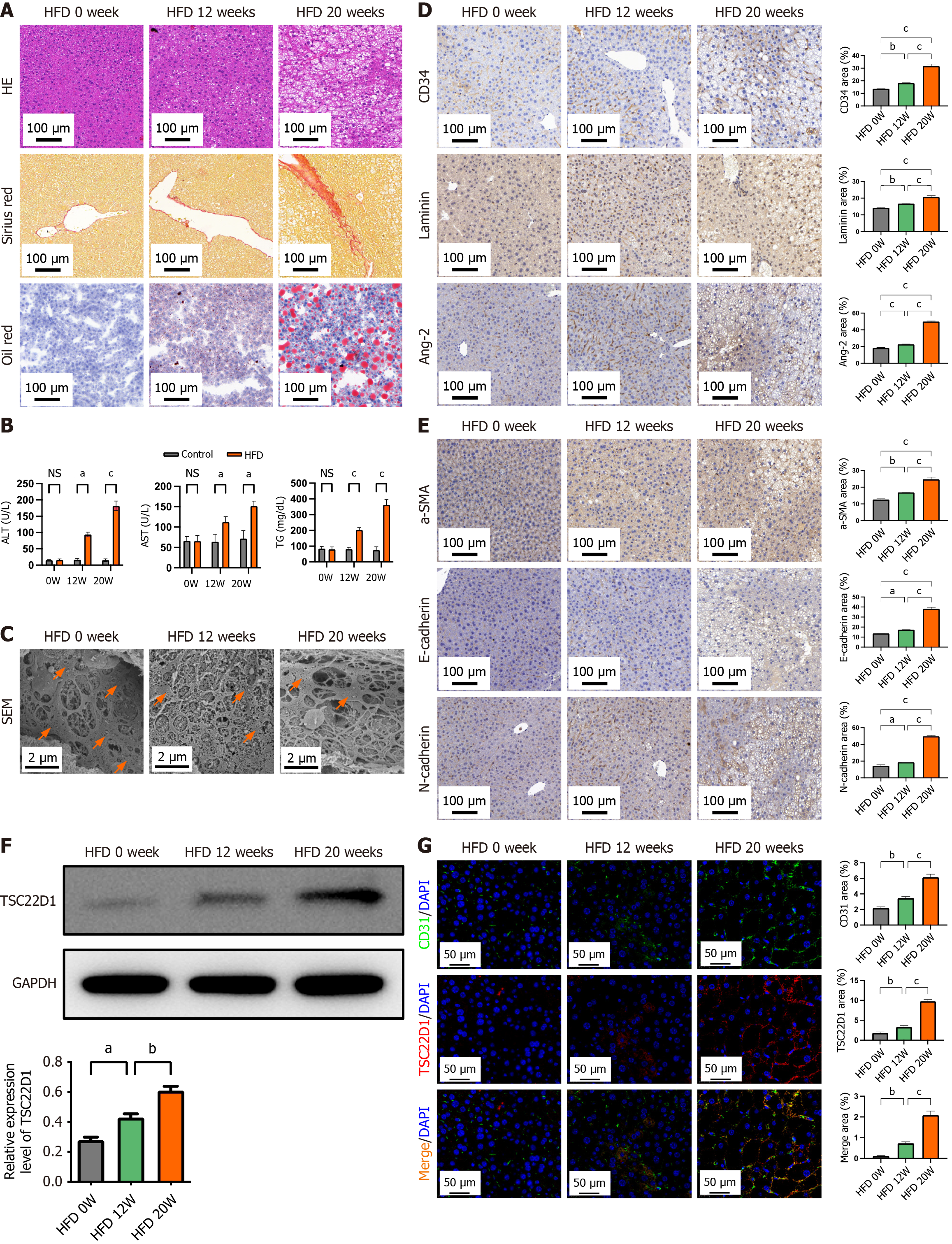
Figure 3 High-fat diet upregulates TSC22D1 expression and promotes liver sinusoidal endothelial cell dysfunction.
