©The Author(s) 2025.
World J Gastroenterol. Jul 28, 2025; 31(28): 105089
Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.105089
Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.105089
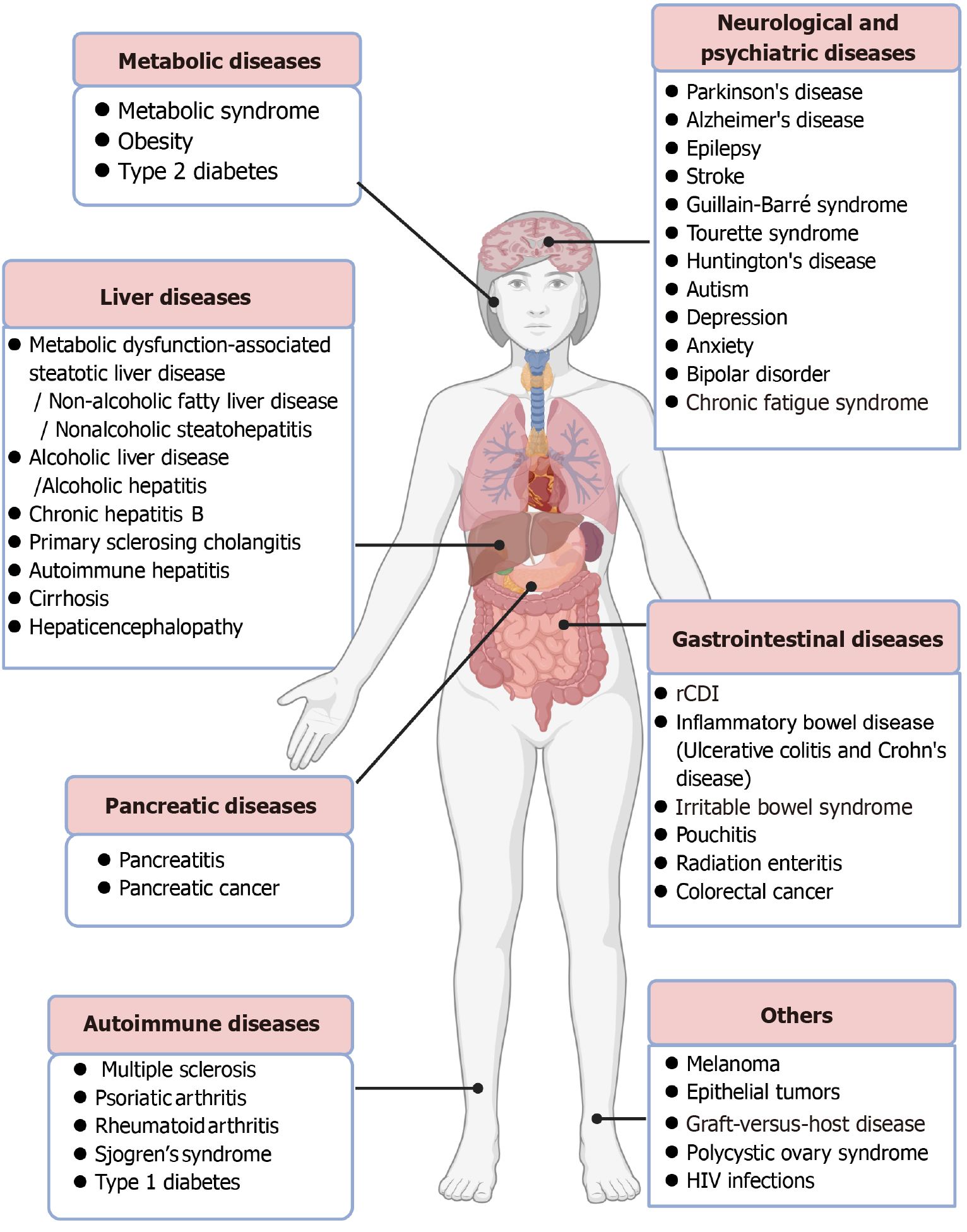
Figure 1 Applications of fecal microbiota transplantation in infectious and noninfectious diseases.
