Published online May 14, 2024. doi: 10.3748/wjg.v30.i18.2418
Revised: February 6, 2024
Accepted: April 17, 2024
Published online: May 14, 2024
Processing time: 819 Days and 20.1 Hours
Colorectal surgeons are well aware that performing surgery for rectal cancer becomes more challenging in obese patients with narrow and deep pelvic cavities. Therefore, it is essential for colorectal surgeons to have a comprehensive under
To evaluate predictive parameters for technical challenges encountered during laparoscopic radical sphincter-preserving surgery for rectal cancer.
We retrospectively gathered data from 162 consecutive patients who underwent laparoscopic radical sphincter-preserving surgery for rectal cancer. Three-dimensional reconstruction of pelvic bone and soft tissue parameters was conducted using computed tomography (CT) scans. Operative difficulty was categorized as either high or low, and multivariate logistic regression analysis was employed to identify predictors of operative difficulty, ultimately creating a nomogram.
Out of 162 patients, 21 (13.0%) were classified in the high surgical difficulty group, while 141 (87.0%) were in the low surgical difficulty group. Multivariate logistic regression analysis showed that the surgical approach using laparoscopic intersphincteric dissection, intraoperative preventive ostomy, and the sacrococcygeal distance were independent risk factors for highly difficult laparoscopic radical sphincter-sparing surgery for rectal cancer (P < 0.05). Conversely, the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance was identified as a protective factor (P < 0.05). A nomogram was subsequently constructed, demonstrating good predictive accuracy (C-index = 0.834).
The surgical approach, intraoperative preventive ostomy, the sacrococcygeal distance, and the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance could help to predict the difficulty of laparoscopic radical sphincter-preserving surgery.
Core Tip: This retrospective cohort study developed a nomogram to predict technical difficulty prior to laparoscopic sphincter-preserving radical resection for rectal cancer. Significant predictive factors were identified through multivariate logistic regression, including the surgical approach using laparoscopic intersphincteric dissection, intraoperative preventive ostomy, the sacrococcygeal distance, and the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance. The nomogram’s clinical value lies in enabling surgeons to preoperatively evaluate expected difficulty and customize surgical approaches accordingly. It aids in individualized surgical planning.
- Citation: Zhou XC, Guan SW, Ke FY, Dhamija G, Wang Q, Chen BF. Construction of a nomogram model to predict technical difficulty in performing laparoscopic sphincter-preserving radical resection for rectal cancer. World J Gastroenterol 2024; 30(18): 2418-2439
- URL: https://www.wjgnet.com/1007-9327/full/v30/i18/2418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i18.2418
Colorectal surgeons performing laparoscopic radical rectal cancer surgery often find it more challenging in male patients compared to female patients. This may be attributed to the narrower and deeper pelvic anatomy, increased curvature of the pelvis, and thicker rectal mesentery in male patients. Currently, there is no international consensus on which pelvic diameter, angle and anatomical factors of soft tissue impact the procedure of laparoscopic radical rectal cancer surgery. During radical surgery for rectal cancer, especially for mid-low rectal cancer, although experienced surgeons are well aware that the female pelvis is usually easier for the surgical procedure than the male pelvis[1], they still cannot draw a definite conclusion on which bone and soft tissue parameters in the pelvic cavity are the main factors determining this difference.
At present, the majority of literature reports that mention the pelvic bone and soft tissue parameters are relatively few, and the measurement indicators cannot be completely unified. Therefore, it is impossible to draw relatively consistent conclusions[2-6]. Based on the above controversial issues, in order to comprehensively evaluate the pelvic spatial anatomical structure and surrounding soft tissue structure that may affect laparoscopic rectal cancer radical surgery, we attempted to assess the predictive value of 16 pelvic bone parameters, 7 soft tissue parameters, and numerous clinicopathological parameters, particularly the impact of pelvic bone parameters on the difficulty of laparoscopic radical sphincter-preserving rectal cancer surgery. Based on this evaluation, we constructed a predictive nomogram model to visualize and facilitate individualized preoperative assessment of the difficulty of laparoscopic radical rectal cancer surgery.
We collected complete clinical, pathological, and radiological data from 162 eligible patients who underwent laparoscopic radical rectal cancer surgery at the Wenzhou Central Hospital from February 2013 to June 2022. The surgical approaches were classified as laparoscopic anterior resection (L-AR), laparoscopic low or ultralow anterior resection (L-LAR), collectively referred to as L-LAR for simplicity, and laparoscopic intersphincteric resection (L-ISR) through the trans-anal approach, all utilizing double stapling techniques except for L-ISR. Of these patients, there were 20 cases of L-AR, 135 cases of L-LAR, and 7 cases of L-ISR. The combined analysis of L-AR, L-LAR, and L-ISR was referred to as laparoscopic sphincter-preserving procedure for assessing the overall difficulty of sphincter preservation surgery, including 105 male patients and 57 female patients.
Inclusion criteria: Tumors with a distance (tumor height) from the lower edge of the tumor to the anal verge ≤ 15 cm. Tumor height denotes the measured distance between the anal verge and the lowermost margin of the tumor mass, assessed via digital rectal examination and/or colonoscopy visualization. Confirmation of the adenocarcinoma was established across all cases, initially through preoperative colonoscopic biopsy and subsequently reinforced by postoperative pathology assessments. Preoperative tumor staging was evaluated using plain computed tomography (CT) scan with contrast enhancement and/or magnetic resonance imaging (MRI) and endorectal ultrasound. The pathological tumor staging was T4a or below, the pathological Tumor node metastasis (TNM) staging was stage III or below, and there was no extensive infiltration into surrounding tissues. The surgeries were performed by the same skilled surgical team.
Exclusion criteria: Patients undergoing laparoscopic Hartmann’s procedure, laparoscopic trans-anal total mesorectal excision (TME), laparoscopic natural orifice specimen extraction surgery, or laparoscopic abdominoperineal resection (APR) procedures, those who had received previous radiotherapy or chemotherapy, patients undergoing neoadjuvant therapy, individuals with iodine contrast agent allergies, those with complications such as intestinal obstruction or perforation, a history of pelvic bone fractures, abnormal coagulation function, multiple primary or recurrent cancers, tumor invasion of adjacent organs, presence of ipsilateral pelvic lymph node metastasis or distant metastasis, and cases with combined organ resection that may affect the accuracy of pelvic measurements.