A: Hematoxylin eosin, Sirius red, and Oil red O staining of liver tissues from three groups of mice; B: Biochemical indices of the three groups of mice; C: Electron microscopy showing fenestrae in liver tissues with orange arrows; D: Immunohistochemical staining for cluster of differentiation (CD) 34, laminin, and angiotensin-2; E: Immunohistochemical staining for α-smooth muscle actin, E-cadherin, and N-cadherin; F: Western blot analysis for TSC22D1 expression; G: Immunofluorescence staining for CD31 and TSC22D1. Scale bar 100 μm, 100 × magnification. Scale bar 50 μm, 200 × magnification. Scale bar 2 μm, 5000 × magnification. n = 6 mice each group. aP < 0.05; bP < 0.01; cP < 0.001. One-way analysis of variance. HE: Hematoxylin eosin; HFD: High-fat diet; CD: Cluster of differentiation; Ang-2: Angiotensin-2; NS: No significant; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TG: Triglyceride; SEM: Scanning electron microscopy; SMA: Smooth muscle actin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; DAPI: 4’,6-diamidino-2-phenylindole.
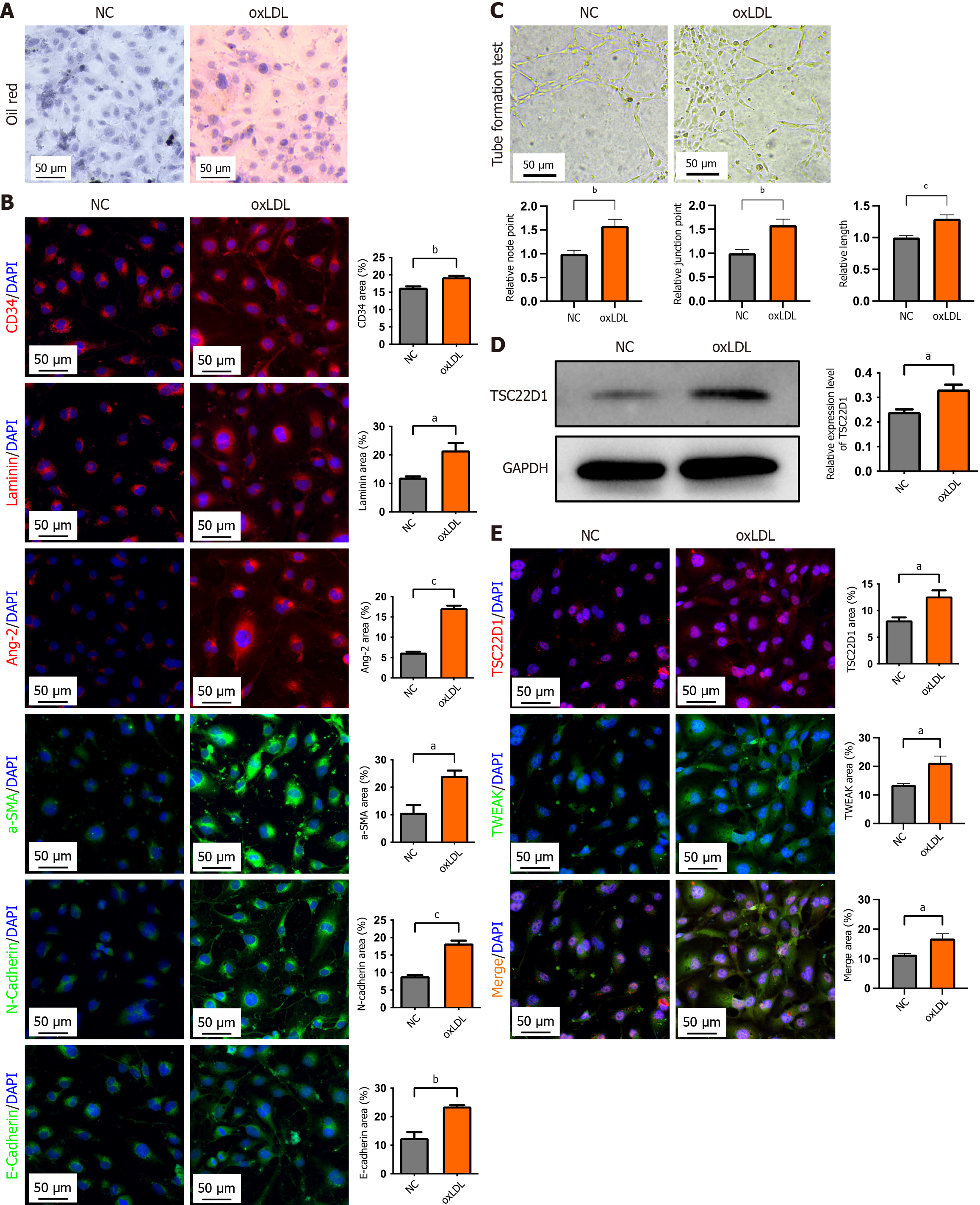
Figure 4 Oxidized low-density lipoprotein upregulates TSC22D1 expression and promotes microvascular and endothelial-mesenchymal transition of liver sinusoidal endothelial cells.
