Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.110004
Revised: June 13, 2025
Accepted: July 8, 2025
Published online: August 7, 2025
Processing time: 70 Days and 0.7 Hours
Ultra-low rectal cancer (ULRC), defined as a lesion located within 5 cm of the anal verge, poses considerable clinical challenges because the treatment decision must balance oncological eradication with preservation of anal function. Historically, abdominoperineal resection (APR) has served as a standard approach for tumor eradication in these patients, but a permanent stoma significantly reduces pa
To address a persistent debate in ULRC management, we compared ISR and APR outcomes through rigorous methodology.
A retrospective analysis of patients undergoing surgery at three centers in China between 2012 and 2023 was performed with propensity score matching (PSM).
A total of 803 patients (435 in the ISR group and 368 in the APR group) met the inclusion criteria, with 289 comprising each of the two groups after PSM. Over a median follow-up of 47.2 months, the absolute 5-year overall survival (OS) improved by 6.7% with ISR (80.8% vs 74.1%, P = 0.032). Cox regression analysis confirmed ISR (HR = 0.554, 95%CI: 0.371-0.828, P = 0.004) as an independent protective factor for OS and reduced local recurrence (9.5% vs 12.9%, P = 0.019). With respect to short-term complications, despite higher anastomotic leakage rates (11.4% vs 1.0%), ISR significantly reduced total complications (29.4% vs 42.2%, P = 0.001) and hospitalization duration (9.8 days vs 12.9 days, P < 0.001). Moreover, incision infection, urinary retention, circumferential resection margins, and hospitalization time were greater in the APR group (P < 0.05).
The long-term prognosis of ULRC treated with ISR is excellent, with no increase in overall surgical complications or hospital stay duration, indicating that ISR is a feasible alternative to APR for managing ULRC.
Core Tip: As the largest propensity score-matched study comparing intersphincteric resection (ISR) and abdominoperineal resection (APR), we minimized selection bias by balancing 13 covariates across 803 patients (289 matched pairs). ISR demonstrated a 6.7% absolute improvement in 5-year overall survival (80.8% vs 74.1%, HR = 0.554, P = 0.004) and reduced local recurrence (9.5% vs 12.9%, P = 0.019), establishing its oncologic superiority. Despite higher anastomotic leakage rates (11.4% vs 1.0%), ISR significantly reduced total complications (29.4% vs 42.2%, P = 0.001) and hospitalization duration (9.8 days vs 12.9 days, P < 0.001), supporting its role as the preferred sphincter-preserving alternative to APR.
- Citation: Wang GC, Chen JX, Pan HF, Ye K, Guo YC, Huang Y. Long-term efficacy and short-term outcomes of intersphincteric resection vs abdominoperineal resection in patients with ultra-low rectal cancer. World J Gastroenterol 2025; 31(29): 110004
- URL: https://www.wjgnet.com/1007-9327/full/v31/i29/110004.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i29.110004
Ultra-low rectal cancer (ULRC), defined as a lesion located within 5 cm of the anal verge, poses considerable clinical challenges because the treatment decision must balance oncological eradication with preservation of anal function[1]. Historically, abdominoperineal resection (APR) has served as a standard approach for tumor eradication in these patients, but a permanent stoma significantly reduces patients’ quality of life[2]. In contrast, intersphincteric resection (ISR) can maintain anal function, thereby improving quality of life; however, the debate surrounding short-term postoperative complications and long-term prognosis has not been fully resolved[3,4].
Several studies have shown that ISR confers a better quality of life in the short term[2,4]. However, postoperative complications in patients treated with ISR—especially anastomotic leakage and functional impairment—remain key limiting barriers to the widespread use of ISR[5]. Klose et al[6] reported that urinary incontinence was the most important complication following ISR, whereas Luvisetto et al[7] reported that patients treated with APR had a higher rate of postoperative complications and a longer hospitalization. These discordant findings highlight ongoing controversies that warrant further investigation. Furthermore, some studies have concluded that ISR and APR differ minimally in terms of long-term efficacy[8,9]. One of the largest meta-analyses, which included 20 nonrandomized controlled studies with a total of 1217 patients who underwent ISR and 1135 patients who underwent APR, reported no significant difference between the two groups in terms of 5-year overall survival (OS; HR = 0.93, 95%CI: 0.60-1.46; P = 0.76)[10]. Conversely, other studies have indicated better survival in patients who undergo sphincter preservation than in those treated with APR[3,11].
Since most of these studies were either single-center or retrospective in design and featured limited sample sizes and variable inclusion criteria, substantial selection bias may persist, restricting generalizability. Although prospective randomized controlled trials provide high-quality evidence, randomization to ISR or APR remains difficult owing to ethical and practical barriers[12]. Indeed, such trials are unlikely to be conducted, leading to a lack of existing studies in this area. Therefore, large-scale multicenter retrospective cohort studies are crucial to address this issue and provide more reliable data.
Accordingly, in this study, 435 ISR patients and 368 APR patients from three major colorectal centers were retrospectively evaluated. By employing propensity score matching (PSM) to reduce selection bias, we aimed to compare both short-term complications and long-term survival, thereby offering additional evidence for clinical decision-making and future research.
This was a multicenter, retrospective cohort study that included patients who underwent ISR or APR at three institutions in China (Zhangzhou Affiliated Hospital of Fujian Medical University, Fujian Medical University Union Hospital, and the Second Affiliated Hospital of Fujian Medical University) between January 2012 and December 2023. All the participating surgical centers are high-volume colorectal diagnosis and treatment centers that perform many laparoscopic total mesorectal excision (TME) surgeries and are fully competent in ISR or APR surgeries. This study was approved by the ethics committees of all three participating centers and complied with the principles of the Declaration of Helsinki; patient confidentiality was protected through deidentification during data analysis, and additional informed consent was therefore waived. The study was registered at the Chinese Clinical Trial Registry, registration number ChiCTR2500096635.
