©The Author(s) 2024.
World J Gastroenterol. May 14, 2024; 30(18): 2440-2453
Published online May 14, 2024. doi: 10.3748/wjg.v30.i18.2440
Published online May 14, 2024. doi: 10.3748/wjg.v30.i18.2440
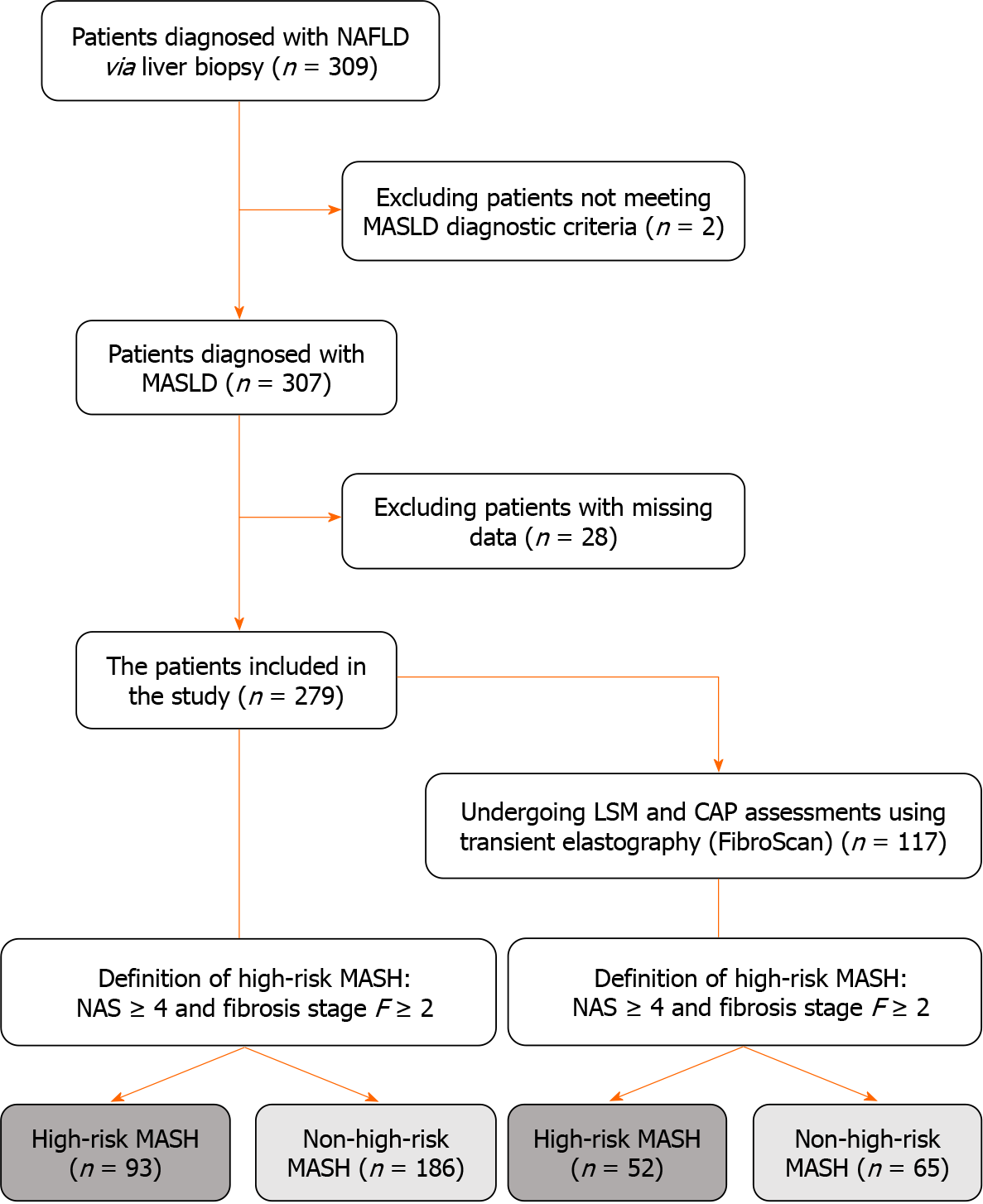
Figure 1 Flow diagram of patient selection process.