Selection of clinical pathological parameters: The following clinical pathological parameters were collected for each patient: demographics including age, gender, body mass index (BMI), medical history including underlying comorbidities (such as diabetes, hypertension, heart disease, pulmonary disease, stroke, and other comorbidities), history of previous abdominal surgery; preoperative assessments including American Society of Anesthesiologists physical status classification, preoperative albumin level, preoperative total protein level, preoperative albumin to globulin ratio (AGR), preoperative hemoglobin level, prognostic nutritional index [PNI, PNI = serum albumin (g/L) + 5 × peripheral lymphocyte count (109/L)], preoperative CEA level, preoperative CA199 Level; tumor characteristics including maximum tumor diameter, distance from the lower edge of the tumor to the anal verge (tumor height), tumor infiltration depth (T staging), lymph node metastasis (N staging), tumor staging (TNM staging), tumor differentiation grade, number of lymph nodes removed, presence of tumor nodules, presence of vascular tumor emboli, presence of neural invasion; surgical factors including conversion to open surgery, surgical approach (L-AR, L-LAR, and L-ISR), number of stapler nail cartridges used, length of stapler cartridges (mm), surgical duration, intraoperative blood loss; postoperative outcomes including postoperative anal evacuation time, postoperative resumption of semi-liquid food time, duration of postoperative hospital stay, and Clavien-Dindo (CD) grade of complications within 30 d after surgery.
Definitions: BMI = weight (kg)/height2 (m²). Complication grading: Surgical complications after rectal cancer surgery were graded according to the CD grade system[7]. Tumor staging followed the 8th edition TNM staging system of the American Joint Committee on Cancer[8]. The surgical duration was calculated from the time of skin incision to completion of abdominal closure, including the time for ileostomy or colostomy in some L-LAR and L-ISR cases. Intraoperative blood loss was calculated by measuring the amount of blood suctioned into the collection container and considering the use of gauze before abdominal and pelvic lavage.
The definition criteria for the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer, as referenced by Escal et al[9] and Yamamoto et al[10], have been appropriately modified. They used six indicators, including surgical duration, intraoperative blood loss, intraoperative use of trans-anal approach, conversion to open surgery, postoperative complications classified as CD grade II or higher, and duration of postoperative hospital stay, to comprehensively assess the grading criteria for high and low difficulty surgeries. A scoring system was assigned (total score of 12, with ≥ 5 indicating high-difficulty surgery), as follows: duration of postoperative hospital stay > 15 d, assigned 2 points; surgical duration > 250 min, assigned 3 points; blood loss > 100 mL, assigned 1 point; conversion to open surgery, assigned 3 points; intraoperative use of trans-anal approach, assigned 2 points; and postoperative complications classified as CD grade II or higher, assigned 1 point (Table 1).
| Criteria | Score |
| Duration of postoperative hospital stay > 15 d | 2 |
| Surgical duration > 250 min | 3 |
| Intraoperative blood loss > 100 mL | 1 |
| Conversion to open surgery | 3 |
| Intraoperative use of trans-anal approach | 2 |
| Postoperative complications classified as CD grade II or above | 1 |
Blood samples were collected 1-2 wk prior to surgery to assess serum nutritional parameters, including total serum protein levels, serum albumin levels, preoperative albumin to globulin ratio (AGR = serum albumin/total serum protein - serum albumin), preoperative serum hemoglobin levels, and PNI (PNI = serum albumin (g/L) + 5 × peripheral blood lymphocyte count (109/L))[11].
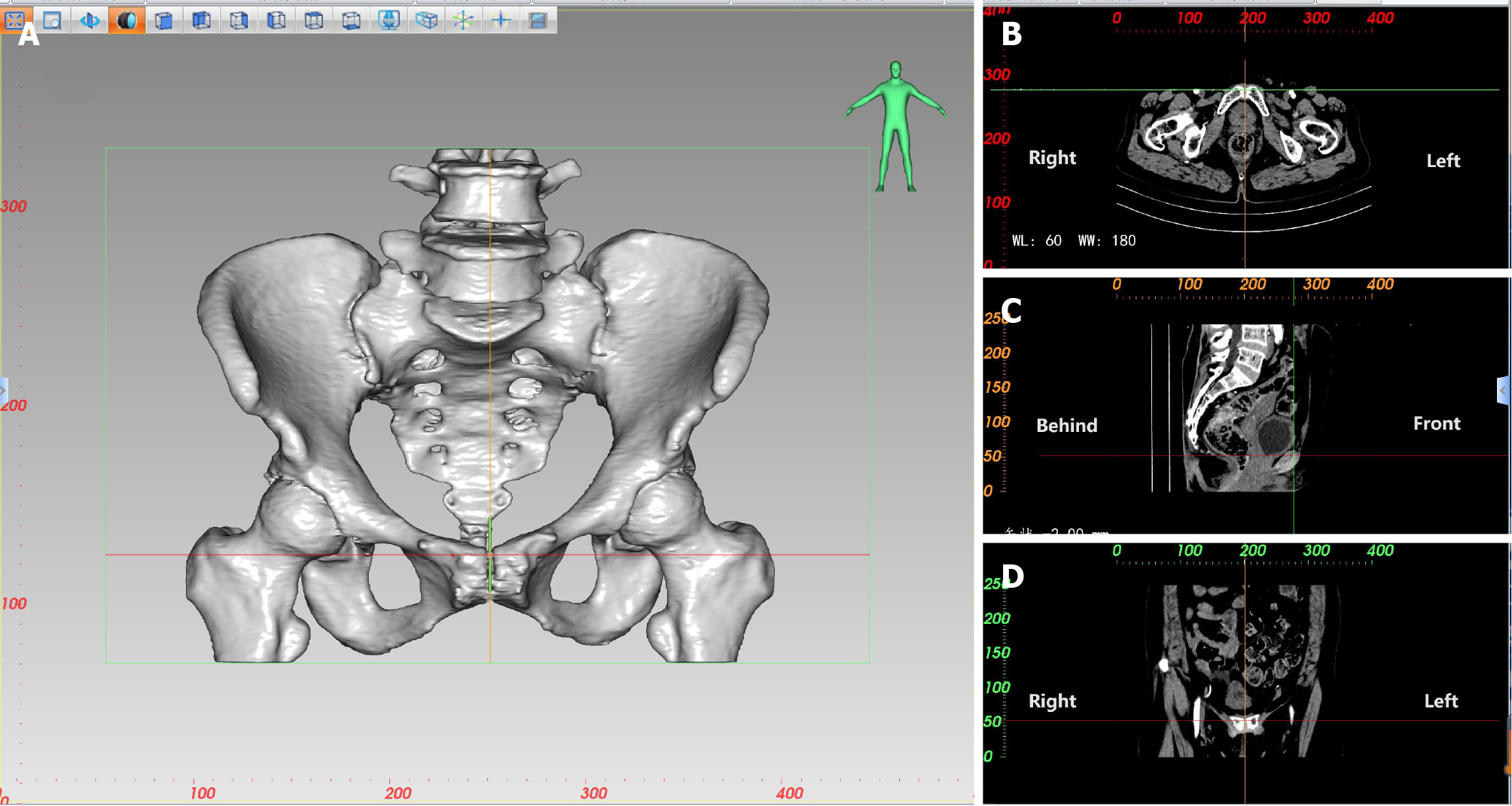
Based on CT thin-slice scans, CT three-dimensional (3D) reconstruction software was used for multiplanar reconstruction. Image segmentation of the bones can be performed using thresholding techniques to establish a 3D digital model of the bones. Different planes, such as transverse, coronal, and sagittal planes, can be used to identify landmarks and measure distances and angles. To find uppermost level of the pubic symphysis (Figure 1), we first locate the pubic symphysis in the sagittal plane by looking at CT scan layers one by one. Each time we click the mouse, two vertical lines appear. When we find uppermost level of the pubic symphysis, a line (X-axis) goes exactly through the top part of the pubis in the coronal plane, while two other lines (Y-axis and Z-axis) are positioned in the middle of the coronal and transverse planes. We use the same method to find another bony landmark, and the software calculates the exact distance between these two points, accurate to 0.01 mm. We can adjust the Window width and level to see the bones for better identification.