Patients included in this study met the following criteria: (1) Had a histologically confirmed diagnosis of rectal cancer with a tumor located ≤ 5 cm from the anal verge; (2) Received a standardized preoperative evaluation that included pelvic magnetic resonance imaging, thoracic and abdominal computed tomography, and tumor biomarker detection and indicated no distant metastasis (DM) before treatment (cM0); (3) Met the eligibility criteria for radical ISR or APR according to the principle of TME; and (4) Had an American Society of Anesthesiologists (ASA) score ≤ 3 and complete clinical and pathological data were available.
Exclusion criteria for this study were the following: (1) Evidence of distant metastases (cM1); (2) A diagnosis of other malignant tumors or benign rectal lesions; (3) A history of prior rectal or anal surgery; (4) No-R0 resection; and (5) Severe comorbidities or active infections contraindicative to surgery.
Before PSM, all the data were thoroughly cleaned and preprocessed. Missing values for key variables (e.g., serum CEA or CA19-9 values) were addressed using multiple imputation by chained equations. Outliers in continuous variables [e.g., body mass index (BMI) or tumor diameter] were identified using boxplots and Z scores and then adjusted or replaced where appropriate.
Following transabdominal separation under TME, patients underwent one of two different operative strategies[13]. In the ISR group, if the tumor was less than 1 cm from the anal canal rectal muscle ring, the ISR technique was used to separate the sphincter space to obtain sufficient distal margins, with separation extending at least 2 cm below the tumor margin. The ISR procedure is typically performed via a complete pelvic approach. When the pelvic approach cannot provide an adequate margin or cannot fully ensure a negative distal margin, a hybrid ISR approach is employed, i.e., after completing the pelvic approach, the surgeon switches to an anal approach to measure the lower margin and then performs an anatomic incision of the mucosal and submucosal layers until the full thickness is incised, reconnecting with the pelvic approach. In all patients, the proximal colon was anastomosed to the anal canal postoperatively. In the APR group, APR was selected when the tumor was either < 2 cm from the anal verge or had invaded the external sphincter, precluding sphincter preservation. The anal canal, external sphincter, and surrounding soft tissues were resected via a perineal incision to ensure specimen integrity. A permanent sigmoid colostomy was subsequently created in the left lower abdomen.
The primary endpoint was OS, defined as the time from surgery to death from any cause. The secondary endpoints were disease-free survival (DFS), local recurrence (LR), and DM. LR and DM were defined as the times to first local or distant relapse, respectively. DFS was defined as the time from the beginning of surgery to disease recurrence (including LR and DM) or death from any cause. The 30-day short-term postoperative complication rates included AL, incisional infection, bleeding, and bowel obstruction.
PSM analysis is commonly used in retrospective studies to construct a randomized experiment-like situation and minimize selection bias between the two groups of patients[14]. We performed PSM analysis using a logistic regression model to estimate the propensity score for each patient. The matched variables included the following: (1) Demographic characteristics: Sex, age, BMI, and ASA; (2) Tumor characteristics: CEA levels, CA19-9 Levels, tumor diameter, pT stage, pN stage, pTNM stage, histopathological type, and tumor deposits; and (3) Therapeutic characteristics: Neoadjuvant chemoradiotherapy (nCRT) and surgical procedures. The caliper was set to 0.2, and matching was performed at a 1:1 ratio.
The clinicopathological characteristics of the two groups of patients before and after PSM were compared. Continuous variables are expressed as the means ± SD. Independent-sample t-tests were used for between-group comparisons, whereas χ2 or Fisher's exact tests were used for comparisons. OS, DFS, LR, and DM were estimated with the Kaplan-Meier method and compared via log-rank tests. A Cox proportional hazards model was then employed to identify independent predictors of OS, with ISR and APR as covariates. All tests were two-sided, and a difference in the data with P < 0.05 was considered significant. All the statistical analyses were performed using SPSS 28.0 (IBM, Chicago, IL, United States) and R 4.4.4 (http://www.r-project.org) software.
