Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.109630
Revised: June 18, 2025
Accepted: July 16, 2025
Published online: August 21, 2025
Processing time: 94 Days and 4.9 Hours
Hepatocellular carcinoma (HCC) is a major health concern in Thailand, with most patients diagnosed at the intermediate stage. Transarterial chemoembolization (TACE) is the standard treatment; however, postembolization syndrome (PES) remains a common complication. Although both dexamethasone (DEXA) and N-acetylcysteine (NAC) have shown efficacy in reducing PES, no study has directly compared their effects.
To compare the incidence of PES between DEXA and NAC in intermediate-stage HCC patients undergoing conventional TACE (cTACE).
A randomized, double-blind, controlled trial was conducted at two tertiary hospitals in Thailand from November 2024 to April 2025. Eligible HCC patients (aged 18-70 years) were randomized (1:1) to receive either NAC (150 mg/kg/hour loading dose, followed by 50 mg/kg over 4 hours, then 6.25 mg/kg/ hour for 48 hours post-cTACE) or DEXA (8 mg IV 1 hour before cTACE). cTACE was performed by blinded interventional radiologists. The primary outcome was PES occurrence within 48 hours, assessed using South West Oncology Group toxicity coding and the Common Terminology Criteria for Adverse Events. The secondary outcomes were post-cTACE liver decompensation and the dynamic changes in the albumin-bilirubin (ALBI) score.
A total of 56 intermediate-stage HCC patients were included (DEXA, n = 28; NAC, n = 28). Most had preserved liver function, with 92.9% classified as Child-Pugh A. The maximum tumor size was 6.2 cm, and 85.7% had multiple lesions. Additionally, 39 patients (69.6%) met the beyond up-to-7 criteria. Overall, 27 patients (48.2%) developed PES. After adjusting for confounding factors, the NAC group had a significantly lower incidence of PES than the DEXA group (32.1% vs 64.3%; adjusted odds ratio = 0.17, 95% confidence interval: 0.03-0.87, P = 0.033). Only two patients (3.6%) developed post-cTACE liver decompensation. Furthermore, 51.8% patients experienced worsening ALBI scores within 48 hours post-procedure; however, the rate of ALBI score worsening did not significantly differ between the groups.
Compared with DEXA, NAC significantly reduces the incidence of PES, regardless of its impact on liver function recovery. Therefore, NAC is a preferable option for reducing PES in Barcelona Clinic Liver Cancer-B stage HCC patients with preserved liver function.
Core Tip: N-acetylcysteine significantly reduces the incidence of postembolization syndrome compared with dexamethasone in patients with intermediate-stage hepatocellular carcinoma undergoing transarterial chemoembolization; however, both treatments have comparable effects on liver function recovery.
- Citation: Koonsiripaiboon P, Ruamtawee W, Simasingha N, Tanasoontrarat W, Claimon T, Sethasine S. Efficacy of N-acetylcysteine vs dexamethasone in preventing postembolization syndrome post-transarterial chemoembolization in hepatocellular carcinoma: A randomized controlled trial. World J Gastroenterol 2025; 31(31): 109630
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/109630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.109630
Hepatocellular carcinoma (HCC) is a significant health concern in Thailand, with over a thousand patients affected and approximately 75% receiving their initial diagnosis at the intermediate stage[1]. Transarterial chemoembolization (TACE) is the standard treatment for intermediate-stage HCC and has been shown to improve patient survival. TACE works by embolizing the blood vessels that supply the tumor, thereby inducing tumor necrosis. A common complication following TACE is postembolization syndrome (PES), which not only affects patient recovery but also serves as a predictor of long-term outcomes and helps determine the appropriateness of subsequent TACE sessions[2]. The reported incidence of PES ranges from 48.2% to 80%[3-6].
Previous studies have investigated the use of dexamethasone (DEXA) to prevent PES at varying doses and durations[6-10]. Notably, a randomized, double-blind study by Ogasawara et al[11] reported that DEXA effectively prevents PES during TACE treatment. This finding was supported by Sainamthip et al[6], who showed that a single intravenous dose of DEXA prior to TACE reduced the incidence of PES. N-acetylcysteine (NAC), a hepatoprotective antioxidant agent[12,13], has also been shown to decrease the incidence of PES in previous studies. Siramolpiwat et al[5] conducted an open-label randomized controlled trial demonstrating that premedication with NAC at 150 mg/kg, compared with placebo, resulted in a 50% reduction in PES incidence.
A recent study by Simasingha et al[4], an open-label randomized controlled trial involving 100 HCC patients undergoing conventional TACE (cTACE), found that the combination of high-dose DEXA and NAC significantly reduced the incidence of PES-from 80% in the placebo group to 6% in the DEXA–NAC group.
The hypothesis posits that both drugs are effective in preventing PES; however, no study has directly compared the efficacy of NAC and DEXA. The primary objective of this study was to compare the efficacy of NAC vs DEXA in preventing PES among HCC patients undergoing cTACE. Secondary objectives included assessing the incidence of post-cTACE liver decompensation and analyzing dynamic changes in the albumin-bilirubin (ALBI) score between the two groups.