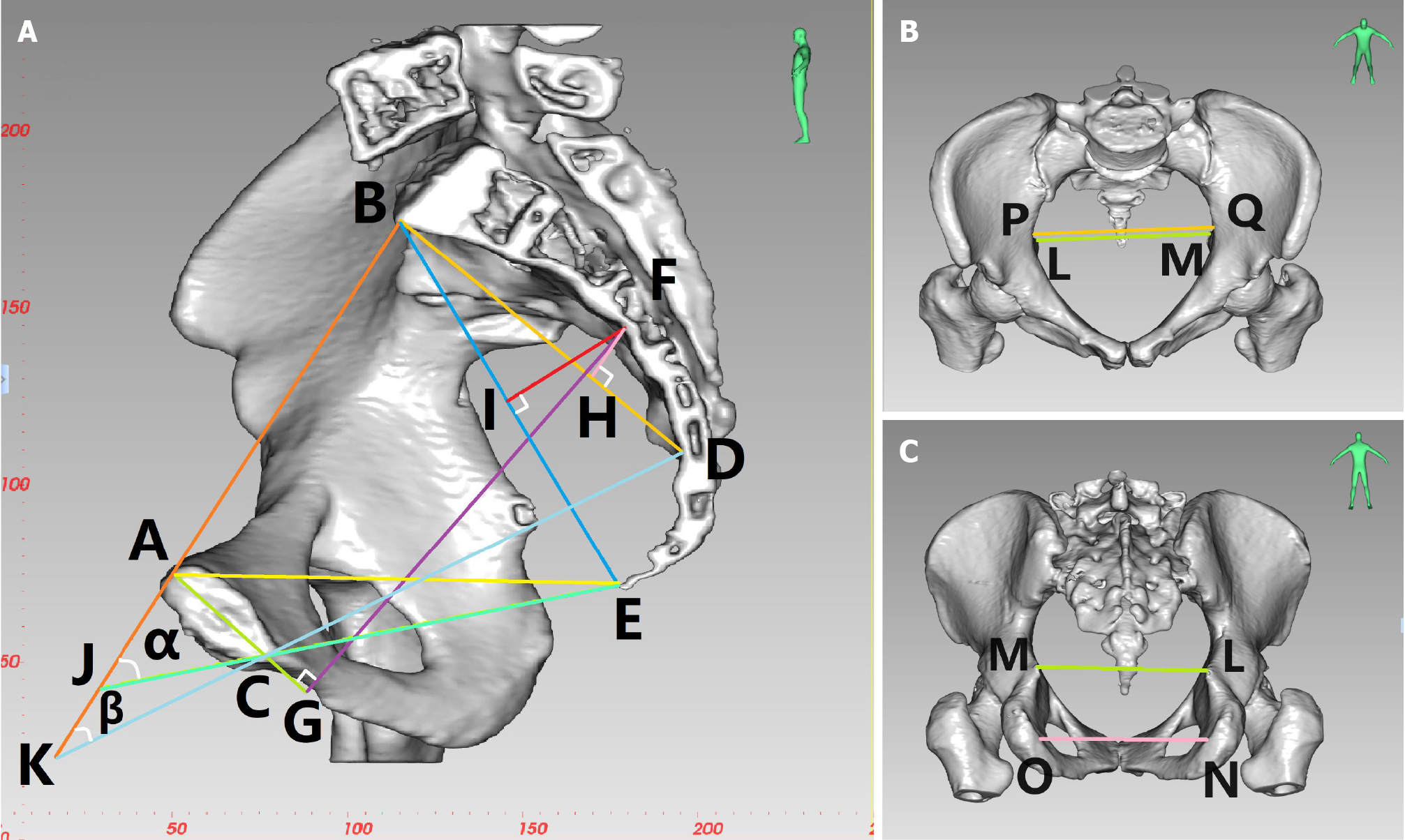
In the same way, 3D measurements of the pelvis are used to locate the midpoint of the anterior margin of the sacral promontory, the maximum distance between the left and right iliac crests, the coccyx tip, the ischial spines, and the ischial tuberosities. A number of bone parameters are measured using 3D CT reconstruction (a selection of pelvic diameters and angles), including the following: anterior-posterior diameter of pelvic inlet (AB), anterior-posterior diameter of mid-pelvis (CD), anterior-posterior diameter of pelvic outlet (CE), superior pubococcygeal diameter (AE), anterior-posterior sacropubic distance (FG), transverse diameter of pelvic inlet (PQ), interischial spine diameter (LM), interischial tuberosity diameter (NO), superior-inferior diameter of the pubic symphysis (AC), superior-inferior diameter of sacrum (BD), sacrococcygeal distance (BE), sacral curature height (FH), sacrococcygeal curvature height (FI), sacrococcygeal-pubic angle (α), and sacropubic angle (β; Table 2 and Figure 2).