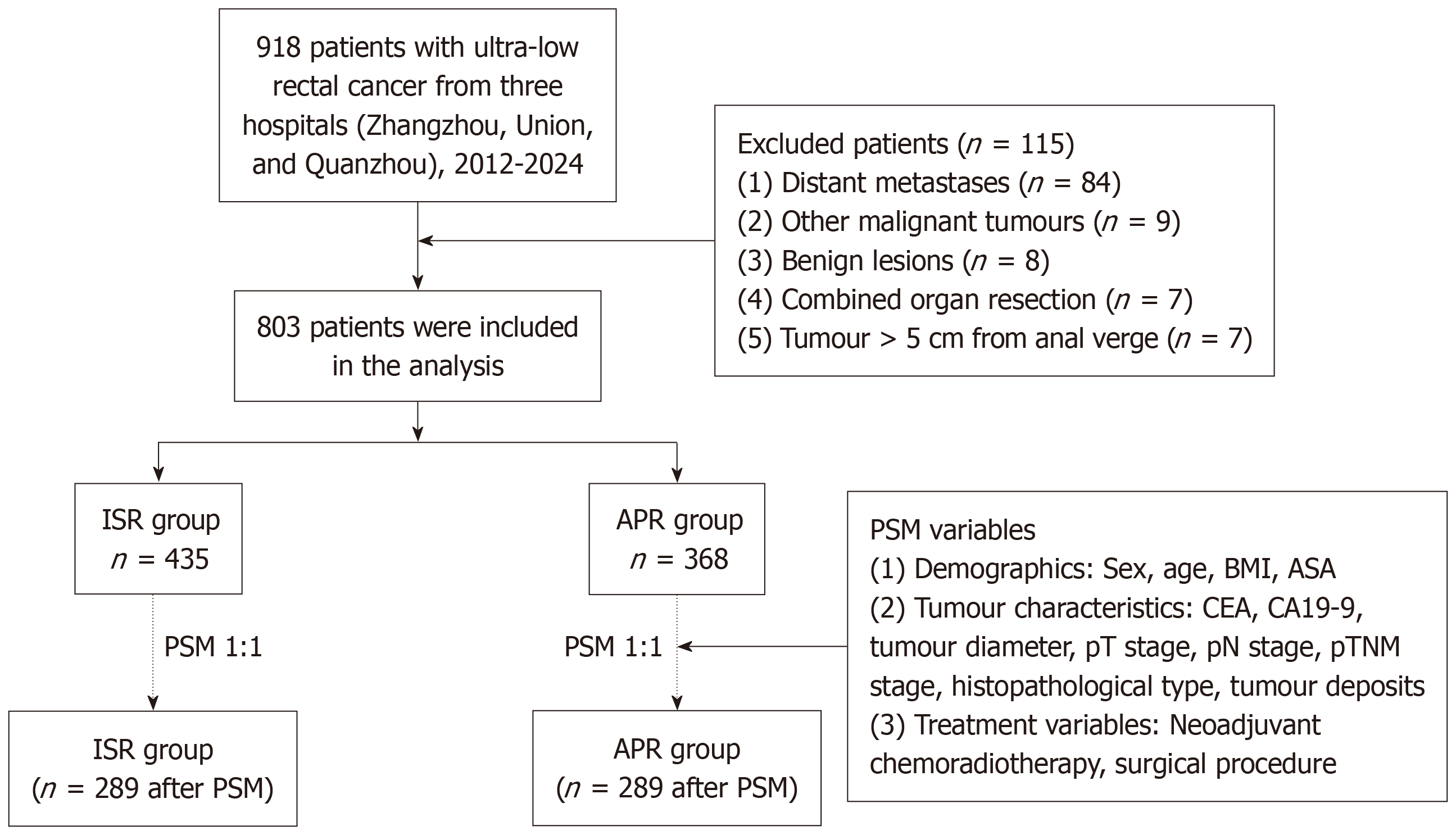
A total of 918 ULRC patients from three centers were initially screened, and 115 patients were excluded, including those with remote metastases (n = 84), other types of malignant tumors (n = 8), benign tumors (n = 7), combined organ resection (n = 7), and a tumor > 5 cm from the anal verge (n = 7). A total of 894 patients remained, comprising 525 in the ISR group and 368 in the APR group (Figure 1).
Before PSM, there were statistically significant differences between the two groups in terms of ASA grade; tumor diameter; CEA levels; CA19-9 Levels; and pT, pN and pTNM stages. To ensure comparability and reduce bias, 289 patients were retained in each group after PSM (ISR: 249 complete trans-pelvic, 40 hybrid approaches; APR: 289). The 13 variables, including demographic characteristics, tumor attributes, and treatment factors, were similar across the matched cohorts (Table 1).
| Variables | Before PSM | After PSM | ||||
| ISR group (n = 435) | APR group (n = 368) | P value | ISR group (n = 289) | APR group (n = 289) | P value | |
| Sex | 0.575 | 0.559 | ||||
| Female | 245 (56.3) | 200 (54.3) | 160 (55.4) | 153 (52.9) | ||
| Male | 190 (43.7) | 168 (45.7) | 136 (47.1) | 136 (47.1) | ||
| Age (years) | 0.084 | 0.676 | ||||
| ≤ 50 | 106 (24.4) | 71 (19.3) | 55 (19.0) | 59 (20.4) | ||
| > 50 | 329 (75.6) | 297 (80.7) | 234 (81.0) | 230 (79.6) | ||
| BMI (kg/m2) | 0.435 | 0.400 | ||||
| ≤ 25 | 350 (80.5) | 304 (82.6) | 237 (82.0) | 229 (79.2) | ||
| > 25 | 85 (19.5) | 64 (17.4) | 52 (18.0) | 60 (20.8) | ||
| ASA grade | 0.025 | 0.970 | ||||
| I | 41 (9.4) | 27 (7.3) | 22 (7.6) | 22 (7.6) | ||
| II | 350 (80.5) | 281 (76.4) | 228 (78.9) | 230 (79.6) | ||
| III | 44 (10.1) | 60 (16.3) | 39 (13.5) | 37 (12.8) | ||
| Tumour diameter (cm) | < 0.001 | 0.491 | ||||
| ≤ 3 | 225 (51.7) | 119 (32.2) | 103 (35.6) | 111 (38.4) | ||
| > 3 | 210 (48.3) | 249 (67.7) | 186 (64.4) | 178 (61.6) | ||
| CEA (ng/mL) | < 0.001 | 0.846 | ||||
| ≤ 5 | 353 (81.1) | 261 (70.9) | 218 (75.4) | 220 (76.1) | ||
| > 5 | 82 (18.9) | 107 (29.1) | 71 (24.6) | 69 (23.9) | ||
| CA19-9 (U/mL) | < 0.001 | 0.883 | ||||
| ≤ 37 | 410 (94.3) | 316 (85.9) | 264 (91.3) | 263 (91.0) | ||
| > 37 | 25 (5.7) | 52 (14.1) | 25 (8.7) | 26 (9.0) | ||
| pT stage | < 0.001 | 0.819 | ||||
| ≤ T3 | 388 (89.2) | 271 (73.6) | 243 (84.1) | 245 (84.8) | ||
| T4 | 47 (10.8) | 97 (26.4) | 46 (15.2) | 44 (15.2) | ||