A randomized, double-blind, controlled trial was conducted at two tertiary centers-the Gastroenterology and Liver Unit, Vajira Hospital, Navamindradhiraj University, and Udonthani Hospital, Thailand-from November 2024 to April 2025. The study protocol adhered to the ethical standards of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Vajira Hospital, Navamindradhiraj University (COA 115/2567). The trial was registered prospectively in the Thai Clinical Trials Registry (TCTR 20240816008).
Patients aged 18-70 years who were diagnosed with intermediate-stage HCC according to the Barcelona Clinic Liver Cancer (BCLC) criteria and had an Eastern Cooperative Oncology Group performance status of 0-1 were eligible. HCC diagnosis was confirmed either via histological examination or by identifying characteristic radiological features of lesions in cirrhotic livers. Baseline ALBI grades were categorized as follows: Grade 1 (≤ -2.60), grade 2 (> -2.60 to ≤ -1.39), and grade 3 (> -1.39)[14]. Exclusion criteria included decompensated cirrhosis (Child-Pugh score ≥ 9); major vascular tumor invasion; history of cTACE refractoriness; congestive heart failure with or without respiratory failure; uncontrolled diabetes or a hemoglobin A1c (HbA1c) level > 8.5; renal impairment with glomerular filtration rate < 45 mL/min/1.73 m2; sepsis; severe allergy or anaphylaxis/anaphylactoid reaction to NAC or drug interactions with nitroglycerin; pregnancy; and prior use of nonsteroidal anti-inflammatory drugs, steroids, or NAC within 21 days. Informed consent was obtained from all patients prior to enrollment.
To evaluate the efficacy of NAC vs DEXA in preventing PES among BCLC stage B HCC patients undergoing cTACE, the sample size was calculated using a two-sample test of equality of proportions in Stata version 13.0 (Stata Corporation, College Station, TX, United States). Based on a previous study, the expected incidence of PES was 25% with NAC[5] and 65% with low-dose DEXA. Assuming 80% power and a two-sided significance level of 5%, with a 1:1 randomization ratio, the required sample size was determined to be 56 participants.
All patients were admitted at least 24 hours before the scheduled cTACE. Baseline evaluations included a medical history, physical examination, and blood tests-complete blood count, liver function tests, coagulation profile, plasma glucose levels, HbA1c levels, renal function tests, and alpha-fetoprotein levels. Randomization was computer-generated in blocks of four, stratified by Child-Pugh class (A or B), and conducted in a blinded manner (1:1) for assignment to the NAC or DEXA group. Drug allocation was managed independently and not by the study investigators. A double-blind approach was maintained throughout both the interventional and post-procedural periods.
In the NAC group, NAC was administered in 5% dextrose, starting with a 150 mg/kg/hour loading dose for 1 hour, followed by 50 mg/kg over 4 hours, and then a continuous infusion of 6.25 mg/kg/hour for 48 hours post-cTACE. In the DEXA group, a single 8 mg intravenous dose of DEXA was administered 1 hour before cTACE, followed by a continuous infusion of normal saline for 48 hours post-cTACE. Drug dosages were based on previous studies[4,6]. These protocols were uniformly applied across all participants. In cases of mild to moderate allergic reactions to NAC, the infusion was paused for 1 hour and an intravenous antihistamine was administered. Treatment resumed once symptoms subsided. The trial was discontinued for patients who withdrew consent or developed severe anaphylactoid reactions, in which case standard protocols for managing severe allergic reactions were followed.
cTACE procedures were performed by two blinded interventional radiologists. All patients received a single intra
All patients were hospitalized for at least 48 hours following the procedure. Symptoms of PES were monitored, and blood tests were repeated at 24 and 48 hours. Patients were discharged if afebrile. On day 7 post-intervention, patients were interviewed regarding nausea, vomiting, and fever. If signs of PES were identified during the interview, analgesics or antiemetics were administered to symptomatic patients. In cases where fever > 38°C occurred within 24-48 hours and could not be clearly distinguished from sepsis or infection, standard sepsis management protocols, including hydration and antipyretics, were followed until culture results confirmed the absence of infection.
The primary outcome was the occurrence of PES within 48 hours after cTACE. PES was diagnosed based on the presence of any symptoms listed in the South West Oncology Group toxicity coding-fever, nausea, vomiting, and/or abdominal pain-occurring within 48 hours, with a calculated sum score of > 2 or any symptom rated above grade I according to the Common Terminology Criteria for Adverse Events (CTCAE) (Supplementary Tables 1 and 2). The secondary outcome was post-cTACE liver decompensation, defined as an increase in the Child-Pugh score by > 2 points, an increase in serum total bilirubin level > 2 mg/dL from baseline, progression in ALBI stage, or the development of other decompensating events.