Besides recurrent Clostridium difficile infec
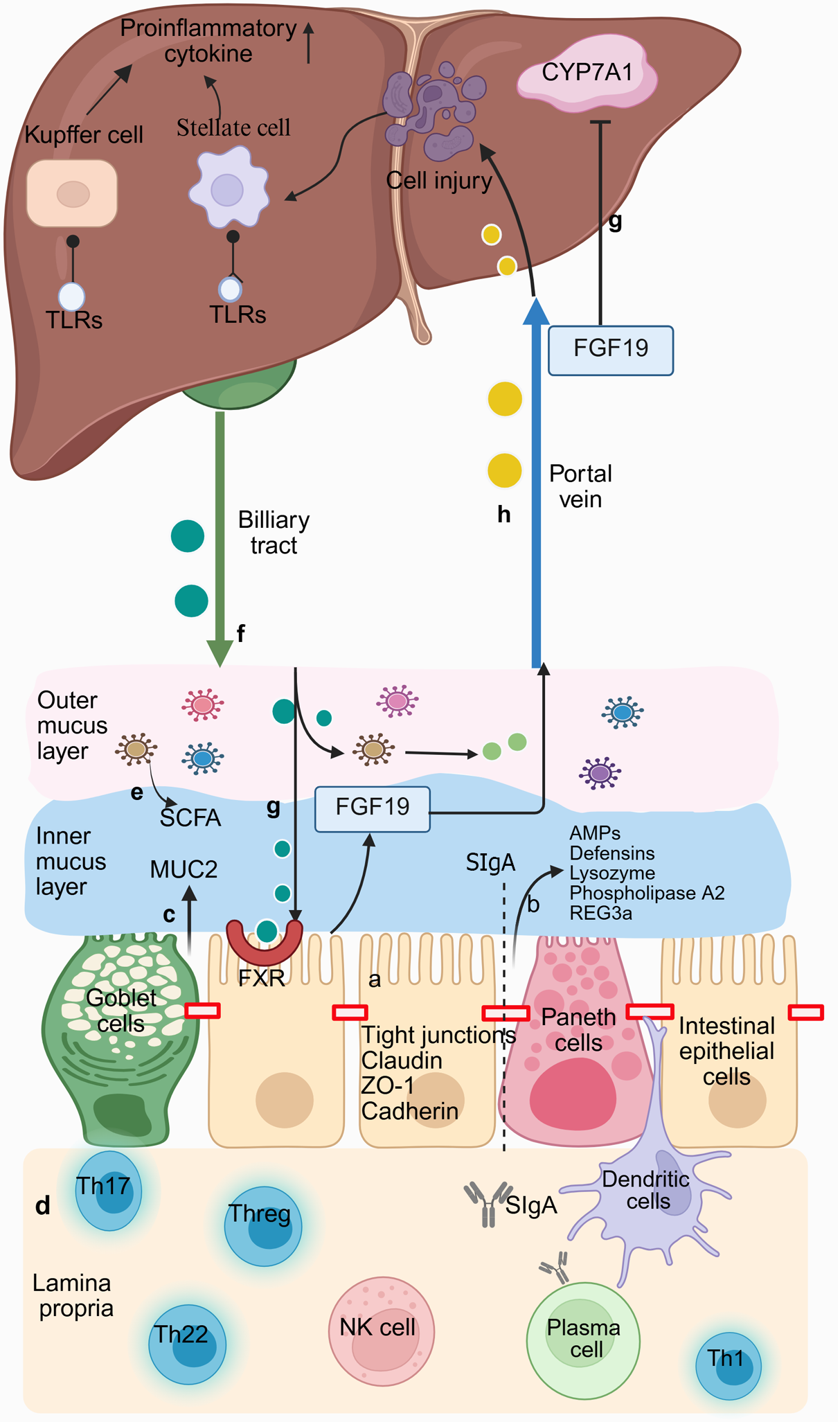
Figure 2 Bidirectional communication between the intestine and liver.
a: Intestinal epithelial cells are tightly connected to adjacent cells through apical ligand proteins to seal the intercellular space between them; b: Paneth cells can secrete a range of antimicrobial peptides (AMPs); c: Goblet cells can produce mucin 2 that is made of the mucus barrier. The mucus barrier comprises two layers: An inner dense layer (in blue) close to the epithelial cells, where the inner mucus is almost sterile because of AMPs, and a loose outer layer (in pink) colonized by intestinal microbiota; d: The lamina propria is rich in immune cells. Dendritic cells can capture luminal bacteria and antigens by inserting dendrites between tight junctions. Plasma cells can promote the secretion of dimer IgA. Secretory immunoglobulin A is transported through epithelial cells to the intestinal lumen, where it can limit the colonization and proliferation of potential pathogens; e: Short-chain fatty acids, a major metabolite of intestinal microbiota, can maintain the integrity of the intestinal barrier; f: The liver secretes primary bile acids (BAs) through the biliary duct into the intestinal lumen. Then, colonic bacteria partially convert them into secondary BAs; g: BAs can induce the transcription of fibroblast growth factor 19 (FGF19) by binding to the farnesoid X receptor of enterocytes in the intestine. Then, FGF19 reaches the liver via the portal vein. It can downregulate the synthesis of new BAs by inhibiting cholesterol 7a-hydroxylase in hepatocytes; h: Microbe-associated molecular patterns, the metabolites of intestinal microbiota, enter the liver through the portals vein and activate Toll-like receptors (TLRs) on Kupffer and hepatic stellate cells when the cells are damaged. Activated TLRs promote the upregulation of downstream pro-inflammatory cytokines. CYP7A1: Cholesterol 7a-hydroxylase; FGF19: Fibroblast growth factor 19; FXR: Farnesoid X receptor; MUC2: Mucin 2; REG3α: Recombinant regenerating islet-derived protein 3 alpha; SCFA: Short-chain fatty acids; SIgA: Secretory immunoglobulin A; TLR: Toll-like receptor; ZO-1: Occlusion band 1. Created with BioRender (Supplementary material).
- Citation: Ma L, Zhang MH, Xu YF, Hao YX, Niu XX, Li Y, Xing HC. Fecal microbiota transplantation: A promising treatment strategy for chronic liver disease. World J Gastroenterol 2025; 31(28): 105089
- URL: https://www.wjgnet.com/1007-9327/full/v31/i28/105089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i28.105089