| pN stage | 0.045 | 0.724 | ||||
| N0 | 300 (69.0) | 229 (62.2) | 191 (66.1) | 195 (67.5) | ||
| N+ | 135 (31.0) | 139 (37.8) | 98 (33.9) | 94 (32.5) | ||
| pTNM stage | < 0.001 | 0.705 | ||||
| 0 | 36 (14.0) | 36 (9.8) | 31 (10.7) | 31 (10.7) | ||
| I | 135 (31.0) | 77 (20.9) | 86 (29.8) | 77 (26.6) | ||
| II | 104 (23.9) | 116 (31.5) | 74 (25.6) | 85 (29.4) | ||
| III | 135 (31.0) | 139 (37.8) | 98 (33.9) | 94 (32.5) | ||
| Histopathology | 0.719 | 0.761 | ||||
| Adenocarcinoma | 426 (97.9) | 359 (97.6) | 283 (97.9) | 284 (98.3) | ||
| Mucinous adenocarcinoma | 9 (2.1) | 9 (2.4) | 6 (2.1) | 5 (1.7) | ||
| nCRT | 0.383 | 0.244 | ||||
| No | 197 (45.3) | 178 (48.4) | 151 (52.2) | 137 (47.4) | ||
| Yes | 238 (54.7) | 190 (51.6) | 138 (47.8) | 152 (52.6) | ||
| Surgical procedures | 0.714 | 0.864 | ||||
| Laparoscopy | 391 (89.9) | 337 (91.6) | 263 (91.7) | 265 (91.7) | ||
| Open | 34 (7.8) | 24 (6.5) | 18 (6.2) | 18 (6.2) | ||
| Robotic | 10 (2.3) | 7 (1.9) | 8 (2.8) | 6 (2.1) | ||
| ISR approach | -1 | -1 | ||||
| Complete trans-pelvic | 372 (85.5) | 0 (0.0) | 249 (86.2) | 0 (0.0) | ||
| Mixed | 63 (14.5) | 0 (0.0) | 40 (13.8) | 0 (0.0) | ||
| APR | 0 (0.0) | 368 (100.0) | 0 (0.0) | 289 (100.0) | ||
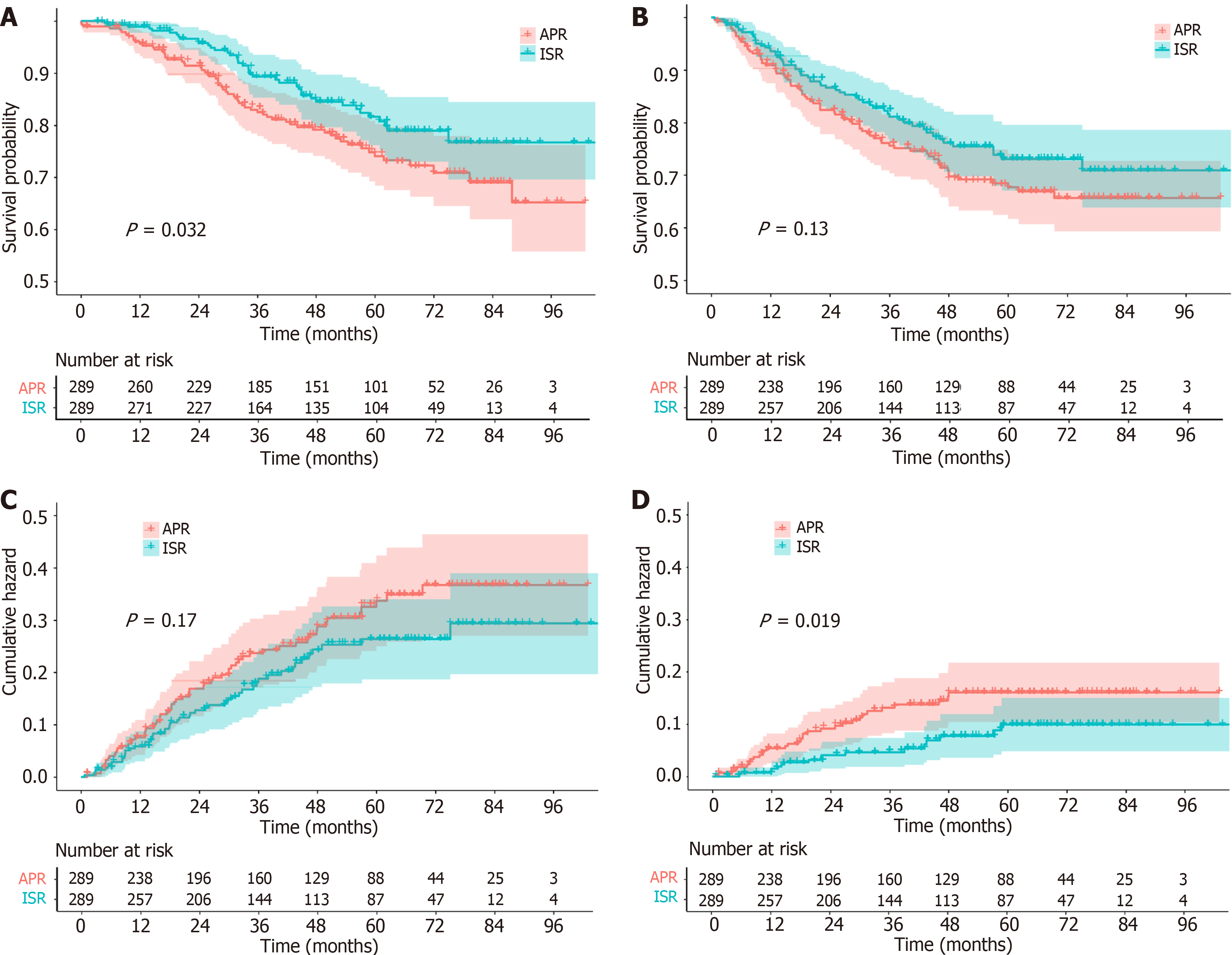
We used the Kaplan-Meier method to estimate survival functions and the log-rank test to compare survival differences between the two groups. Figure 2A shows the OS of the two groups of patients, with 5-year OS rates of 80.8% in the ISR group and 74.1% in the APR group, with a P value of 0.032, indicating a statistically significant difference in survival between the two groups. Figure 2B shows the DFS rates for the two groups of patients, with a 5-year DFS rate of 73.1% for the ISR group and 67.7% for the APR group, with a P value of 0.13, indicating that the difference in DFS between the two groups was not statistically significant. Figure 2C shows the DM rates of the two groups of patients. The 5-year DM rate was 23.2% in the ISR group and 28.6% in the APR group, with a P value of 0.17, indicating that the difference in DM between the two groups was not statistically significant. Figure 2D shows the LR rates for the two groups of patients. The 5-year LR rate for ISR was 9.5%, and the APR rate was 12.9%, with a P value of 0.019, indicating a significant difference between the two groups (Figure 2).