Descriptive statistics were used to summarize participant characteristics. Categorical variables are expressed as frequencies and percentages, whereas continuous variables are expressed as means and standard deviations. The χ2 test was used to compare the incidence of PES between the two groups. Univariable logistic regression was used to analyze the association between PES, treatment group (NAC vs DEXA), and tumor burden. If baseline differences were identified between groups, multivariable logistic regression was employed to adjust for potential confounders. Multilevel random intercept and slope linear regression models were applied to assess mean differences (MD) in ALBI score changes between groups on days 2 and 28 post-cTACE. All statistical analyses were conducted using Stata version 13.0, with P < 0.05 considered statistically significant.
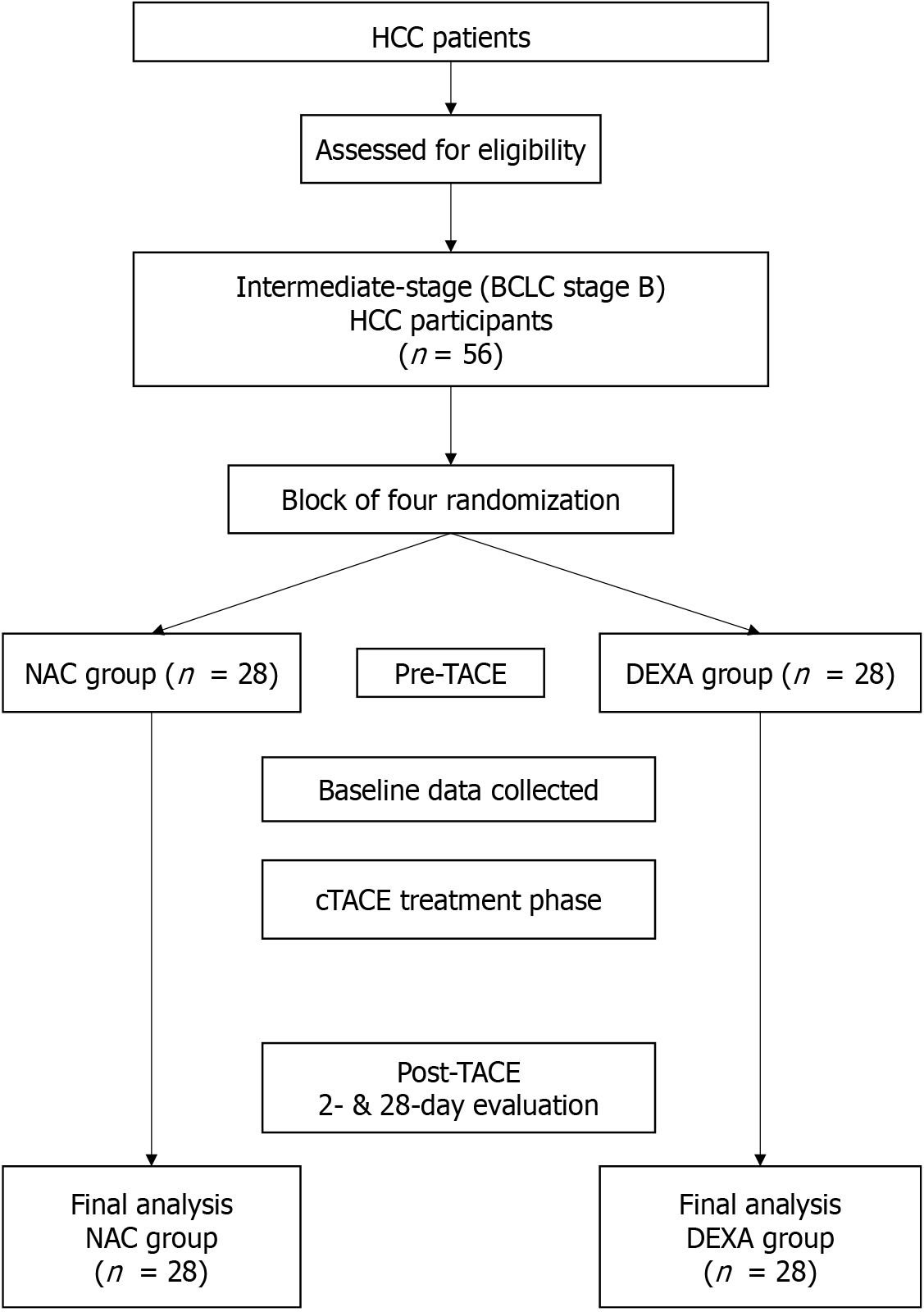
Fifty-six participants with intermediate-stage HCC were enrolled and evenly categorized into two groups: 28 received NAC and 28 received DEXA (Figure 1). Most participants were male (83.9%), with a mean age of 59.7 years. No significant differences were observed between the groups in terms of body mass index; underlying diseases (except diabetes mellitus); cirrhosis etiology; or baseline Child-Pugh, ALBI, and model for end-stage liver disease scores. However, the DEXA group had a significantly higher proportion of patients with diabetes mellitus (39.3%) than the NAC group (10.7%) (P = 0.029) (Table 1).