A: Oil red O staining for the verification of the oxidized low-density lipoprotein (oxLDL) intervention model; B: Western blot analysis of TSC22D1 expression in normal and oxLDL-treated liver sinusoidal endothelial cells; C: Tube formation assay; D: Immunofluorescence staining for cluster of differentiation 34, laminin, and angiotensin-2, α-smooth muscle actin, E-cadherin, and N-cadherin; E: Fluorescence double staining for TSC22D1 and tumor necrosis factor-like weak inducer of apoptosis. aP < 0.05; bP < 0.01; cP < 0.001. Student’s t test. Scale bar 50 μm, 200 × magnification. NC: Negative control; oxLDL: Oxidized low-density lipoprotein; CD: Cluster of differentiation; DAPI: 4’,6-diamidino-2-phenylindole; Ang-2: Angiotensin-2; SMA: Smooth muscle actin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; TWEAK: Tumor necrosis factor-like weak inducer of apoptosis.
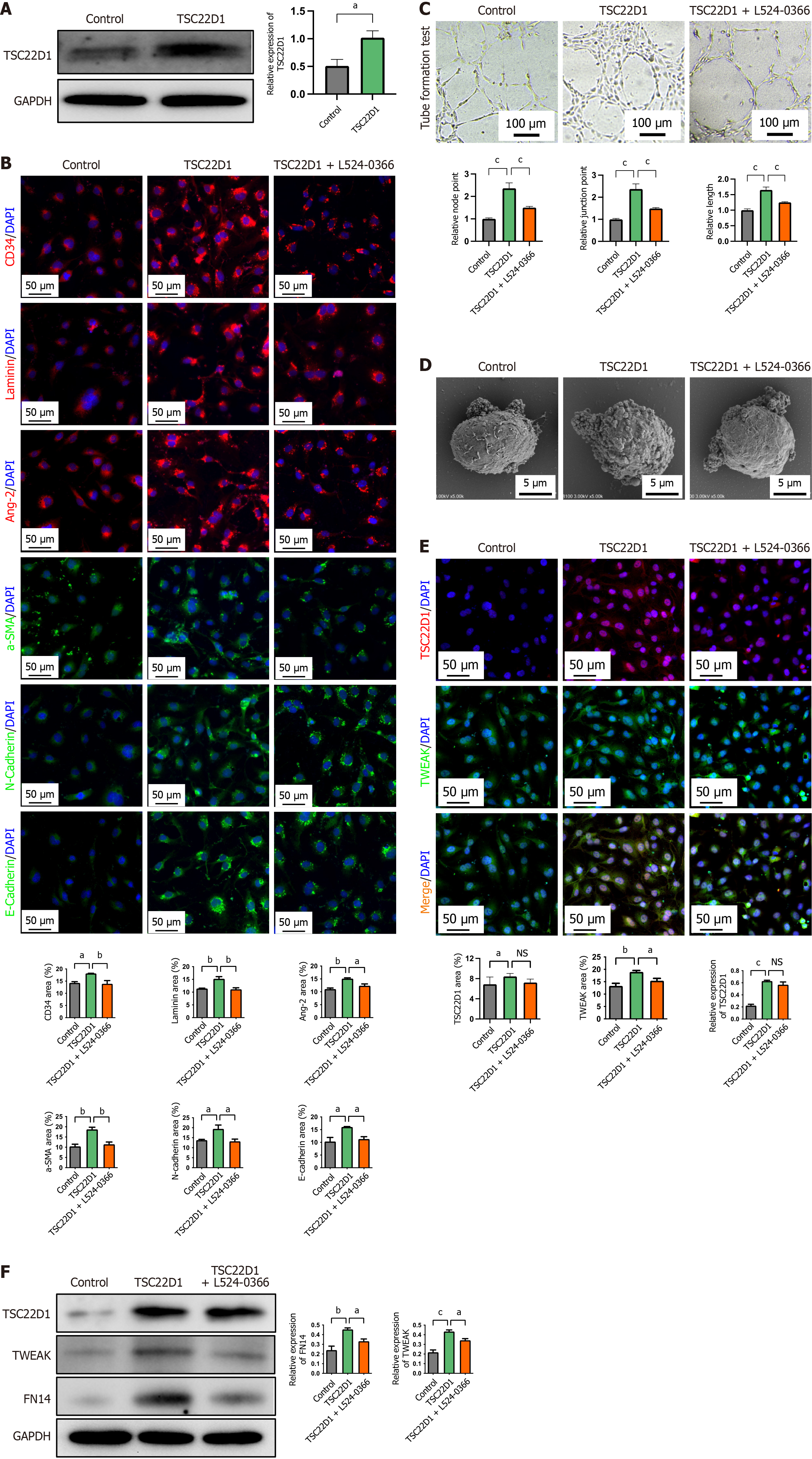
Figure 5 TSC22D1 promotes the microvascular and endothelial-mesenchymal transition of liver sinusoidal endothelial cells via the tumor necrosis factor-like weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway.