The aggregate distant metastasis rate did not differ between the two groups (18.0% vs 22.8%, P = 0.149). Analysis of metastatic sites revealed no meaningful discrepancies in most organ-specific rates, but the APR group had a greater incidence of metastases than did the ISR group in terms of the incidence of pelvic (P = 0.045), lateral lymph node (P = 0.036), and retroperitoneal (P = 0.004) metastases (Table 2).
| Metastatic site | APR group (n = 289) | ISR group (n = 289) | Total (n = 578) | P value |
| Total distant | 66 (22.8) | 52 (18.0) | 118 (20.4) | 0.149 |
| Lung | 33 (11.4) | 28 (9.7) | 61 (10.6) | 0.498 |
| Liver | 15 (5.2) | 14 (4.8) | 29 (5.0) | 0.849 |
| Bone | 12 (4.2) | 13 (4.5) | 25 (4.3) | 0.838 |
| Pelvis | 15 (5.2) | 7 (2.4) | 22 (3.8) | 0.082 |
| Abdominal cavity | 15 (5.2) | 6 (2.1) | 21 (3.6) | 0.045 |
| Lateral lymph nodes | 14 (4.8) | 5 (1.7) | 19 (3.3) | 0.036 |
| Retroperitoneal | 13 (4.5) | 2 (0.7) | 15 (2.6) | 0.004 |
| Brain | 2 (0.7) | 6 (2.1) | 8 (1.4) | 0.286 |
| Inguinal | 4 (1.4) | 0 (0.0) | 4 (0.7) | 0.124 |
| Perineum area | 3 (1.0) | 0 (0.0) | 3 (0.2) | 1.000 |
| Adrenal | 1 (0.3) | 0 (0.0) | 3 (0.5) | 0.559 |
| Not common sites1 | 14 (4.8) | 7 (2.4) | 21 (3.6) | 0.120 |
After PSM, 578 ULRC patients were included. The results of the multivariable Cox regression analysis revealed that ISR was an independent protective factor for OS (HR = 0.554, 95%CI: 0.371-0.828, P = 0.004). Additionally, independent risk factors for OS included CA19-9 Levels (HR = 2.150, 95%CI: 1.194-3.679, P = 0.005), pN stage (HR = 3.621, 95%CI: 2.426-5.404, P < 0.001), and ASA grade (HR = 1.804, 95%CI: 1.194-2.726, P = 0.005; Table 3).
| Variable | Group | n (%) | Univariate analysis HR (95%CI) | P value | Multivariate analysis HR (95%CI) | P value |
| Surgery | APR | 289 (50.0) | ||||
| ISR | 289 (50.0) | 0.546 (0.363-0.820) | 0.004 | 0.554 (0.371-0.828) | 0.004 | |
| Sex | Female | 313 (54.2) | ||||
| Male | 265 (45.8) | 1.108 (0.742-1.656) | 0.616 | |||
| Age (years) | ≤ 50 | 114 (19.7) | ||||
| > 50 | 464 (80.3) | 1.068 (0.614-1.859) | 0.815 | |||
| BMI (kg/m2) | ≤ 25 | 466 (80.6) | ||||
| > 25 | 112 (19.4) | 1.466 (0.909-2.365) | 0.117 | |||
| CEA (ng/mL) | ≤ 5 | 438 (75.8) | ||||
| > 5 | 140 (24.2) | 1.556 (0.990-2.444) | 0.055 | |||
| CA19-9 (U/mL) | ≤ 37 | 527 (91.2) | ||||
| > 37 | 51 (8.8) | 1.771 (1.007-3.113) | 0.047 | 2.150 (1.194-3.679) | 0.005 | |
| pT stage | ≤ T3 | 488 (84.4) | ||||
| T4 | 90 (15.6) | 1.109 (0.632-1.947) | 0.718 | |||
| pN stage | N0 | 386 (66.8) | ||||
| N+ | 192 (33.2) | 2.450 (1.067-5.629) | 0.035 | 3.621 (2.426-5.404) | < 0.001 | |
| pTNM stage | 0-II | 386 (66.8) | ||||
| III | 192 (33.2) | 1.292 (0.826-2.022) | 0.261 | |||
| Histopathology | Adenocarcinoma | 567 (98.1) | ||||
| Mucinous adenocarcinoma | 11 (1.9) | 1.414 (0.532-3.763) | 0.487 | |||
| nCRT | No | 288 (49.8) | ||||
| Yes | 190 (50.2) | 1.687 (1.062-2.679) | 0.027 | |||
| Surgical Procedures | Laparoscopy and Open | 564 (97.6) | ||||
| Robotic | 14 (2.4) | 0.765 (0.432-1.355) | 0.359 | |||
| ASA grade | I-II | 502 (86.9) | ||||
| III | 76 (13.1) | 1.839 (1.130-2.992) | 0.014 | 1.804 (1.194-2.726) | 0.005 | |
| Tumour diameter (cm) | ≤ 3 | 214 (37.0) | ||||
| > 3 | 364 (63.0) | 0.929 (0.598-1.444) | 0.743 |
The postoperative complication profiles of the two groups were distinctly different, with a higher overall complication rate in the APR group than in the ISR group (42.2% vs 29.4%, P = 0.001). Anastomotic leakage/bowel perforation, anastomotic bleeding, positive distal margins, and operative time were greater in the ISR group than in the APR group (P < 0.05). However, incision infection, urinary retention, circumferential resection margins, and hospitalization time were greater in the APR group than in the ISR group (P < 0.05). The details are detailed in Table 4.