| Baseline characteristic | Total (n = 56) | NAC (n = 28) | DEXA (n = 28) | P value |
| Male | 47 (83.9) | 24 (85.7) | 23 (82.1) | 1.0004 |
| Age (years) | 59.7 ± 8.7 | 59.0 ± 6.6 | 60.3 ± 10.4 | 0.2593 |
| Body mass index (kg/m2) | 24.3 ± 3.9 | 24.5 ± 4.5 | 24.1 ± 3.4 | 0.7542 |
| Underlying diseases | ||||
| Hypertension | 25 (44.6) | 13 (46.4) | 12 (42.9) | 0.7881 |
| Diabetes mellitus | 14 (25.0) | 3 (10.7) | 11 (39.3) | 0.0294 |
| Dyslipidemia | 7 (12.5) | 1 (3.6) | 6 (21.4) | 0.1014 |
| Etiology of cirrhosis | ||||
| Chronic hepatitis B | 20 (35.7) | 11 (39.3) | 9 (32.14) | 0.5771 |
| Chronic hepatitis C | 23 (41.1) | 13 (46.4) | 10 (35.7) | 0.4151 |
| Alcohol | 19 (33.9) | 11 (39.3) | 8 (28.6) | 0.3971 |
| MASLD | 10 (17.9) | 2 (7.1) | 8 (28.6) | 0.0784 |
| Child-Pugh score | 1.0004 | |||
| A (5-6) | 52 (92.9) | 26 (92.9) | 26 (92.9) | |
| B (7-8) | 4 (7.1) | 2 (7.1) | 2 (7.1) | |
| MELD score | 0.7781 | |||
| < 10 | 37 (66.1) | 18 (64.3) | 19 (67.9) | |
| ≥ 10 | 19 (33.9) | 10 (35.7) | 9 (32.1) | |
| MELD score | 9.0 ± 1.8 | 9.2 ± 2.0 | 8.8 ± 1.6 | 0.6113 |
| MELD-Na | 9.8 ± 4.4 | 9.4 ± 4.3 | 10.2 ± 4.5 | 0.4592 |
| ALBI grade | 0.0944 | |||
| 1 | 5 (8.9) | 4 (14.3) | 1 (3.6) | |
| 2 | 30 (53.6) | 11 (39.3) | 19 (67.9) | |
| 3 | 21 (37.5) | 13 (46.4) | 8 (28.6) | |
| ALBI score | -2.3 ± 0.5 | -2.2 ± 0.6 | -2.4 ± 0.4 | 0.2623 |
| BCLC stage B | 0.2171 | |||
| B1 | 14 (25.0) | 9 (32.1) | 5 (17.9) | |
| B2 | 42 (75.0) | 19 (67.9) | 23 (82.1) | |
| Maximum tumor size (cm) | 6.2 ± 3.7 | 5.2 ± 2.0 | 7.2 ± 4.6 | 0.1323 |
| Tumor number | 0.3264 | |||
| 1 | 8 (14.3) | 2 (7.1) | 6 (21.4) | |
| 2-5 | 37 (66.1) | 21 (75.0) | 16 (57.1) | |
| > 5 | 11 (19.6) | 5 (17.9) | 6 (21.4) | |
| Tumor size plus number (Beyond up to 7) | 39 (69.6) | 18 (64.3) | 21 (75.0) | 0.3831 |
| AST (U/L) | 68.1 ± 40.3 | 75.5 ± 43.9 | 60.6 ± 35.5 | 0.2483 |
| ALT (U/L) | 44.1 ± 35.2 | 48.5 ± 33.3 | 39.6 ± 37.0 | 0.0703 |
| Total bilirubin (mg/dL) | 0.9 ± 0.5 | 0.9 ± 0.6 | 0.8 ± 0.5 | 0.7243 |
| Albumin (mg/dL) | 3.6 ± 0.5 | 3.5 ± 0.6 | 3.7 ± 0.4 | 0.1812 |
| Mitomycin dose (mL) | 14.5 ± 6.1 | 11.4 ± 6.1 | 17.5 ± 4.4 | < 0.0013 |
| Lipiodol dose (mL) | 9.4 ± 5.6 | 8.4 ± 7.3 | 10.4 ± 3.0 | 0.0313 |
Tumor characteristics, including maximum size, number of lesions, Beyond Up-to-7 criteria, and BCLC substaging, were comparable between the groups. However, the mitomycin dose was significantly higher in the DEXA group (17.5 ± 4.4 mL) than in the NAC group (11.4 ± 6.1 mL) (P < 0.001). Similarly, the lipiodol dose was higher in the DEXA group (10.4 ± 3.0 mL) than in the NAC group (8.4 ± 7.3 mL) (P = 0.031).