| Parameter name | Definition |
| Anterior-posterior diameter of pelvic inlet (AB) | Distance between the midpoint of the superior pubic symphysis and the midpoint of the anterior margin of the sacral promontory |
| Transverse diameter of pelvic inlet (PQ) | Maximum distance between the left and right iliopectineal line |
| Anterior-posterior diameter of mid-pelvis (CD) | Distance between the midpoint of the inferior pubic symphysis and the midpoint of the sacrococcygeal junction |
| Anterior-posterior diameter of pelvic outlet (CE) | Distance between the midpoint of the inferior pubic symphysis and the tip of the coccyx |
| Interischial spine diameter (LM) | Shortest distance between the ischial spines |
| Interischial tuberosity diameter (NO) | Shortest distance between the ischial tuberosities |
| Superior-inferior diameter of the pubic symphysis (AC) | Distance between the superior and inferior borders of the pubic symphysis |
| Sacrococcygeal distance (BE) | Distance between the midpoint of the anterior margin of the sacral promontory and the tip of the coccyx |
| Superior-inferior diameter of sacrum (BD) | Distance between the midpoint of the anterior margin of the sacral promontory and the midpoint of the sacrococcygeal junction |
| Sacrococcygeal-pubic angle (α) | The angle between the extended lines of the anterior-posterior diameter of pelvic inlet and the anterior-posterior diameter of pelvic outlet |
| Sacropubic angle (β) | Angle between the extended lines of the anteroposterior diameter of pelvic inlet and the anterior-posterior diameter of mid-pelvis |
| Sacrococcygeal curvature height (FI) | Vertical distance from the most convex point of the sacrococcygeal curve to the sacrococcygeal distance |
| Sacral curvature height (FH) | Vertical distance from the most convex point of the sacral curve to the superior-inferior diameter of sacrum |
| Superior pubococcygeal diameter (AE) | Distance between the midpoint of the superior pubic symphysis and the tip of the coccyx |
| Anterior-posterior sacropubic distance (FG) | Vertical distance from the most convex point of the sacrococcygeal curve to the superior-inferior diameter of pubic symphysis or its extended line |
Among the 16 pelvic diameter and angle parameters, the pelvic anterior and posterior diameter lines that reflect pelvic width include 5 parameters: anterior-posterior diameter of pelvic inlet (AB), anterior-posterior diameter of mid-pelvis (CD), anterior-posterior diameter of pelvic outlet (CE), superior pubococcygeal diameter (AE), and anterior-posterior sacropubic distance (FG); the left and right pelvic diameter lines that reflect the width of the pelvis include 3 parameters: transverse diameter of pelvic inlet (PQ), interischial spine diameter (LM), and interischial tuberosity diameter (NO); and the superior and inferior diameter lines that reflect the depth of the pelvis include 3 parameters: superior-inferior diameter of the pubic symphysis (AC), superior-inferior diameter of sacrum (BD) and sacrococcygeal distance (BE). Reflecting the degree of curvature of the sacrococcygeal bone includes 2 parameters: Sacrococcygeal curvature height (FI) and sacral curvature height (FH); comprehensively measuring the arc length of the sacrococcygeal bone (sacrum), the size of the bending degree, the size of the superior and inferior diameters of the pubic symphysis, and the distance between the pubis and the sacrococcygeal bone (sacrum), including two parameters: Sacrococcygeal-pubic angle (α) and sacropubic angle (β). Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance reflects the fact that the greater the width of the pelvis, and the shallower the depth of the pelvis, the greater the ratio of anterior-posterior diameter of pelvic inlet/sacrococcygeal distance.
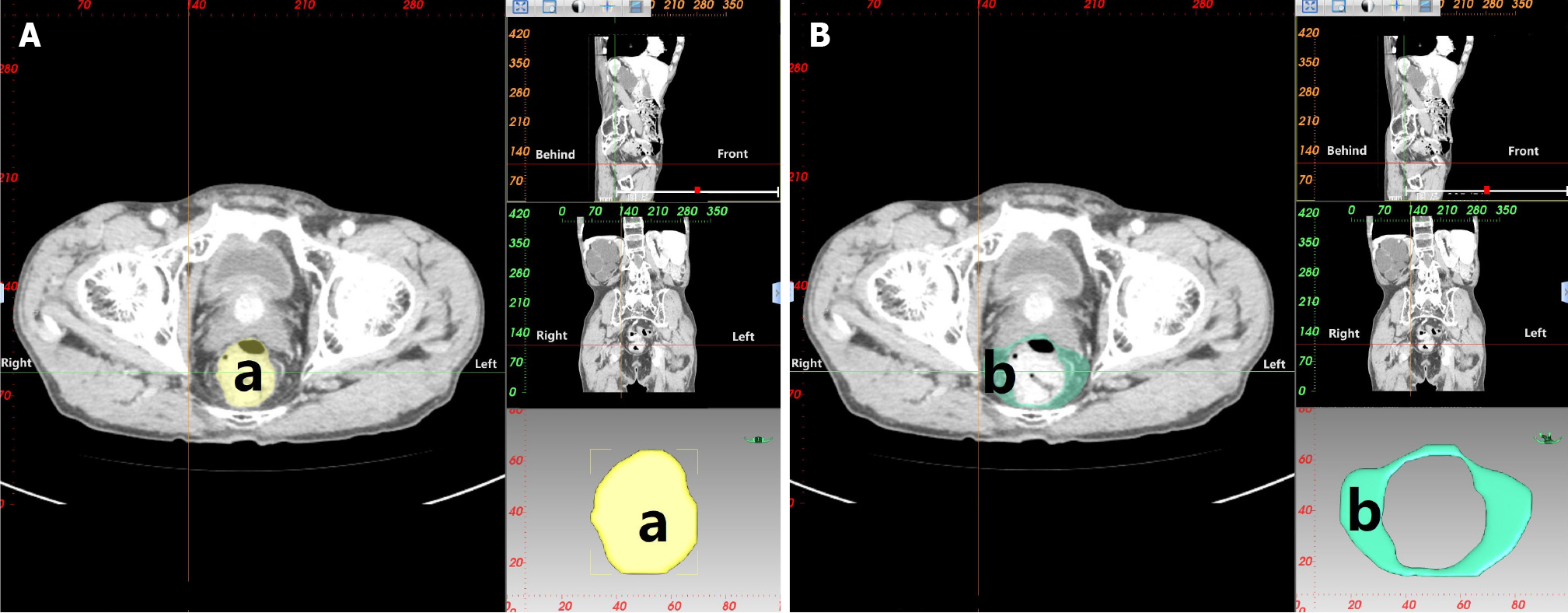
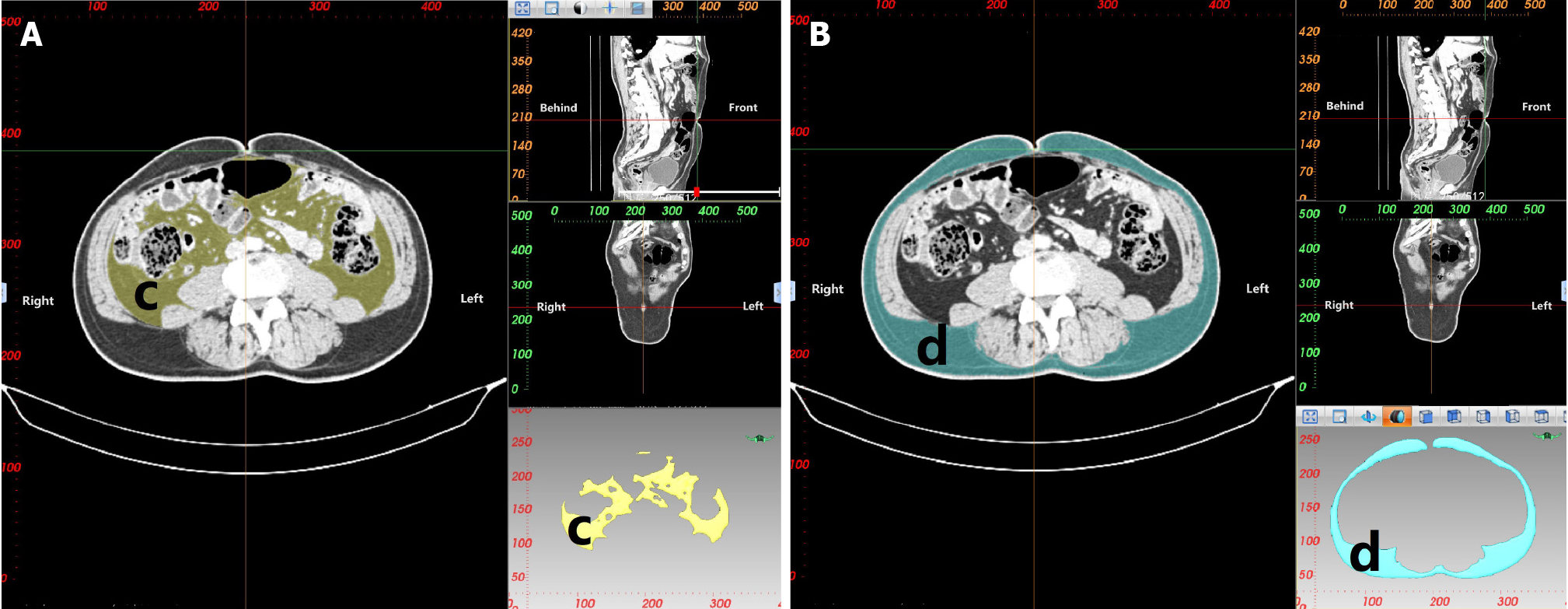
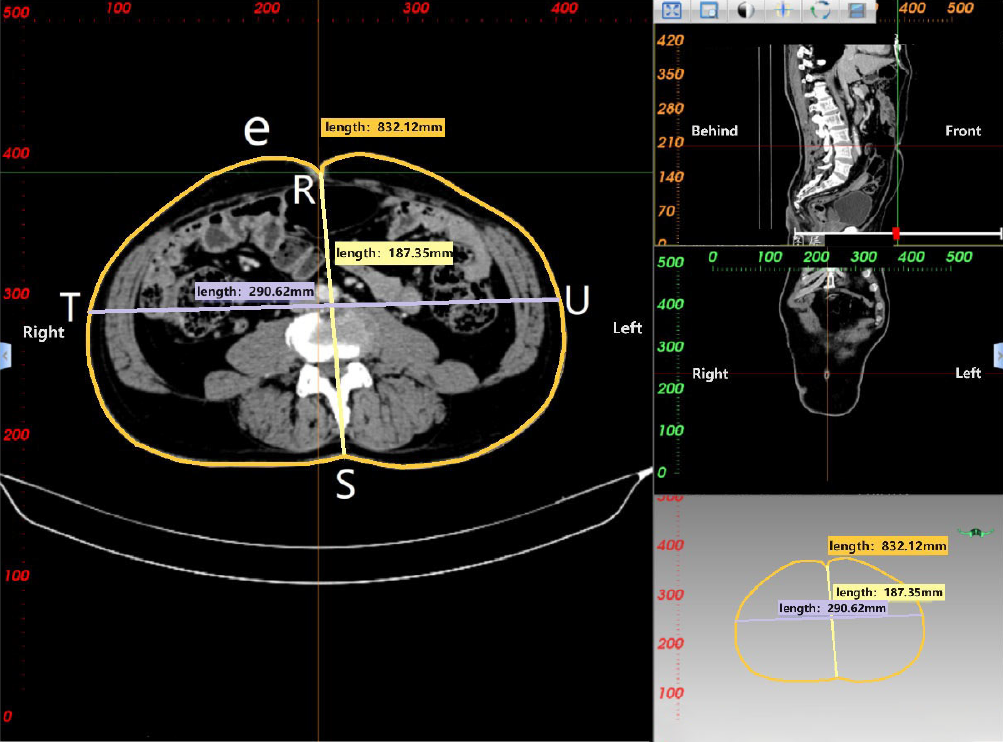
CT 3D reconstruction soft tissue measurement parameters include: rectal area (a), mesorectal fat area (b), visceral fat area (c), subcutaneous fat area (d), waist circumference (e), anterior-posterior abdominal diameter (RS), and transverse abdominal diameter (TU; Table 3 and Figures 3-5).
| Parameter name | Definition |
| Rectal area (a) | Area of the rectum at the level of the ischial spine |
| Mesorectal fat area (b) | Area of the mesorectal fat at the level of the ischial spine |
| Visceral fat area (c) | Area of visceral fat at the level of the umbilicus |
| Subcutaneous fat area (d) | Area of subcutaneous fat at the level of the umbilicus |
| Waist circumference (e) | Circumference of the waist at the level of the umbilicus |
| Anterior-posterior abdominal diameter (RS) | Measurement distance from the umbilicus to the spine-wall intersection in the abdominal plane |
| Transverse abdominal diameter (TU) | Measurement distance along the horizontal line of the lumbar vertebrae anterior margin at the level of the umblicus |
The experimental data were analyzed using R software (version 4.2.1) for statistical analysis, with a significance level 0.05. A P value less than 0.05 (P < 0.05) was considered statistically significant. For univariate analysis, the Shapiro-Wilk test was used to assess the normality of continuous variables. Normally distributed data were presented as mean ± SD and between-group comparisons were performed using t-tests (paired t-tests or analysis of variance for two-sample mean comparisons, depending on the assumptions of normality and homogeneity of variances). Non-normally distributed data were shown as median (Q1, Q3). To compare between-groups, we used the non-parametric Mann-Whitney U test. Categorical and ordinal data were analyzed using the non-parametric Mann-Whitney U test for one-way ordered data and the chi-square test, continuity-corrected chi-square test, or Fisher’s exact test for two-way unordered data. Variables with a P value less than 0.05 in the univariate analysis were included in the multivariate analysis. Logistic regression analysis was performed to identify independent risk and protective factors using a stepwise approach, and a final regression equation was established. Risk and protective factors for the difficulty level of laparoscopic radical sphincter-preserving surgery for rectal cancer were analyzed.
General information on the clinical and pathological details of 162 patients that undergo laparoscopic radical sphincter-preserving surgery to treat rectal cancer is summarized in Table 4.