| Complications | APR group (n = 289) | ISR group (n = 289) | P value |
| Overall complications | 122 (42.2) | 85 (29.4) | 0.001 |
| Pelvic infection | 42 (14.5) | 42 (14.5) | 1.000 |
| Bowel obstruction | 22 (7.6) | 25 (8.7) | 0.648 |
| Incision infection | 46 (15.9) | 3 (1.0) | < 0.001 |
| Urinary retention | 29 (10.0) | 8 (2.8) | < 0.001 |
| Anastomotic leakage/bowel perforation | 3 (1.0) | 33 (11.4) | < 0.0011 |
| Anastomotic leak grade | < 0.001 | ||
| Grade A | 0 (0.0) | 3 (1.0) | |
| Grade B | 1 (0.3) | 29 (10.0) | |
| Grade C | 2 (0.7) | 1 (0.3) | |
| Pneumonia | 10 (3.5) | 15 (5.2) | 0.307 |
| Circumferential resection margin | 12 (4.2) | 3 (1.0) | 0.019 |
| Chylous leakage | 4 (1.4) | 8 (2.8) | 0.243 |
| Anastomotic bleeding | 0 (0.0) | 7 (2.4) | 0.015 |
| Distal margin positive | 0 (0.0) | 4 (1.4) | 0.124 |
| Other rare complications2 | 7 (2.4) | 6 (2.1) | 0.779 |
| Surgical time (min) | 205.1 ± 95.8 | 229.8 ± 95.4 | 0.001 |
| Hospitalization time (days) | 12.9 ± 8.8 | 9.8 ± 5.2 | < 0.001 |
We compared the outcomes of ISR and APR performed for ULRC in different centers in China, with a focus on long-term prognosis and postoperative complications. Our results revealed that, in the treatment of ULRC, there was an advantage in 5-year OS with ISR compared with APR (80.8% vs 74.1%, P = 0.032), as well as a trend toward a lower rate of LR (9.5% vs 12.9%, P = 0.019), which was also an independent protective factor for OS (HR = 0.541, 95%CI: 0.362-0.810, P = 0.003). Similarly, previous studies have shown that patients with ULRC who undergo sphincter-preserving surgery have a higher survival rate than those who undergo APR (HR = 0.78, P = 0.01)[3]. Similar results have been obtained in studies in different countries, and ISR is considered a surgical procedure that does not compromise the chances of a cure and contributes to quality of life[15,16].
Surgical treatment for ULRC is challenging and requires consideration of whether preserving the sphincter while maintaining tumor safety is possible. The surgery must be performed in the extremely narrow pelvic cavity, necessitating increased surgical visualization, exposure levels, and meticulous manipulation[17]. Park and Kim[18] summarized recent literature and concluded that ISR, with LR rates ranging from 0% to 12% after surgery, is a safe sphincter-salvage procedure and does not compromise the oncological outcome of resection, thus providing an important alternative to APR. Although these findings suggest the potential superiority of ISR for local control, some still contend that APR might confer an advantage due to wider tissue resection. Our data may reflect more refined tissue identification and sphincter-saving techniques in ISR. Moreover, variations in surgical expertise, operator experience, and intraoperative decisions can also influence LR and distant metastasis[19].
With the advancement of laparoscopic equipment, the application of laparoscopic techniques has increased annually, and the opportunity to preserve the sphincter has become increasingly achievable[20]. Since the introduction of ISR, the APR rate has decreased to 4.9%[21]. In this study, only 6.2% of the open surgeries allowed for ISR. Additionally, Saito et al[22] compared the outcomes of these two surgical approaches and reported that ISR was significantly superior to APR in terms of the margin recurrence rate (3% vs 11.4%; P = 0.017) and 5-year OS (80% vs 61.5%; P = 0.033). APR may be associated with higher rates of perforation, LR, and positive margins[12]. Warschkow et al[23] compared ISR with APR, and the poorer tumor prognosis in APR patients was attributed to differences in patient characteristics rather than the surgery itself (HR = 0.85, 95%CI: 0.56-1.29, P = 0.456). ISR surgical techniques, such as more precise lymph node dissection and better vascular preservation, have also continued to improve, and these technical advancements may have a positive impact on survival rates. It is believed that ISR treatment for ULRC does not compromise the oncological prognosis[24].
Although it has been reported that urinary incontinence is the most important complication following ISR surgery, the most significant benefit of ISR surgery is that it can help patients with low rectal cancer effectively avoid permanent ostomy[6]. Postoperatively, APR is most commonly associated with excretory problems that cause physical damage and impose additional psychological burdens on patients[25]. Furthermore, APR can lead to various postoperative issues, including male sexual dysfunction, reduced confidence in achieving and maintaining erections, and exacerbated urinary symptoms, all of which contribute to a decline in quality of life[2]. Unfortunately, our study lacked timely assessments of quality of life, and further details could not be disclosed. Future prospective studies should address this shortcoming.