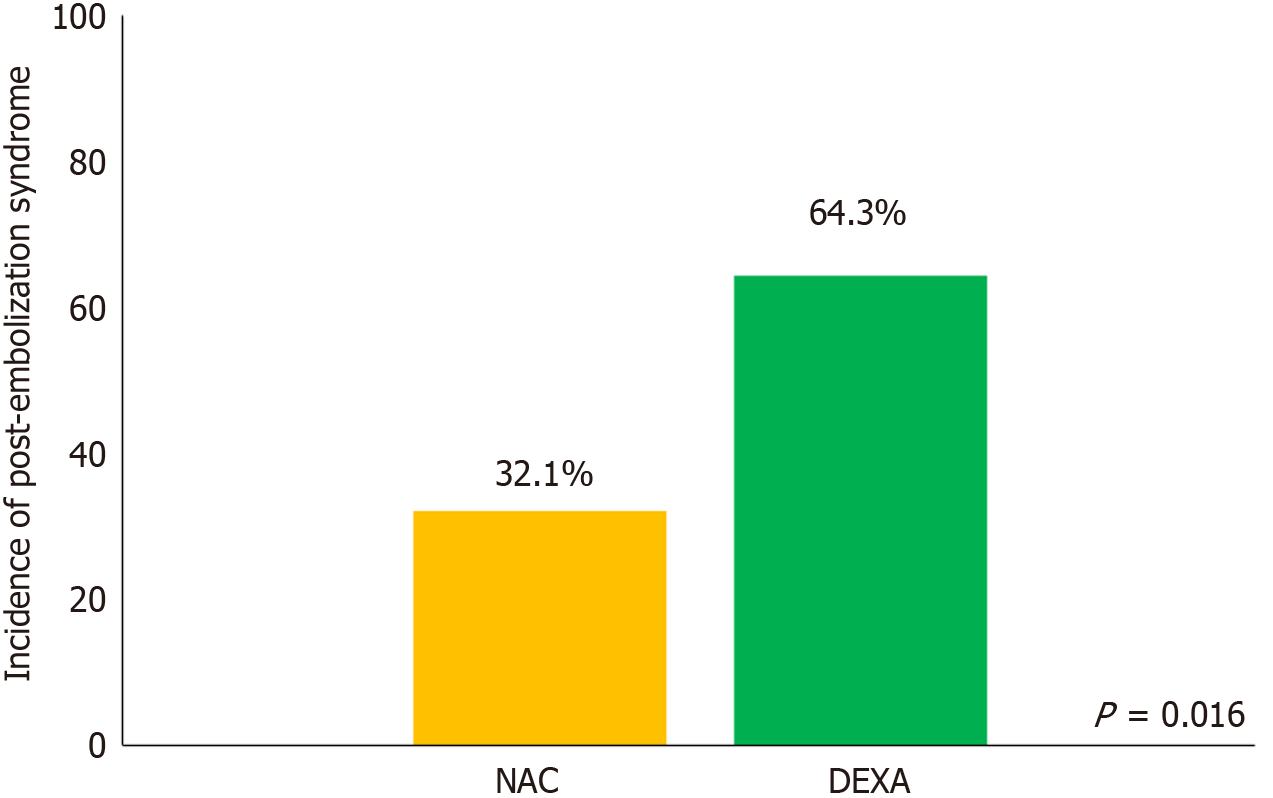
PES occurred in 48.2% of participants within 48 hours post-cTACE. The incidence of PES was significantly lower in the NAC group (32.1%) than in the DEXA group (64.3%) (P = 0.016) (Figure 2). Multivariable analysis, adjusted for confounding variables, showed that NAC significantly reduced the likelihood of developing PES [adjusted odds ratio (OR) = 0.17; 95% confidence interval (CI): 0.03-0.87; P = 0.033]. Tumor burden based on the “Beyond Up-to-7 criteria” was not associated with PES (adjusted OR = 1.00; 95%CI: 0.16-6.30; P = 1.000) (Table 2).