NAFLD: Non-alcoholic fatty liver disease; MASLD: Metabolic dysfunction-associated steatotic liver disease; LSM: Liver stiffness measurement; CAP: Controlled attenuation parameter; MASH: Metabolic dysfunction-associated steatohepatitis; NAS: Non-alcoholic fatty liver disease activity score.
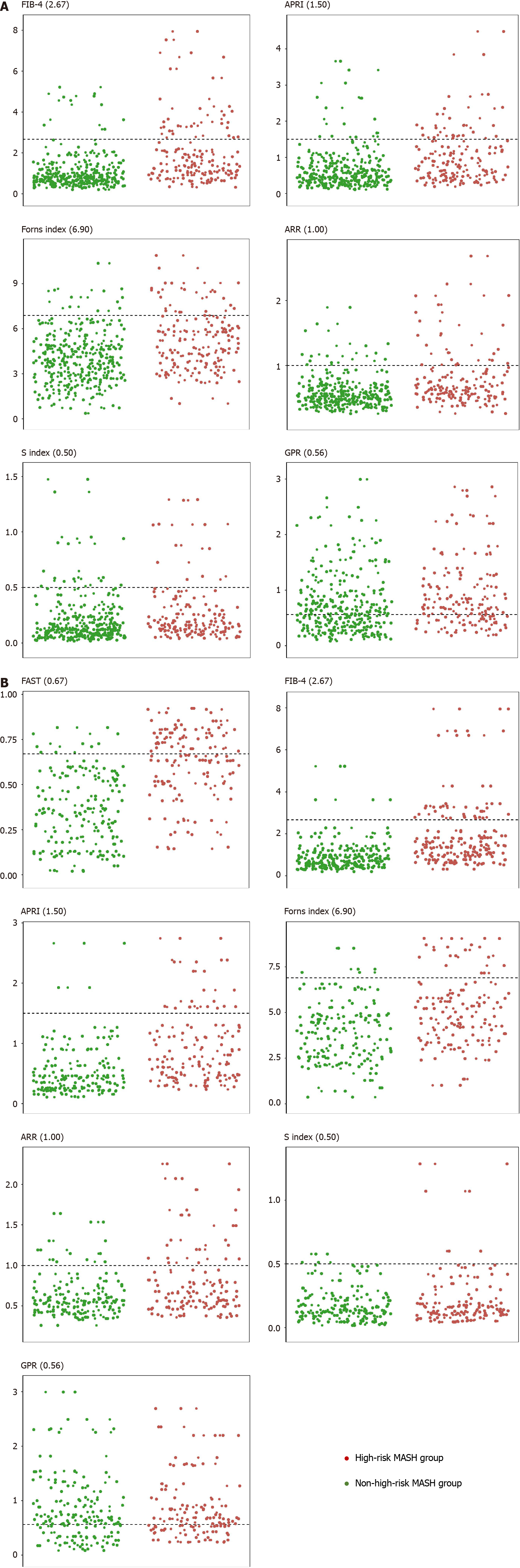
Figure 2 The distribution of seven non-invasive models based on high threshold criteria.
A: The distribution of non-invasive models in the overall population; B: The distribution of non-invasive models in the subgroup population. MASH: Metabolic dysfunction-associated steatohepatitis; FAST: FibroScan-aspartate transaminase; FIB-4: Fibrosis-4; APRI: Aspartate transaminase to platelet ratio index; ARR: Aspartate transaminase to alanine transaminase ratio; GPR: Gamma-glutamyl transpeptidase to platelet ratio.