A: Western blot analysis for the verification of TSC22D1 overexpression plasmid; B: Immunofluorescence staining for cluster of differentiation 34, laminin, angiotensin-2, α-smooth muscle actin, E-cadherin, and N-cadherin; C: Tube formation assay; D: Electron microscopy showing fenestrae density in liver sinusoidal endothelial cells; E: Fluorescence double staining for TSC22D1 and tumor necrosis factor-like weak inducer of apoptosis (TWEAK); F: Western blot analysis for TSC22D1 and TWEAK/fibroblast growth factor-inducible 14 pathway. Scale bar 100 μm, 100 × magnification. Scale bar 50 μm, 200 × magnification. Scale bar 10 μm, 1000 × magnification. aP < 0.05; bP < 0.01; cP < 0.001. One-way analysis of variance. NS: No significant; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; CD: Cluster of differentiation; DAPI: 4’,6-diamidino-2-phenylindole; Ang-2: Angiotensin-2; SMA: Smooth muscle actin; TWEAK: Tumor necrosis factor-like weak inducer of apoptosis; FN14: Fibroblast growth factor-inducible 14.
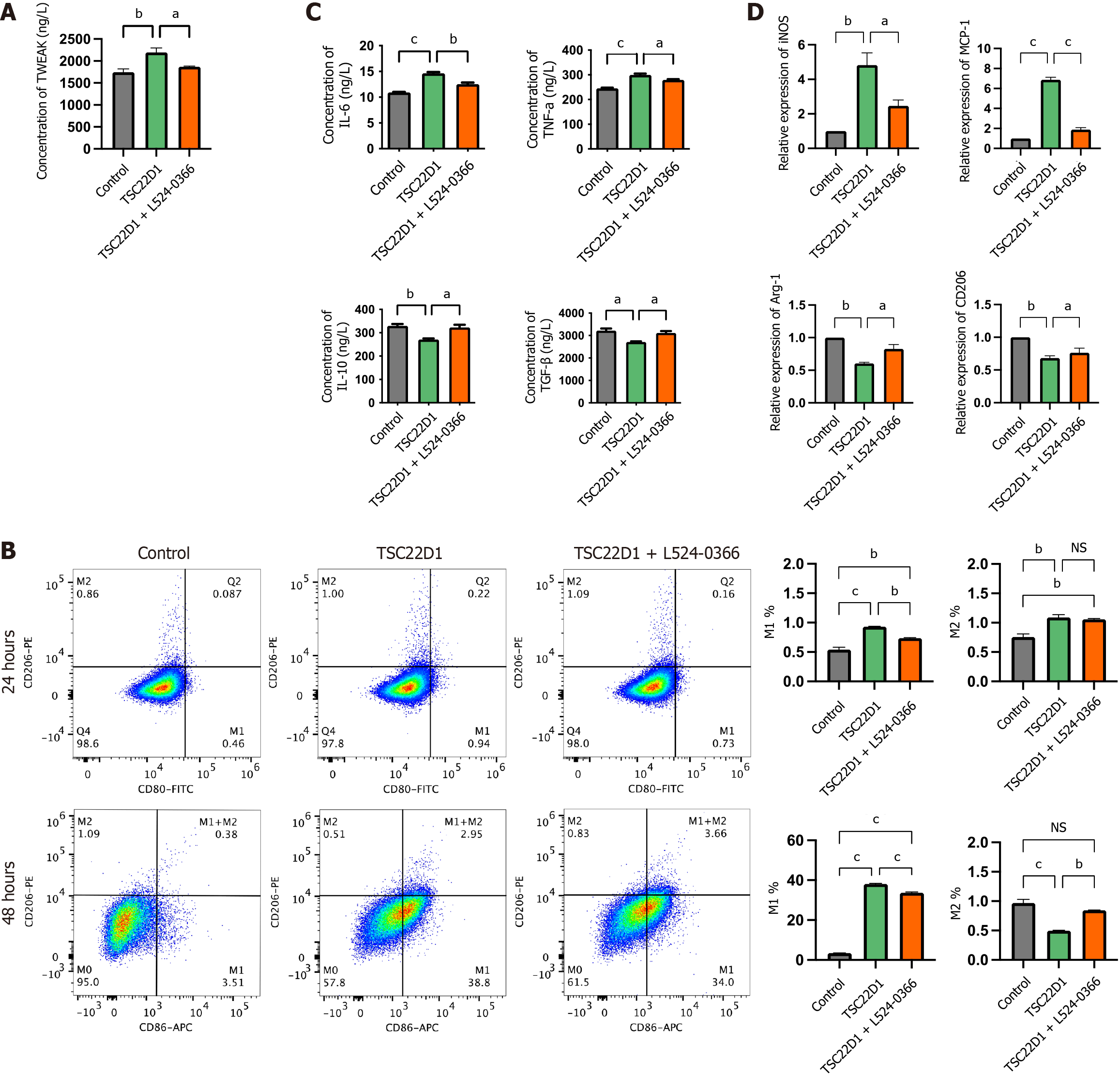
Figure 6 Tumor necrosis factor-like weak inducer of apoptosis promotes macrophage M1 polarization secreted by liver sinusoidal endothelial cells overexpressing TSC22D1 via the tumor necrosis factor-like weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway.
A: Enzyme-linked immunosorbent assay (ELISA) analysis of tumor necrosis factor-like weak inducer of apoptosis in the supernatants of liver sinusoidal endothelial cells (LSECs); B: Flow cytometry analysis detecting the expression levels of M1 and M2 macrophages; C: ELISA analysis of