| Variables | PES (n = 27) | No PES (n = 29) | OR (95%CI) | P value | Adjusted OR | P value |
| Treatment group | ||||||
| N-acetylcysteine | 9 (32.1) | 19 (67.9) | 0.26 (0.09- 0.80) | 0.018 | 0.17 (0.03- 0.87) | 0.0331 |
| Dexamethasone | 18 (64.3) | 10 (35.7) | Reference | Reference | ||
| Beyond up to 7 | ||||||
| Yes | 22 (56.4) | 17 (43.6) | 3.11 (0.92-10.52) | 0.069 | 1.00 (0.16-6.30) | 1.0002 |
| No | 5 (29.4) | 12 (70.6) | Reference | Reference |
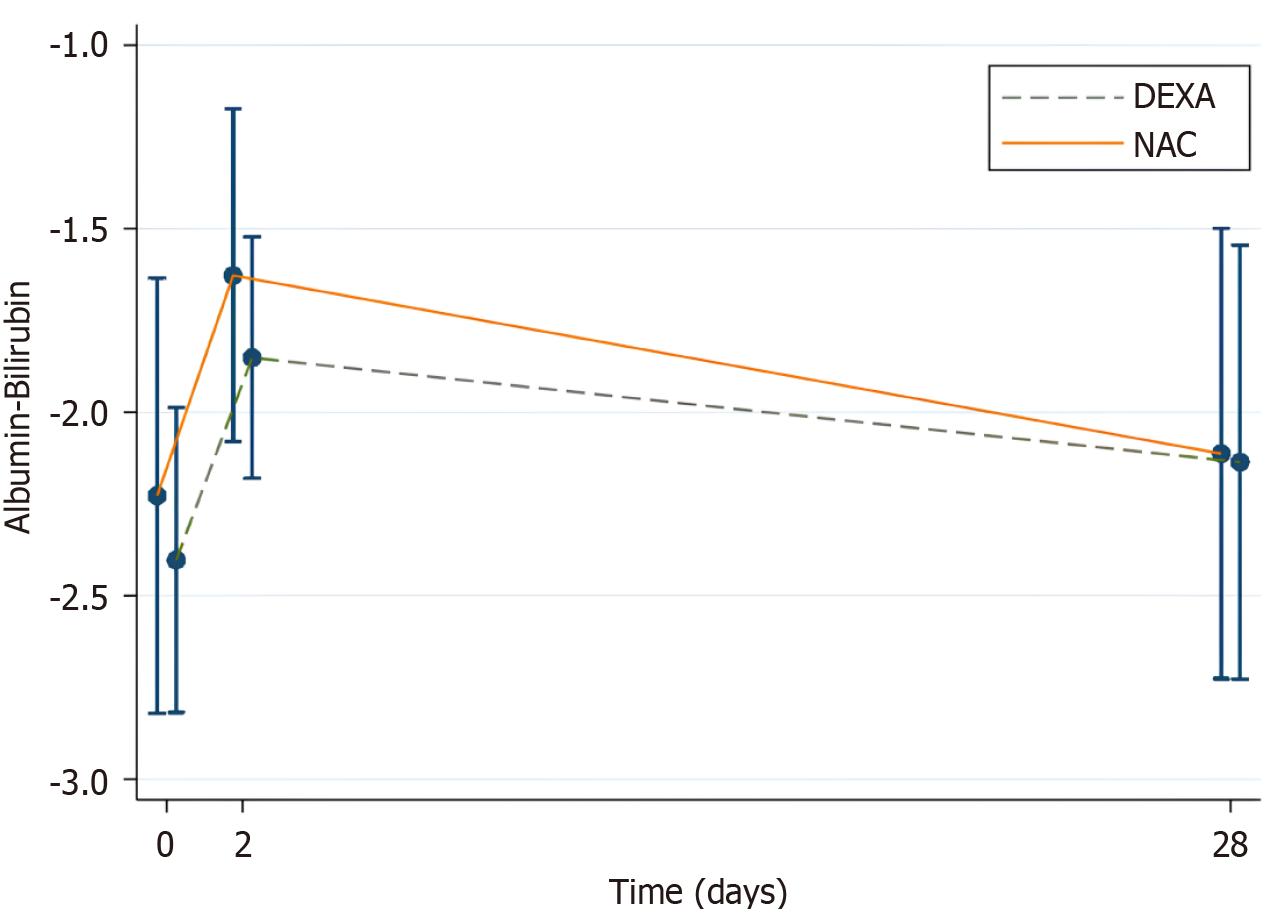
Only two patients developed post-cTACE liver decompensation. To evaluate liver function dynamics, changes in ALBI scores were assessed. ALBI scores worsened on day 2 post-cTACE in both groups but improved by day 28 (Figure 3).
From baseline to day 2, both groups showed significant changes in ALBI scores (NAC: MD 0.60; 95%CI: 0.48-0.72, P < 0.001; DEXA: MD 0.55; 95%CI: 0.43-0.67, P < 0.001). However, the difference in ALBI score change between the groups was not statistically significant (MD 0.05; 95%CI: -0.12 to 0.22, P = 0.568) (Table 3; Figure 3).
| Endpoints | NAC group (n = 28) | DEXA group (n = 28) | Mean ALBI change (NAC group)2 | P value | Mean ALBI change (DEXA group)2 | P value | Difference of mean ALBI change between the two group3 | P value |
| ALBI | 0.18 (-0.07 to 0.42), P = 0.1581 | |||||||
| Day 0 | -2.2 ± 0.6 | -2.4 ± 0.4 | Reference | Reference | Reference | |||
| Day 2 | -1.6 ± 0.5 | -1.9 ± 0.3 | 0.60 (0.48 – 0.72) | < 0.001 | 0.55 (0.43 – 0.67) | < 0.001 | 0.05 (-0.12 – 0.22) | 0.568 |
| Day 28 | -2.1 ± 0.6 | -2.1 ± 0.6 | 0.11 (-0.04 – 0.27) | 0.140 | 0.27 (0.11 – 0.42) | < 0.001 | -0.15 (-0.36 – 0.06) | 0.167 |
By day 28, ALBI scores had returned to baseline in the NAC group (MD 0.11; 95%CI: -0.04 to 0.27, P = 0.140) but remained elevated in the DEXA group (MD 0.27; 95%CI: 0.11 to 0.42, P < 0.001). The between-group difference in ALBI score change at day 28 was also not statistically significant (MD -0.15; 95%CI: -0.36 to 0.06, P = 0.167) (Table 3; Figure 3). Although post-TACE ALBI score alterations were observed in both groups, these changes were generally insufficient to warrant an ALBI stage shift, except in two participants (one from each group) who experienced liver decompensation. Therefore, these alterations are unlikely to influence decisions regarding subsequent TACE sessions.
