©The Author(s) 2025.
World J Gastroenterol. Aug 7, 2025; 31(29): 109947
Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.109947
Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.109947
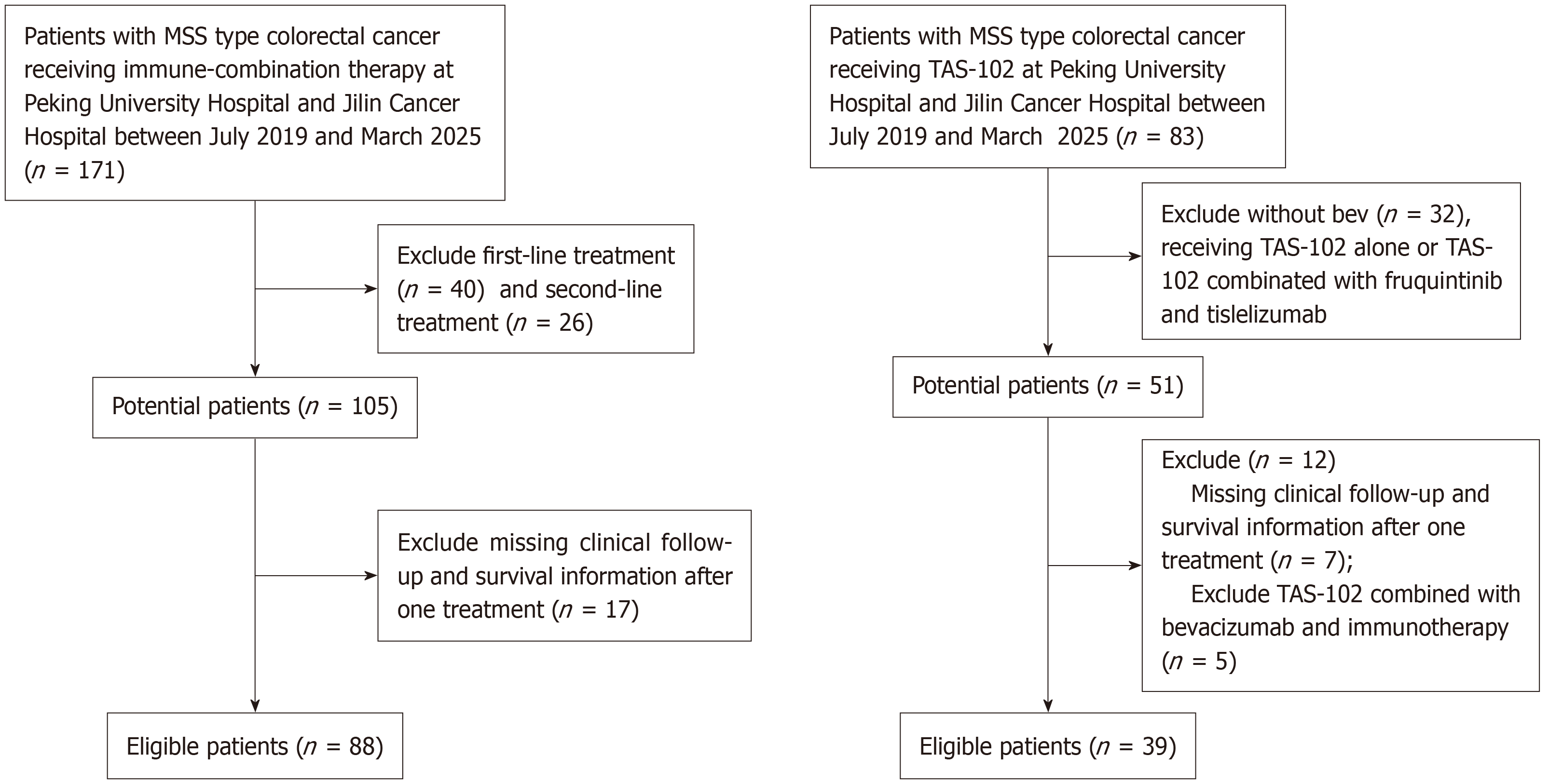
Figure 1 Flow chart of patient inclusion.
MSS: Microsatellite stable; TAS-102: Trifluridine/tipiracil.