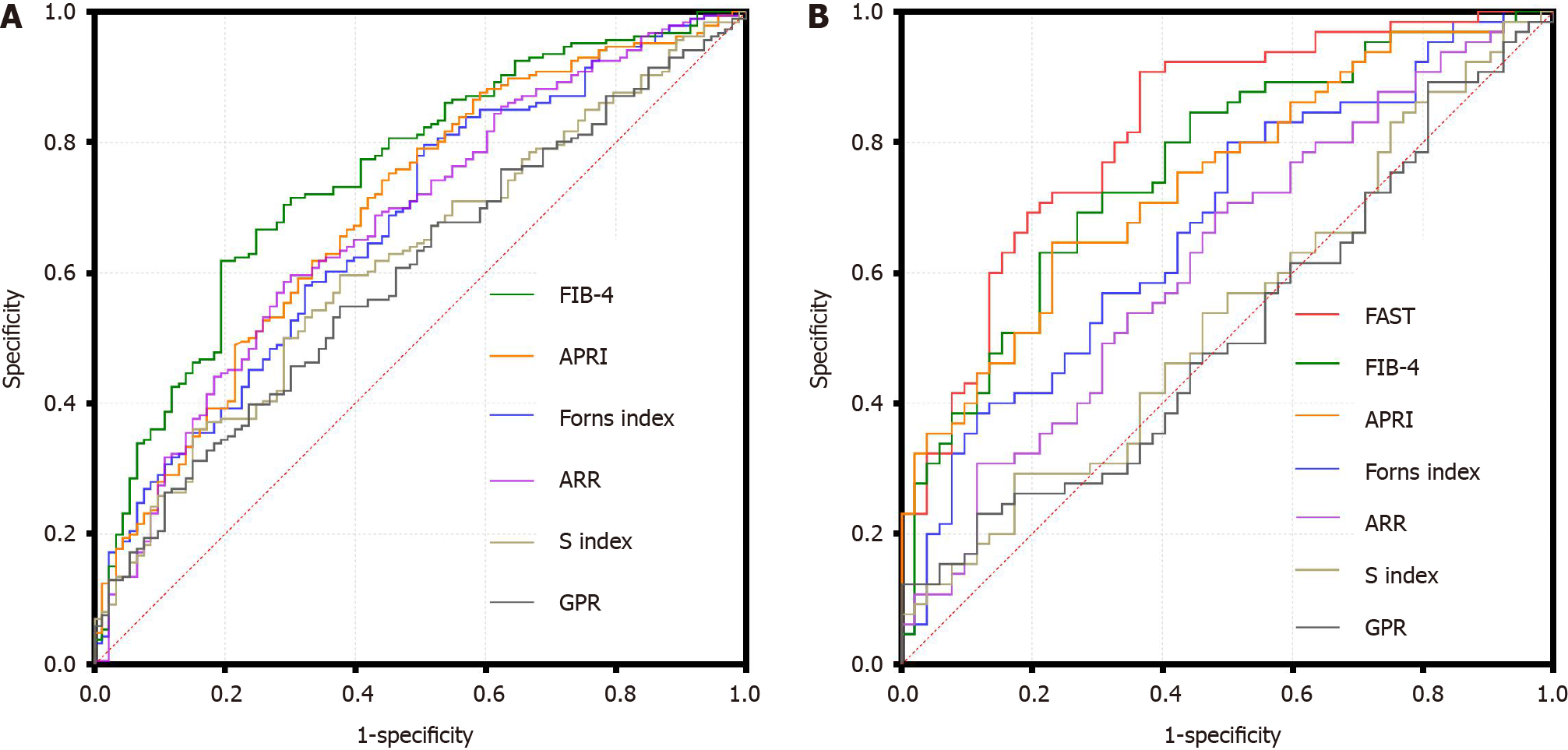
Figure 3 The evaluation of seven non-invasive models by receiver operating characteristic curves.
A: The receiver operating characteristic curves (ROC) of the overall population; B: The ROC of the subgroup population. FAST: FibroScan-aspartate transaminase; FIB-4: Fibrosis-4; APRI: Aspartate transaminase to platelet ratio index; ARR: Aspartate transaminase to alanine transaminase ratio; GPR: Gamma-glutamyl transpeptidase to platelet ratio.
- Citation: Yin JY, Yang TY, Yang BQ, Hou CX, Li JN, Li Y, Wang Q. FibroScan-aspartate transaminase: A superior non-invasive model for diagnosing high-risk metabolic dysfunction-associated steatohepatitis. World J Gastroenterol 2024; 30(18): 2440-2453
- URL: https://www.wjgnet.com/1007-9327/full/v30/i18/2440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i18.2440