No serious adverse events were reported. Five participants in the DEXA group developed grade 3 hyperglycemia according to the CTCAE, which was managed with insulin. Among the 27 patients with hepatitis B, no cases of HBV reactivation were observed during the follow-up period.
Most studies investigating the use of DEXA for PES prevention have compared it against placebo, consistently demonstrating its efficacy in reducing PES incidence[6,8,11,15]. Sainamthip et al[6] reported that a single 8 mg dose of DEXA administered before cTACE effectively prevented PES in patients with HCC, improving comfort and potentially shortening hospital stays. Our study employed a similar regimen; however, the incidence of PES remained high at 62%, suggesting reduced effectiveness in our patient population.
A likely explanation is the difference in cancer stage distribution. Sainamthip et al[6] included a higher proportion (23.4%) of BCLC stage A patients, who are at lower risk of PES, resulting in a lower PES incidence of 36.7%. In contrast, our cohort included only BCLC stage B patients, who are at greater risk. Supporting this, Yang et al[9] reported a 78.0% incidence of PES in BCLC stage B patients who received 12 mg of DEXA prior to cTACE, highlighting cancer stage as a potential factor influencing PES risk.
Although variations in chemotherapeutic dosage could potentially influence PES incidence, our multivariable analysis showed no significant impact after adjusting for dosage. However, heterogeneity in tumor burden may have influenced dosage selection, which was determined based on the clinical judgment of experts aiming to minimize the risk of PES.
No severe adverse events related to DEXA, such as hyperglycemia or HBV reactivation, were observed in our study, consistent with previous findings[6,8,11]. The dose and duration of DEXA administration are important considerations. Kuwaki et al[8] used varied regimens over 1-3 days, which effectively reduced PES. Notably, Ogasawara et al[11] de
In addition to the anti-inflammatory effects of DEXA, NAC acts as a glutathione precursor with antioxidant, anti-inflammatory, and mitochondrial-protective properties, helping to reduce cytokine production (e.g., IL-6, TNF-α) and hepatocyte apoptosis[12,16]. Chughlay et al[17] confirmed that the NAC dosage used for liver injury aligns with the regimen employed in our study. We demonstrated that NAC significantly reduced the incidence of PES, even after controlling for tumor burden, baseline liver function, and chemotherapeutic dosing. These findings are consistent with those of Siramolpiwat et al[5], who reported that NAC reduced PES incidence compared with no treatment.
In a recent study[4], we reported promising outcomes with the combination of high-dose DEXA and NAC, observing a substantial reduction in PES incidence, mitigated liver dysfunction, and shortened hospital stays. However, the individual contribution of each agent to PES reduction could not be clearly determined. Biolato et al[18] cautioned that high-dose DEXA may increase metabolic risks. These considerations highlight the potential utility of NAC as a safer monotherapy for PES prevention.
Although NAC is generally well tolerated, clinicians should be mindful of dose- and duration-dependent adverse effects, particularly during the loading phase. Rare but notable side effects include anaphylactoid reactions, flushing, angioedema, bronchospasm, and hypotension[12]. In our study, adverse effects of NAC were mild and manageable, consistent with the findings of a previous study[19]. However, HCC patients with a known allergy to NAC or with severe asthma are not suitable candidates for NAC treatment.
Post-cTACE liver dysfunction was minimal, likely due to the predominance of Child–Pugh A patients and the expertise of the interventional radiologists. We used ALBI score dynamics to detect subtle changes in liver function, as the ALBI score is more sensitive than the Child–Pugh score in assessing hepatic reserve[20].
A major strength of our study is its pioneering head-to-head comparison of NAC and DEXA for the prevention of PES, thereby addressing a notable gap in prior research. In addition to evaluating PES incidence, the study incorporated dynamic ALBI scoring to assess changes in liver function and to validate drug safety from multiple perspectives. These clinical outcomes provide valuable insights for optimizing peri-procedural pharmacologic strategies. Moreover, by focusing exclusively on HCC patients at BCLC stage B-a population at high risk for PES-we minimized potential confounding effects from early or advanced disease stages.
However, our study is limited by its small sample size, which may restrict the generalizability of the findings. A larger cohort is needed to confirm the observed benefits and ensure external validity. Additionally, the absence of a placebo control group limits the ability to isolate the true effects of NAC and DEXA. Future research should explore additional variables such as tumor location, embolization technique (super-selective vs selective or non-selective), and other procedural factors that may influence PES risk. A better understanding of these elements will help determine whether PES prevention should be universally applied or tailored to specific patient subgroups.