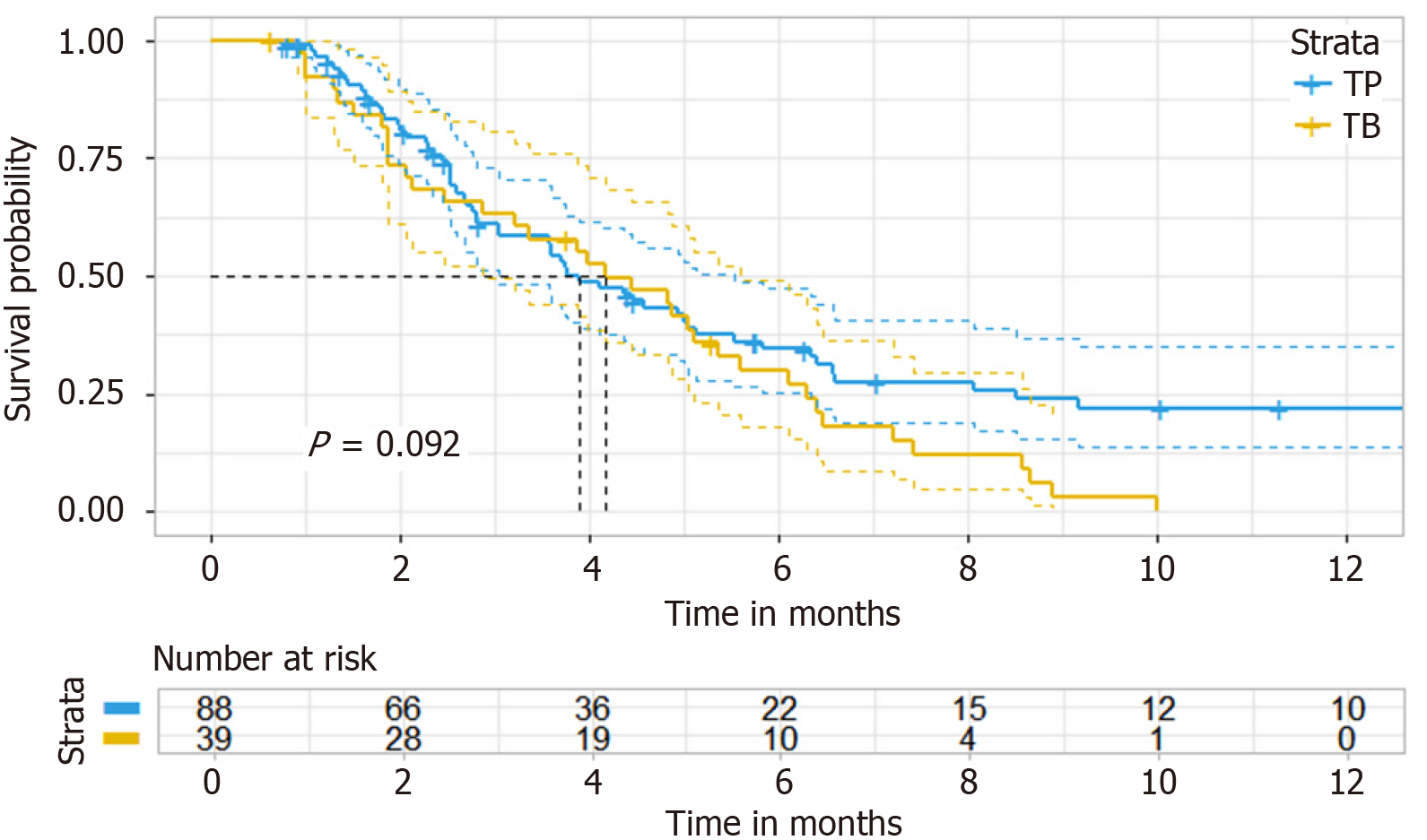
Figure 2 Kaplan-Meier curve of original cohort (progression-free survival).
TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
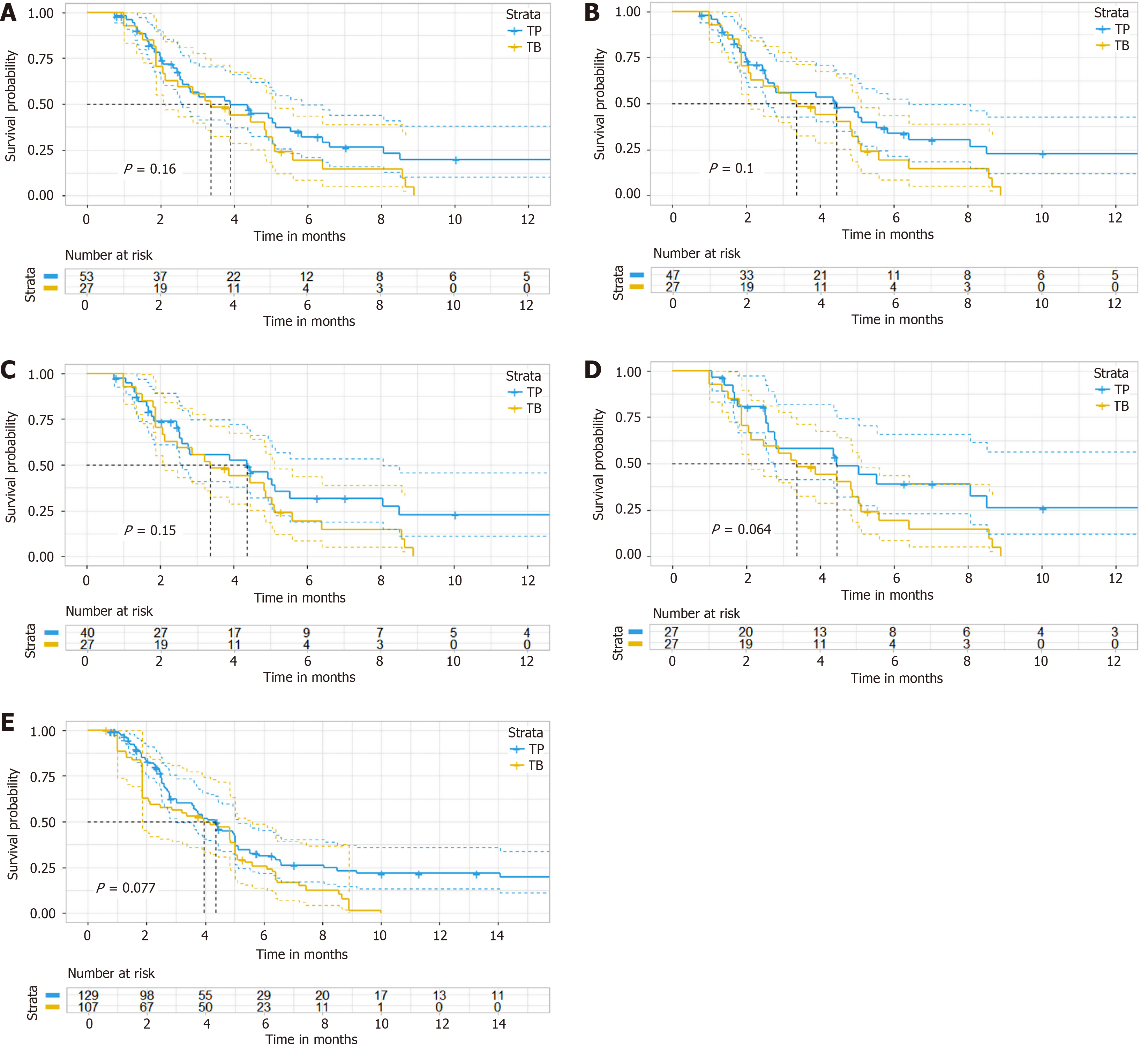
Figure 3 Propensity score-matching analysis in progression-free survival.