| Range | mean ± SD | No. of cases [n (%)] | |
| Age (yr) | (32.00, 89.00) | 65 (59, 72) | |
| Gender | |||
| Male | 105 (64.81) | ||
| Female | 57 (35.19) | ||
| BMI (kg/m2) | (15.94, 31.25) | 22.75 ± 3.25 | |
| Underlying comorbidities | 72 (44.44) | ||
| Diabetes | 26 (16.05) | ||
| Hypertension | 58 (35.8) | ||
| Heart disease | 8 (4.94) | ||
| Pulmonary disease | 3 (1.85) | ||
| Stroke | 5 (3.09) | ||
| Other | 1 (0.62) | ||
| ASA grade | |||
| I | 18 (11.11) | ||
| II | 137 (84.57) | ||
| III | 7 (4.32) | ||
| History of previous abdominal surgery | 15 (9.26) | ||
| Preoperative total protein (g/L) | (53.8, 81) | 65.45 (62.4, 68.83) | |
| Preoperative albumin (g/L) | (29, 48.5) | 38.64 ± 3.32 | |
| Preoperative albumin to globulin ratio | (0.9, 2.1) | 1.4 (1.3, 1.6) | |
| Preoperative hemoglobin (g/L) | (89, 166) | 134.5 (119, 143.75) | |
| PNI | (35, 58) | 47.23 ± 5.02 | |
| Preoperative CEA (ug/L) | (0.4, 49.9) | 2.9 (1.9, 5.18) | |
| Preoperative CA199 (kU/L) | (0.6, 128.1) | 8.55 (5.18, 17.98) | |
| Tumor height (cm) | (2, 15) | 8 (6.25, 10) | |
| Tumor Location | |||
| Upper rectum (10 cm < distance ≤ 15 cm) | 30 (18.52) | ||
| Midrectum (5 cm < distance ≤ 10 cm) | 103 (63.58) | ||
| Lower rectum (distance ≤ 5 cm) | 29 (17.9) | ||
| Maximum tumor diameter (cm) | (0.8, 10) | 3.8 (3, 4.88) | |
| Surgical approach | |||
| L-AR | 20 (12.35) | ||
| L-LAR | 135 (83.33) | ||
| L-ISR | 7 (4.32) | ||
| Intraoperative preventive ostomy | 39 | ||
| L-AR + preventive ostomy | 0 | ||
| L-LAR + preventive ostomy | 32 (82.05) | ||
| L-ISR + preventive ostomy | 7 (17.95) | ||
| pT staging | |||
| Tis | 4 (2.47) | ||
| T1 | 19 (11.73) | ||
| T2 | 26 (16.05) | ||
| T3 | 109 (67.28) | ||
| T4a | 4 (2.47) | ||
| pN staging | |||
| N0 | 94 (58.02) | ||
| N1 | 36 (22.22) | ||
| N2 | 32 (19.75) | ||
| pTNM staging | |||
| 0 | 4 (2.47) | ||
| I | 38 (23.46) | ||
| II | 52 (32.1) | ||
| III | 68 (41.98) | ||
| Tumor differentiation grade | |||
| Well-differentiated | 9 (5.56) | ||
| Moderately-differentiated to well-differentiated | 20 (12.35) | ||
| Moderately differentiated | 112 (69.14) | ||
| Moderately differentiated to poorly differentiated | 17 (10.49) | ||
| Poorly-differentiated | 4 (2.47) | ||
| Tumor nodules | 16 (9.88) | ||
| Vascular tumor emboli | 47 (29.01) | ||
| Neural invasion | 40 (24.69) | ||
| Number of lymph nodes removed | (3, 36) | 15 (13, 18) | |
| Surgical duration (min) | (78, 465) | 216.5 (179.25, 260) | |
| Intraoperative blood loss (ml) | (10, 300) | 50 (20, 100) | |
| Postoperative complications (CD grade) | |||
| No complications | 125 (77.16) | ||
| Grade I | 7 (4.32) | ||
| Grade II | 21 (12.96) | ||
| Grade III | 8(4.94) | ||
| Grade IV | 1(0.62) | ||
| Postoperative anastomotic fistula grading | |||
| No anastomotic fistula | 152 (93.83) | ||
| Grade A | 0 | ||
| Grade B | 6 (3.7) | ||
| Grade C | 4 (2.47) | ||
| Duration of postoperative hospital stay (d) | (6, 56) | 14 (12, 16) | |
| Intraoperative use of transanal approach (n) | 7 (4.32) | ||
| Conversion to open surgery | 1 (0.62) | ||
| Postoperative anal evacuation time (d) | (1, 7) | 2 (2, 3) | |
| Postoperative resumption of semi-liquid food time (d) | (3, 25) | 8 (5, 9) | |
| Anastomosis method | |||
| Manual anastomosis (Hand-sewn anastomosis) | 7 (4.32) | ||
| Stapler anastomosis | 155 (95.68) | ||
| Number of staple nail cartridges used in surgery (n) | |||
| L-AR | (1, 2) | 2 (1, 2) | |
| L-LAR | (1, 4) | 2 (1, 2) | |
| L-ISR | 0 | 0 | |
| Length of stapler cartridge used in surgery (mm) | |||
| L-AR | (45, 120) | 90 (56.25, 90) | |
| L-LAR | (45, 180) | 90 (60, 90) | |
| L-ISR | 0 | 0 |
General information on the pelvic bone parameters and soft tissue parameters for the total of 162 patients is summarized in Table 5.
| Measurement | mean ± SD or median (Q1, Q3) (range) |
| Anterior-posterior diameter of pelvic inlet (mm) | 115.23 ± 11.79 |
| Transverse diameter of pelvic inlet (mm) | 123.52 ± 8.14 |
| Anterior-posterior diameter of mid-pelvis (mm) | 111.83 (106.36, 117.74) |
| Anterior-posterior diameter of pelvic outlet (mm) | 88.97 (82.35, 96.86) |
| Interischial spine diameter (mm) | 96.06 (87.3, 104.27) |
| Interischial tuberosity diameter (mm) | 106.63 ± 14.15 |
| Superior-inferior diameter of pubic symphysis (mm) | 42.25 (39.31, 45.42) |
| Sacrococcygeal distance (mm) | 123.19 ± 11.95 |
| Superior-inferior diameter of sacrum (mm) | 105.77 ± 10.17 |
| Sacrococcygeal curvature height (mm) | 39.61 (36.15, 42.71) |
| Sacral curvature height (mm) | 20.82 ± 5.87 |
| Superior pubococcygeal diameter (mm) | 120.26 ± 9.56 |
| Anterior-posterior sacropubic distance (mm) | 119.84 (113.14, 126.49) |
| Sacrococcygeal-pubic angle (°) | 48.05 ± 7.01 |
| Sacropubic angle (°) | 33.04 ± 5.43 |
| Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance | 0.94 (0.86, 1) |
| Rectal area (cm2) | 16.67 (12.23, 21.48) |
| Mesorectal fat area (cm2) | 39.26 (27.41, 51.62) |
| Visceral fat area (cm2) | 215.9 (126.21, 274.02) |
| Subcutaneous fat area (cm2) | 258.24 (198.25, 340.77) |
| Waist circumference (cm) | 82.37 ± 9.15 |
| Anterior-posterior abdominal diameter (cm) | 18.42 ± 2.91 |
| Transverse abdominal diameter (cm) | 28.84 ± 2.79 |
Perioperative outcomes, baseline pathological characteristics, and univariate analysis of laparoscopic radical sphincter-preserving surgery for rectal cancer.