NAC significantly reduces the incidence of PES compared with DEXA, regardless of its impact on liver function recovery. These results suggest that NAC is a preferable option for PES prevention in BCLC-B stage HCC patients with preserved liver function. Future research should focus on larger and more specific subgroups within the intermediate HCC stage to further clarify the advantages of NAC, particularly during liver ischemia–reperfusion injury.
| 1. | Sethasine S, Simasingha N, Ratana-Amornpin S, Mahachai V. Real world for management of hepatocellular carcinoma: a large population-based study. Scand J Gastroenterol. 2023;58:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Mason MC, Massarweh NN, Salami A, Sultenfuss MA, Anaya DA. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Agrawal R, Majeed M, Aqeel SB, Wang Y, Haque Z, Omar YA, Upadhyay SB, Gast T, Attar BM, Gandhi S. Identifying predictors and evaluating the role of steroids in the prevention of post-embolization syndrome after transarterial chemoembolization and bland embolization. Ann Gastroenterol. 2021;34:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Simasingha N, Tanasoontrarat W, Claimon T, Sethasine S. Efficacy of dexamethasone and N-acetylcysteine combination in preventing post-embolization syndrome after transarterial chemoembolization in hepatocellular carcinoma. World J Gastroenterol. 2023;29:890-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 5. | Siramolpiwat S, Punjachaipornpon T, Pornthisarn B, Vilaichone RK, Chonprasertsuk S, Tangaroonsanti A, Bhanthumkomol P, Phumyen A, Yasiri A, Kaewmanee M. N-Acetylcysteine Prevents Post-embolization Syndrome in Patients with Hepatocellular Carcinoma Following Transarterial Chemoembolization. Dig Dis Sci. 2019;64:3337-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Sainamthip P, Kongphanich C, Prasongsook N, Chirapongsathorn S. Single dose dexamethasone prophylaxis of postembolisation syndrome after chemoembolisation in hepatocellular carcinoma patient: A randomised, double-blind, placebo-controlled study. World J Clin Cases. 2021;9:9059-9069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Lu H, Zheng C, Liang B, Xia X. Efficacy and safety analysis of dexamethasone + palonosetron in prevention of post-embolization syndrome after D-TACE: A retrospective study. Medicine (Baltimore). 2023;102:e35433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Kuwaki K, Nouso K, Miyashita M, Makino Y, Hagihara H, Moriya A, Adachi T, Wada N, Yasunaka Y, Yasunaka T, Takeuchi Y, Onishi H, Nakamura S, Ikeda F, Shiraha H, Takaki A, Okada H. The Efficacy and Safety of Steroids for Preventing Postembolization Syndrome after Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma. Acta Med Okayama. 2019;73:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, Chun HJ, Jang JW, Bae SH, Choi JY, Yoon SK. Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Study. J Vasc Interv Radiol. 2017;28:1503-1511.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Kogut MJ, Chewning RH, Harris WP, Hippe DS, Padia SA. Postembolization syndrome after hepatic transarterial chemoembolization: effect of prophylactic steroids on postprocedure medication requirements. J Vasc Interv Radiol. 2013;24:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Nagai K, Nakagawa T, Sugawara T, Hanaoka H, Kanai F, Yokosuka O. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 12. | Tenório MCDS, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel). 2021;10:967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 13. | Sun Y, Pu LY, Lu L, Wang XH, Zhang F, Rao JH. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J Gastroenterol. 2014;20:15289-15298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2172] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 15. | Ishikawa T. Prevention of post-embolization syndrome after transarterial chemoembolization for hepatocellular carcinoma-is prophylactic dexamethasone useful, or not? Hepatobiliary Surg Nutr. 2018;7:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Dludla PV, Nkambule BB, Mazibuko-Mbeje SE, Nyambuya TM, Marcheggiani F, Cirilli I, Ziqubu K, Shabalala SC, Johnson R, Louw J, Damiani E, Tiano L. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants (Basel). 2020;9:1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Chughlay MF, Kramer N, Werfalli M, Spearman W, Engel ME, Cohen K. N-acetylcysteine for non-paracetamol drug-induced liver injury: a systematic review protocol. Syst Rev. 2015;4:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Biolato M, Pompili M. Dexamethasone and N-acetylcysteine before transarterial chemoembolization in hepatocellular carcinoma: A Western perspective. World J Gastroenterol. 2024;30:3635-3639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Hu J, Zhang Q, Ren X, Sun Z, Quan Q. Efficacy and safety of acetylcysteine in "non-acetaminophen" acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015;39:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Navadurong H, Thanapirom K, Wejnaruemarn S, Prasoppokakorn T, Chaiteerakij R, Komolmit P, Treeprasertsuk S. Validation of the albumin-bilirubin score for identifying decompensation risk in patients with compensated cirrhosis. World J Gastroenterol. 2023;29:4873-4882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/