A: After propensity score-matching analysis in progression-free survival (PFS) (ratio = 4); B: After propensity score-matching analysis in PFS (ratio = 3); C: After propensity score-matching analysis in PFS (ratio = 2); D: After propensity score-matching analysis in PFS (ratio = 1); E: After inverse probability of treatment weighting analysis (PFS). TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
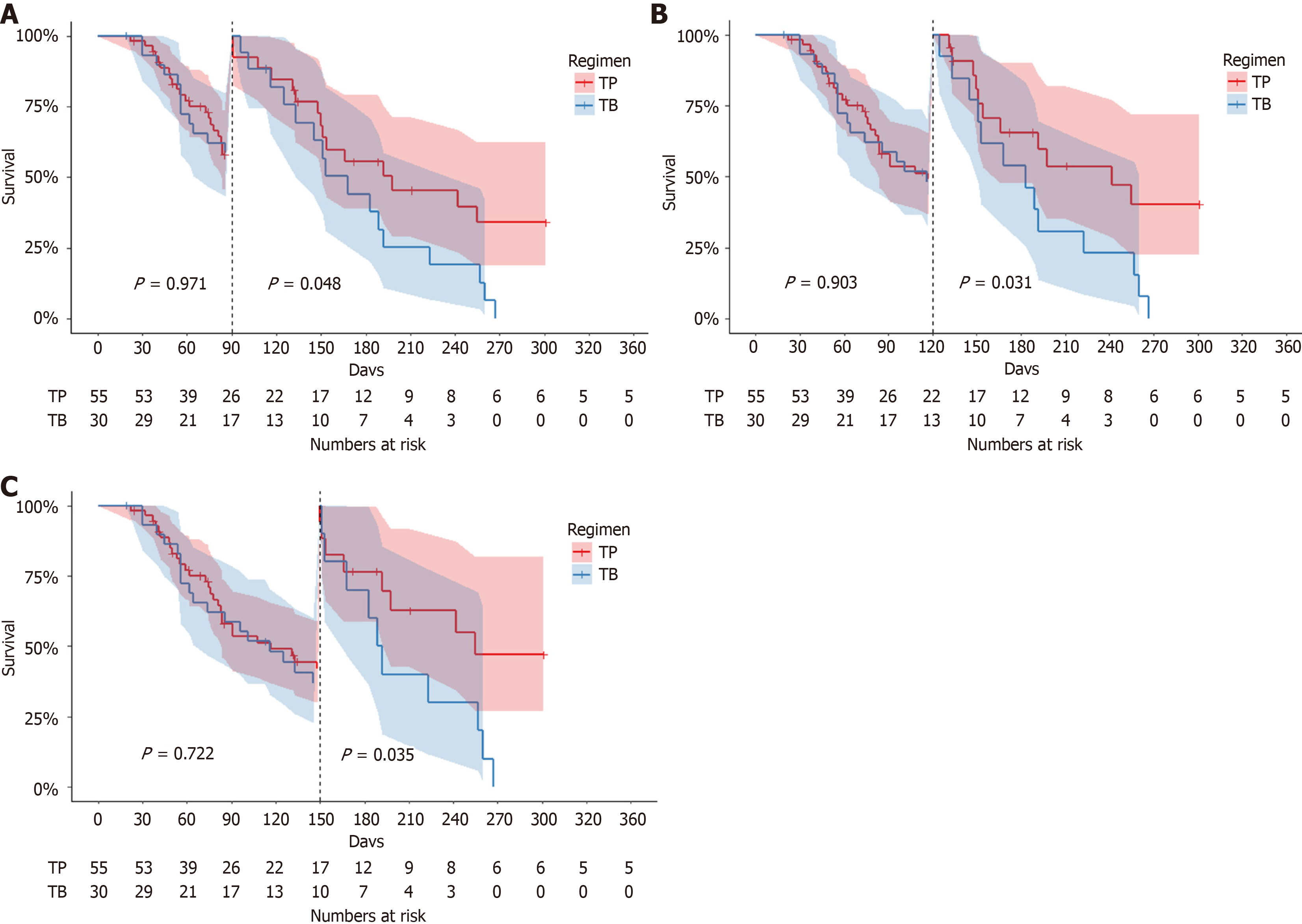
Figure 4 Kaplan-Meier curve of landmark analysis in progression-free survival.
A: Survival curves stratified by a 90-day landmark timepoint; B: Survival curves stratified by a 120-day landmark timepoint; C: Survival curves stratified by a 150-day landmark timepoint. TP: Targeted therapy combined with anti-programmed cell death 1 immunotherapy; TB: Bevacizumab.