The incidence of anastomotic leakage is relatively high in the ISR group, and even the use of a temporary stoma cannot prevent postoperative anastomotic leakage[26]. Studies have identified male sex, advanced tumor stage, perioperative bleeding, nCRT, and lower anastomotic height as independent risk factors for AL[5,27]. In the APR group, there were no concerns regarding anastomotic issues after radical surgery, but our study also reported a small number of postoperative intestinal perforations, which may be related to secondary injuries caused by improper surgical techniques. Nevertheless, our results indicate that ISR still results in fewer total complications and shorter hospital stays (P < 0.001). A meta-analysis involving 2438 patients reported a shorter hospital stay (mean difference of 2.98 days, P < 0.00001) and a lower postoperative complication rate (OR = 0.76, P = 0.04) in the ISR group, which was consistent with our present study[28]. However, other researchers noted no significant difference in complication rates between the two procedures[4], possibly reflecting differing patient populations or surgical techniques.
In our cohort, perineal incision infection (14.9%) and urinary retention (11.4%) were the most frequent major complications following APR, which explains the longer hospital stay and higher complication rate associated with APR. The incidence of perineal incision infection after APR has reached 27.7%-50.6%, which may even compromise long-term outcomes[29,30]. Post-APR perineal defects leave extensive dead space prone to fluid collection in a region with a high bacterial load, where conventional sterilization may not suffice. Additionally, factors such as suture tension, poor soft-tissue coverage, and patient positioning may exacerbate incision breakdown[31-33].
Historically, clinicians have prioritized complete tumor extirpation via APR at the expense of quality of life[25]. Our findings not only offer an important basis for surgical selection but also emphasize the importance of integrating both oncologic safety and functional preservation when considering a surgical approach. Hence, surgeons may favor ISR for patients who value quality of life and sphincter function over the disadvantages of a permanent stoma[15]. Future strategies could further embrace a more patient-centered paradigm, recognizing that survival and quality of life need not be mutually exclusive in ULRC management.
Although this large multicenter study provides robust evidence, certain limitations must be acknowledged. First, as a retrospective study design, selection bias or partial information bias may have been introduced. Although we used the PSM method to address the imbalance in baseline characteristics between the two groups, we must acknowledge that some factors were not considered or fully measured. Therefore, we further employed Cox multivariable regression analysis to validate the factors of interest. This method allows for the independent measurement of a specific factor while avoiding interference from other unknown factors, thereby addressing this limitation[34]. Second, although the multicenter design enhances external validity, there may be potential differences among centers in terms of surgical techniques, treatment protocols, and patient selection. However, the enrolled centers are all high-volume colorectal cancer treatment centers that have fully negotiated the learning curve. Third, the median follow-up time in this study was 47.2 months, which reflects the prognosis in the mid-term postsurgical period. However, this follow-up period has certain limitations in monitoring tumor recurrence, as some recurrences may not be detected until five years or more after surgery. The follow-up period in this study may not have been sufficient to comprehensively assess the risk of recurrence. Therefore, future studies should extend the follow-up period to evaluate postsurgical recurrence more comprehensively. Finally, this study lacked postoperative quality-of-life data for patients who underwent ISR or APR, but previous studies have sufficiently demonstrated superior postoperative quality of life following ISR[2,25]. Future studies should incorporate standardized quality-of-life scales to assess postoperative functional recovery and psychosocial status, enabling a more comprehensive comparison of the impact of the two surgical approaches on quality of life.
In conclusion, the long-term prognosis of ULRC treated with ISR is excellent, with no increases in surgical complications and hospital stay duration. These findings further support that ISR should be actively applied to ULRC treatment as a viable surgical alternative in regions or hospitals where the necessary conditions exist.
| 1. | Lee L, Trepanier M, Renaud J, Liberman S, Charlebois P, Stein B, Fried GM, Fiore J Jr, Feldman LS. Patients' preferences for sphincter preservation versus abdominoperineal resection for low rectal cancer. Surgery. 2021;169:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Kang SB, Cho JR, Jeong SY, Oh JH, Ahn S, Choi S, Kim DW, Lee BH, Youk EG, Park SC, Heo SC, Lee DS, Ryoo SB, Park JW, Park HC, Lee SM, Kang SI, Kim MH, Oh HK, Shin R, Kim MJ, Lee KH, Kim YH, Kim JS, Lee KW, Lee HS, Kim HJ, Park YS, Sohn DK, Park KJ; Seoul Colorectal Research Group (SECOG). Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): A long-term prospective, multicentre, cohort study. Lancet Reg Health West Pac. 2021;6:100087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Zheng K, Hu Q, Yu G, Zhou L, Yao Y, Zhou Y, Wang H, Hao L, Yu E, Lou Z, Zhang Y, Qiu H, Meng R, Zhang W. Trends of sphincter-preserving surgeries for low lying rectal cancer: A 20-year experience in China. Front Oncol. 2022;12:996866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 4. | He Z, Peng B, Chen W, Zhu J, Chen B, Li G, Cao J, Li W. Clinical Efficacy of Intersphincteric Resection for Low Rectal Cancer Compared With Abdominoperineal Resection: A Single-Center Retrospective Study. Am Surg. 2023;89:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Liu J, Wang X, Chen H, Mei S, Qiu W, Tang J. Risk factors, quality of life, and oncological effects of refractory anastomotic leakage for laparoscopic intersphincteric resection. J Gastroenterol Hepatol. 