In the comprehensive analysis of 162 patients who underwent laparoscopic radical sphincter-preserving surgery for rectal cancer, based on the grading criteria for the difficulty of laparoscopic rectal cancer surgery, 21 patients (13.0%) were classified as the high-difficulty surgery group, while 141 patients (87.0%) were classified as the low-difficulty surgery group. The outcome from that univariate analysis revealed that these factors were notably linked with the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer: surgical duration (P < 0.001), intraoperative blood loss (P < 0.001), postoperative complications (CD grade; P = 0.0185), duration of postoperative hospital stay (P < 0.001), intraoperative use of trans-anal approach (P = 0.0004), and postoperative anal evacuation time (P = 0.0284). However, there was no significant correlation between the difficulty of surgery and pathological factors such as pT staging, pN staging, pTNM staging, tumor differentiation grade, tumor nodules, vascular tumor emboli, neural invasion, number of lymph nodes removed, postoperative anastomotic fistula grading, conversion to open surgery, and postoperative resumption of semi-liquid food time (P > 0.05; Table 6).
| Univariate analysis | ||||
| Whole group (n = 162) | Low surgical difficulty group (n = 141) | High surgical difficulty group (n = 21) | P value | |
| pT staging | 0.73901 | |||
| Tis | 4 (2.47) | 4 (2.84) | 0 | |
| T1 | 19 (11.73) | 17 (12.06) | 2 (9.52) | |
| T2 | 26 (16.05) | 20 (14.18) | 6 (28.57) | |
| T3 | 109 (67.28) | 97 (68.79) | 12 (57.14) | |
| T4a | 4 (2.47) | 3 (2.13) | 1 (4.76) | |
| pN staging | 0.43271 | |||
| N0 | 94 (58.02) | 83 (58.87) | 11 (52.38) | |
| N1 | 36 (22.22) | 32 (22.7) | 4 (19.05) | |
| N2 | 32 (19.75) | 26 (18.44) | 6 (28.57) | |
| pTNM staging | 0.58951 | |||
| 0 | 4 (2.47) | 4 (2.84) | 0 | |
| Ⅰ | 38 (23.46) | 33 (23.4) | 5 (23.81) | |
| Ⅱ | 52 (32.1) | 46 (32.62) | 6 (28.57) | |
| III | 68 (41.98) | 58 (41.13) | 10 (47.62) | |
| Degree of tumor differentiation | 0.07301 | |||
| Well differentiated | 9 (5.56) | 9 (6.38) | 0 | |
| High-moderate differentiation | 20 (12.35) | 17 (12.06) | 3 (14.29) | |
| Moderate differentiation | 112 (69.14) | 100 (70.92) | 12 (57.14) | |
| Moderate-Low differentiation | 17 (10.49) | 13 (9.22) | 4 (19.05) | |
| Low differentiation | 4 (2.47) | 2 (1.42) | 2 (9.52) | |
| Tumor nodules | 16 (9.88) | 16 (11.35) | 0 | 0.22952 |
| Vascular tumor emboli | 47 (29.01) | 40 (28.37) | 7 (33.33) | 0.83372 |
| Neural invasion | 40 (24.69) | 36 (25.53) | 4 (19.05) | 0.71012 |
| Number of Lymph Nodes Removed | 15 (13, 18) | 15 (13, 18) | 15 (12, 17) | 0.79291 |
| Surgical duration (min) | 216.5 (179.25, 260) | 205 (176, 238) | 324.19 ± 59.62 | 3.423e-101 |
| Intraoperative Blood Loss (ml) | 50 (20, 100) | 50 (20, 50) | 100 (50, 200) | 4.436e-061 |
| Postoperative complications (CD grade) | 0.01851 | |||
| No complications | 125 (77.16) | 113 (80.14) | 12 (57.14) | |
| Grade I | 7 (4.32) | 5 (3.55) | 2 (9.52) | |
| Grade II | 21 (12.96) | 17 (12.06) | 4 (19.05) | |
| Grade III | 8 (4.94) | 5 (3.55) | 3 (14.29) | |
| Grade IV | 1 (0.62) | 1 (0.71) | 0 | |
| Postoperative anastomotic fistula grading | 0.09071 | |||
| No anastomotic fistula | 152 (93.83) | 134 (95.04) | 18 (85.71) | |
| Grade B | 6 (3.7) | 5 (3.55) | 1 (4.76) | |
| Grade C | 4 (2.47) | 2 (1.42) | 2 (9.52) | |
| Duration of postoperative hospital stay (d) | 14 (12, 16) | 13 (12, 15) | 23 (22, 27) | 6.01e-111 |
| Intraoperative use of trans-anal approach | 7 (4.32) | 2 (1.42) | 5 (23.81) | 0.00041 |
| Conversion to open surgery | 1 (0.62) | 0 | 1 (4.76) | 0.12962 |
| Postoperative anal evacuation time(d) | 2 (2, 3) | 2 (2, 3) | 2 (2, 2) | 0.02841 |
| Postoperative resumption of semi-liquid food time (d) | 8 (5, 9) | 8 (6, 9) | 6 (4, 10) | 0.17981 |
Univariate analysis results demonstrated a significant correlation between the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer and the following factors: tumor height (P = 0.0024), surgical approach (P < 0.001), intraoperative preventive ostomy (P < 0.001), sacrococcygeal distance (P = 0.0378), anterior-posterior diameter of pelvic inlet/sacrococcygeal distance (P = 0.0165), and sacrococcygeal-pubic angle (P = 0.0116). However, no significant correlation was observed between other clinical parameters, pelvic bone parameters, soft tissue parameters, and the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer (P > 0.05; Table 7).