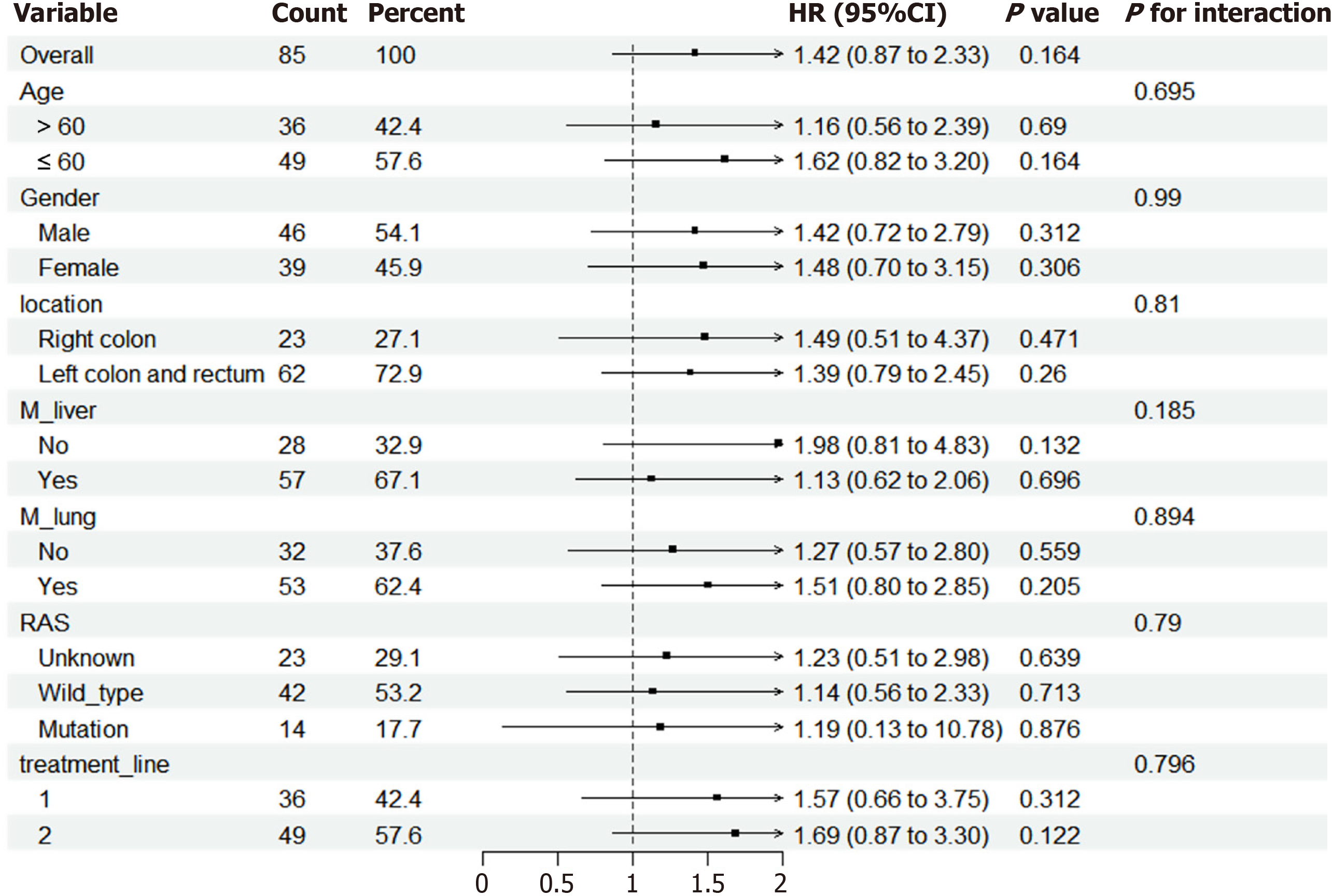
Figure 5 Forest plots depict the hazard ratios and 95% confidence intervals for progression-free survival by subgroup.
HR: Hazard ratio; CI: Confidence interval.
- Citation: Gao Z, Wang XY, Song T, Shen ZG, Wang XY, Wu SK, Jin X. Targeted therapy combined with immunotherapy vs trifluridine/tipiracil with bevacizumab as late-line therapy in metastatic colorectal cancer. World J Gastroenterol 2025; 31(29): 109947
- URL: https://www.wjgnet.com/1007-9327/full/v31/i29/109947.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i29.109947