interleukin (IL)-6, tumor necrosis factor-α, IL-10, and transforming growth factor-β in the supernatants of macrophages cultured in conditioned media from LSECs; D: Quantitative real-time polymerase chain reaction analysis of inducible nitric oxide synthase, monocyte chemoattractant protein-1, arginase-1 and cluster of differentiation 206 in macrophages. aP < 0.05; bP < 0.01; cP < 0.001. One-way analysis of variance. TWEAK: Tumor necrosis factor-like weak inducer of apoptosis; CD: Cluster of differentiation; FITC: Fluorescein Isothiocyanate; APC: Antigen-presenting cell; NS: No significant; IL: Interleukin; TNF: Tumor necrosis factor; TGF: Transforming growth factor; iNOS: Inducible nitric oxide synthase; MCP: Monocyte chemoattractant protein; Arg-1: Arginase-1.
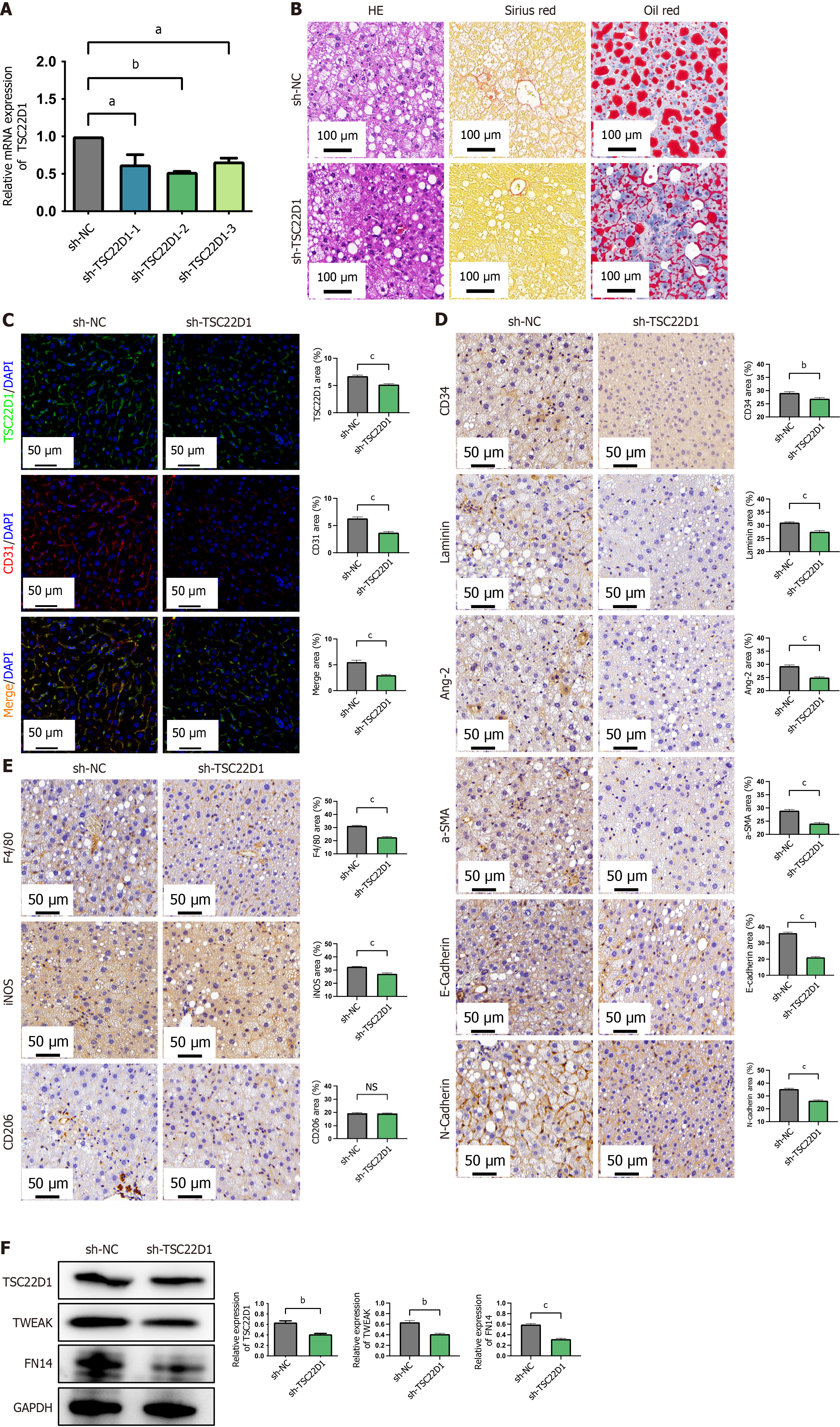
Figure 7 Targeting TSC22D1 alleviates liver sinusoidal endothelial cells dysfunction and inhibits macrophage M1 polarization via the tumor necrosis factor-like weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway in vivo.
A: The interference efficiency of different short hairpin RNAs against TSC22D1 via quantitative real-time polymerase chain reaction analysis; B: Hematoxylin eosin, Sirius red, and Oil red O staining of liver tissues from the adeno-associated virus serotype 8-short hairpin RNA-negative control group and the recombinant adeno-associated virus serotype 8 carrying TSC22D1-targeting short hairpin RNA vector group; C: Fluorescence double staining for cluster of differentiation (CD) 31 and TSC22D1; D: Immunohistochemical staining for CD34, laminin, angiotensin-2, α-smooth