2023;38:1934-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Klose J, Tarantino I, Kulu Y, Bruckner T, Trefz S, Schmidt T, Schneider M, Hackert T, Büchler MW, Ulrich A. Sphincter-Preserving Surgery for Low Rectal Cancer: Do We Overshoot the Mark? J Gastrointest Surg. 2017;21:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Luvisetto F, Shamali A, Rutgers MLW, Flashman K, Khan JS. Sphincter preservation in patients with low rectal cancer: striking the right oncological balance. Discov Oncol. 2021;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Akagi Y, Shirouzu K, Ogata Y, Kinugasa T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg Oncol. 2013;22:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Koyama M, Murata A, Sakamoto Y, Morohashi H, Takahashi S, Yoshida E, Hakamada K. Long-term clinical and functional results of intersphincteric resection for lower rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S422-S428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 10. | Du Q, Yang W, Zhang J, Qiu S, Liu X, Wang Y, Yang L, Zhou Z. Oncologic outcomes of intersphincteric resection versus abdominoperineal resection for lower rectal cancer: a systematic review and meta-analysis. Int J Surg. 2024;110:2338-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Jo WI, Lim DR, Kuk JC, Shin EJ. Comparison of oncologic outcome of abdominoperineal resection versus sphincter saving resection for low lying rectal cancer. Korean J Clin Oncol. 2021;17:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Piozzi GN, Baek SJ, Kwak JM, Kim J, Kim SH. Anus-Preserving Surgery in Advanced Low-Lying Rectal Cancer: A Perspective on Oncological Safety of Intersphincteric Resection. Cancers (Basel). 2021;13:4793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 14. | D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 15. | Rouanet P, Rivoire M, Gourgou S, Lelong B, Rullier E, Jafari M, Mineur L, Pocard M, Faucheron JL, Dravet F, Pezet D, Fabre JM, Bresler L, Balosso J, Taoum C, Lemanski C. Sphincter-saving surgery for ultra-low rectal carcinoma initially indicated for abdominoperineal resection: Is it safe on a long-term follow-up? J Surg Oncol. 2021;123:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Kim HS, Ko S, Oh NG. Long-term results of extended intersphincteric resection for very low rectal cancer: a retrospective study. BMC Surg. 2016;16:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Park IJ, Kim JC. Intersphincteric Resection for Patients With Low-Lying Rectal Cancer: Oncological and Functional Outcomes. Ann Coloproctol. 2018;34:167-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Chi P, Wang XJ. [Clinical application and standardized implementation of intersphincteric resection]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Jiang WZ, Xu JM, Xing JD, Qiu HZ, Wang ZQ, Kang L, Deng HJ, Chen WP, Zhang QT, Du XH, Yang CK, Guo YC, Zhong M, Ye K, You J, Xu DB, Li XX, Xiong ZG, Tao KX, Ding KF, Zang WD, Feng Y, Pan ZZ, Wu AW, Huang F, Huang Y, Wei Y, Su XQ, Chi P; LASRE trial investigators. Short-term Outcomes of Laparoscopy-Assisted vs Open Surgery for Patients With Low Rectal Cancer: The LASRE Randomized Clinical Trial. JAMA Oncol. 2022;8:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Kim JC, Kim CW, Lee JL, Yoon YS, Park IJ, Kim JR, Kim J, Park SH. Complete intersphincteric longitudinal muscle excision May Be key to reducing local recurrence during intersphincteric resection. Eur J Surg Oncol. 2021;47:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y, Yoneyama Y, Nishizawa Y, Minagawa N. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009;33:1750-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 23. | Warschkow R, Ebinger SM, Brunner W, Schmied BM, Marti L. Survival after Abdominoperineal and Sphincter-Preserving Resection in Nonmetastatic Rectal Cancer: A Population-Based Time-Trend and Propensity Score-Matched SEER Analysis. Gastroenterol Res Pract. 2017;2017:6058907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Piozzi GN, Park H, Lee TH, Kim JS, Choi HB, Baek SJ, Kwak JM, Kim J, Kim SH. Risk factors for local recurrence and long term survival after minimally invasive intersphincteric resection for very low rectal cancer: Multivariate analysis in 161 patients. Eur J Surg Oncol. 2021;47:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Scheele J, Lemke J, Wittau M, Sander S, Henne-Bruns D, Kornmann M. Quality of Life after Rectal Cancer Resection Comparing Anterior Resection, Abdominoperineal Resection, and Complicated Cases. Visc Med. 2022;38:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Niu L, Wang J, Zhang P, Zhao X. Protective ileostomy does not prevent anastomotic leakage after anterior resection of rectal cancer. J Int Med Res. 2020;48:300060520946520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 28. | Peng B, Lu J, Wu Z, Li G, Wei F, Cao J, Li W. Intersphincteric Resection Versus Abdominoperineal Resection for Low Rectal Cancer: A Meta-Analysis. Surg Innov. 2020;27:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hawkins AT, Berger DL, Shellito PC, Sylla P, Bordeianou L. Wound dehiscence after abdominoperineal resection for low rectal cancer is associated with decreased survival. Dis Colon Rectum. 2014;57:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Huang W, Wei ZQ, Qiu YH, Tang G, Sun H. Effects of wound infection on prognosis after laparoscopic abdominoperineal resection of rectal cancer. Front Oncol. 2022;12:1036241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Jia L, Zhao H, Liu J. Meta-analysis of postoperative incision infection risk factors in colorectal cancer surgery. Front Surg. 2024;11:1415357. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2014;57:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Khattak MA, Khan AN, Jafferi S, Iqbal Y, Abdulrasheed H, McArthur D. Perineal Wound Healing Following Abdominoperineal Resection of the Rectum. Cureus. 2024;16:e66318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Andrade C. Survival Analysis, Kaplan-Meier Curves, and Cox Regression: Basic Concepts. Indian J Psychol Med. 2023;45:434-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/