muscle actin, E-cadherin, and N-cadherin in liver tissues; E: Immunohistochemical staining for mouse epidermal growth factor-like module-containing mucin-like hormone receptor-like 1, inducible nitric oxide synthase, and CD206; F: Western blot analysis for TSC22D1 and tumor necrosis factor-like weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway. Scale bar 100 μm, 100 × magnification. Scale bar 50 μm, 200 × magnification. aP < 0.05; bP < 0.01; cP < 0.001. Student’s t test. n = 6 mice each group. sh-TSC22D1: The recombinant adeno-associated virus serotype 8 carrying TSC22D1-targeting short hairpin RNA vector; sh-NC: The recombinant adeno-associated virus serotype 8 carrying negative control short hairpin RNA vector; F4/80: Mouse epidermal growth factor-like module-containing mucin-like hormone receptor-like 1; HE: Hematoxylin eosin; CD: Cluster of differentiation; DAPI: 4’,6-diamidino-2-phenylindole; Ang-2: Angiotensin-2; SMA: Smooth muscle actin; iNOS: Inducible nitric oxide synthase; NS: No significant; TWEAK: Tumor necrosis factor-like weak inducer of apoptosis; FN14: Fibroblast growth factor-inducible 14; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
- Citation: Ding W, Xu XQ, Wu LL, Wang Q, Wang YQ, Chen WW, Tan YL, Wang YB, Jiang HJ, Dong J, Yan YM, Xu XZ. TSC22D1 promotes liver sinusoidal endothelial cell dysfunction and induces macrophage M1 polarization in non-alcoholic fatty liver disease. World J Gastroenterol 2025; 31(31): 109605
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/109605.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.109605