| Factors | Entire group | Low difficulty group | High difficulty group | P value |
| Age (yr) | 65 (59, 72) | 65 (58, 73) | 65 (62, 71) | 0.992 |
| Gender | 0.15712 | |||
| Male | 105 (64.81) | 88 (62.41) | 17 (80.95) | |
| Female | 57 (35.19) | 53 (37.59) | 4 (19.05) | |
| BMI (kg/m²) | 22.75 ± 3.25 | 22.7 ± 3.2 | 23.04 ± 3.64 | 0.6965 |
| Underlying comorbidities | 72 (44.44) | 65 (46.1) | 7 (33.33) | 0.38812 |
| ASA grade | 0.83321 | |||
| I | 18 (11.11) | 15 (10.64) | 3 (14.29) | |
| II | 137 (84.57) | 121 (85.82) | 16 (76.19) | |
| III | 7 (4.32) | 5 (3.55) | 2 (9.52) | |
| History of previous abdominal surgery | 15 (9.26) | 15 (10.64) | 0 | 0.22172 |
| Preoperative total Protein (g/L) | 65.45 (62.4, 68.83) | 65.1 (62.4, 68.3) | 66.4 (62.5, 70.2) | 0.32101 |
| Preoperative albumin (g/L) | 38.64 ± 3.32 | 38.54 ± 3.25 | 39.3 ± 3.79 | 0.3892 |
| Preoperative albumin to globulin ratio | 1.4 (1.3, 1.6) | 1.4 (1.3, 1.6) | 1.4 (1.3, 1.6) | 0.70931 |
| Preoperative Hemoglobin (g/L) | 134.5 (119, 143.75) | 134 (119, 143) | 141 (126, 151) | 0.09621 |
| PNI | 47.23 ± 5.02 | 47.02 ± 4.81 | 48.63 ± 6.2 | 0.2637 |
| Preoperative CEA (ug/L) | 2.9 (1.9, 5.18) | 2.9 (1.8, 5.2) | 2.9 (1.9, 4.6) | 0.22571 |
| Preoperative CA199 (kU/L) | 8.55 (5.18, 17.98) | 8.3 (5, 18) | 10.8 (6.9, 17.9) | 0.56631 |
| Tumor height (cm) | 8 (6.25, 10) | 8 (7, 10) | 7 (5, 8) | 0.00241 |
| Tumor maximum diameter (cm) | 3.8 (3, 4.88) | 3.8 (3, 4.5) | 3 (2.8, 5) | 0.55071 |
| Surgical approach | 6.775e-41 | |||
| L-AR | 20 (12.35) | 19 (13.48) | 1 (4.76) | |
| L-LAR | 135 (83.33) | 120 (85.11) | 15 (71.43) | |
| L-ISR | 7 (4.32) | 2 (1.42) | 5 (23.81) | |
| Intraoperative preventive ostomy | 39 (20.07) | 26 (18.44) | 13 (61.90) | 4.644e-052 |
| Intraoperative stapler cartridge count | 2 (1, 2) | 2 (1, 2) | 1 (1, 2) | 0.09531 |
| Intraoperative stapler cartridge length (mm) | 90 (60, 90) | 90 (60, 90) | 60 (45, 90) | 0.09691 |
| Anterior-posterior diameter of pelvic inlet (mm) | 115.23 ± 11.79 | 115.53 ± 11.85 | 113.23 ± 11.49 | 0.4025 |
| Transverse diameter of pelvic inlet (mm) | 123.52 ± 8.14 | 123.98 ± 7.79 | 120.42 ± 9.83 | 0.126 |
| Anterior-posterior diameter of mid-pelvis (mm) | 111.83 (106.36, 117.74) | 112.16 (106.74, 118.12) | 110.69 (103.69, 115.17) | 0.42211 |
| Anterior-posterior diameter of pelvic outlet (mm) | 88.97 (82.35, 96.86) | 89.18 (82.52, 96.92) | 86.53 (81.69, 94.37) | 0.86681 |
| Interischial spine diameter (mm) | 96.06 (87.3, 104.27) | 96.35 (88.76, 104.58) | 90.34 (85.01, 100.56) | 0.06211 |
| Interischial tuberosity diameter (mm) | 104.3 (96.08, 116.95) | 104.09 (96.6, 118.44) | 107.77 (95.7, 112.01) | 0.61451 |
| Superior-inferior diameter of the pubic symphysis (mm) | 42.21 ± 4.75 | 42.14 ± 4.88 | 42.71 ± 3.77 | 0.5341 |
| Sacrococcygeal distance (mm) | 123.19 ± 11.95 | 122.49 ± 12.04 | 127.91 ± 10.42 | 0.0378 |
| Superior-inferior diameter of sacrum (mm) | 105.77 ± 10.17 | 105.36 ± 10.18 | 108.5 ± 9.9 | 0.1881 |
| Sacrococcygeal curvature height (mm) | 39.61 (36.15, 42.71) | 39.63 (36.14, 43.43) | 38.97 (37.68, 40.96) | 0.55961 |
| Sacral curvature height (mm) | 20.82 ± 5.87 | 20.83 ± 6.04 | 34.61 ± 4.11 | 0.9529 |
| Superior pubococcygeal diameter (mm) | 120.26 ± 9.56 | 120.01 ± 9.28 | 121.98 ± 11.35 | 0.4555 |
| Anterior-posterior sacropubic distance (mm) | 119.84 (113.14, 126.49) | 120.04 (113.25, 127.11) | 119.01 (112.86, 121.95) | 0.30551 |
| Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance | 0.94 (0.86, 1) | 0.94 (0.86, 1.02) | 0.87 (0.82, 0.97) | 0.01651 |
| Sacrococcygeal-pubic angle (°) | 48.05 ± 7.01 | 47.64 ± 7.21 | 34.61 ± 4.11 | 0.0116 |
| Sacropubic angle (°) | 33.22 (29.5, 36.66) | 32.83 (28.98, 36.56) | 34.89 (32.05, 36.79) | 0.14131 |
| Rectal area (cm2) | 16.67 (12.23, 21.48) | 16.64 (12.23, 22.57) | 17.07 (10.44, 19.59) | 0.47581 |
| Mesorectal fat area (cm2) | 39.26 (27.41, 51.62) | 38.62 (28.18, 51.35) | 46.32 (24.25, 54.02) | 0.53641 |
| Visceral fat area (cm2) | 215.9 (126.21, 274.02) | 213.65 (126.1, 260.2) | 240.25 (137.54, 320.74) | 0.58341 |
| Subcutaneous fat area (cm2) | 258.24 (198.25, 340.77) | 264.32 (204.34, 341.38) | 240.91 (139.23, 310.51) | 0.30911 |
| Waist circumference (cm) | 82.37 ± 9.15 | 82.36 ± 8.92 | 82.44 ± 10.83 | 0.9741 |
| Anterior-posterior abdominal diameter (cm) | 18.44 (16.38, 20.4) | 18.41 (16.48, 20.25) | 19.11 (16.2, 20.96) | 0.59881 |
| Transverse abdominal diameter (cm) | 28.84 ± 2.79 | 28.83 ± 2.69 | 28.92 ± 3.48 | 0.6991 |
Multivariate logistic regression analysis revealed that the surgical approach using L-ISR, intraoperative preventive ostomy, and sacrococcygeal distance were independent risk factors for the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer (P < 0.05). In contrast, the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance was a protective factor for the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer (P < 0.05; Table 8).
| Variable | β | SE | Wald | Odds ratio (95%CI) | P value |
| Intercept | 1.945 | 6.353 | 0.094 | 6.99 (0.000-1787454.283) | 0.760 |
| Tumor Height | 0.230 | 0.196 | 1.387 | 1.259 (0.858-1.848) | 0.239 |
| Surgical approach (Control group = L-ISR) | |||||
| L-AR | -5.050 | 2.425 | 4.336 | 0.006 (0.000-0.743) | 0.037 |
| L-LAR | -3.417 | 1.311 | 6.789 | 0.033 (0.003-0.429) | 0.009 |
| Intraoperative preventive ostomy | 2.604 | 0.861 | 9.147 | 13.517 (2.500-73.069) | 0.002 |
| Sacrococcygeal distance | 0.086 | 0.04 | 4.567 | 1.09 (1.007-1.180) | 0.033 |
| Sacrococcygeal-pubic angle | -0.115 | 0.082 | 1.959 | 0.891 (0.759-1.047) | 0.162 |
| Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance | -9.328 | 4.151 | 5.050 | 0.00009 (0.000-0.304) | 0.025 |
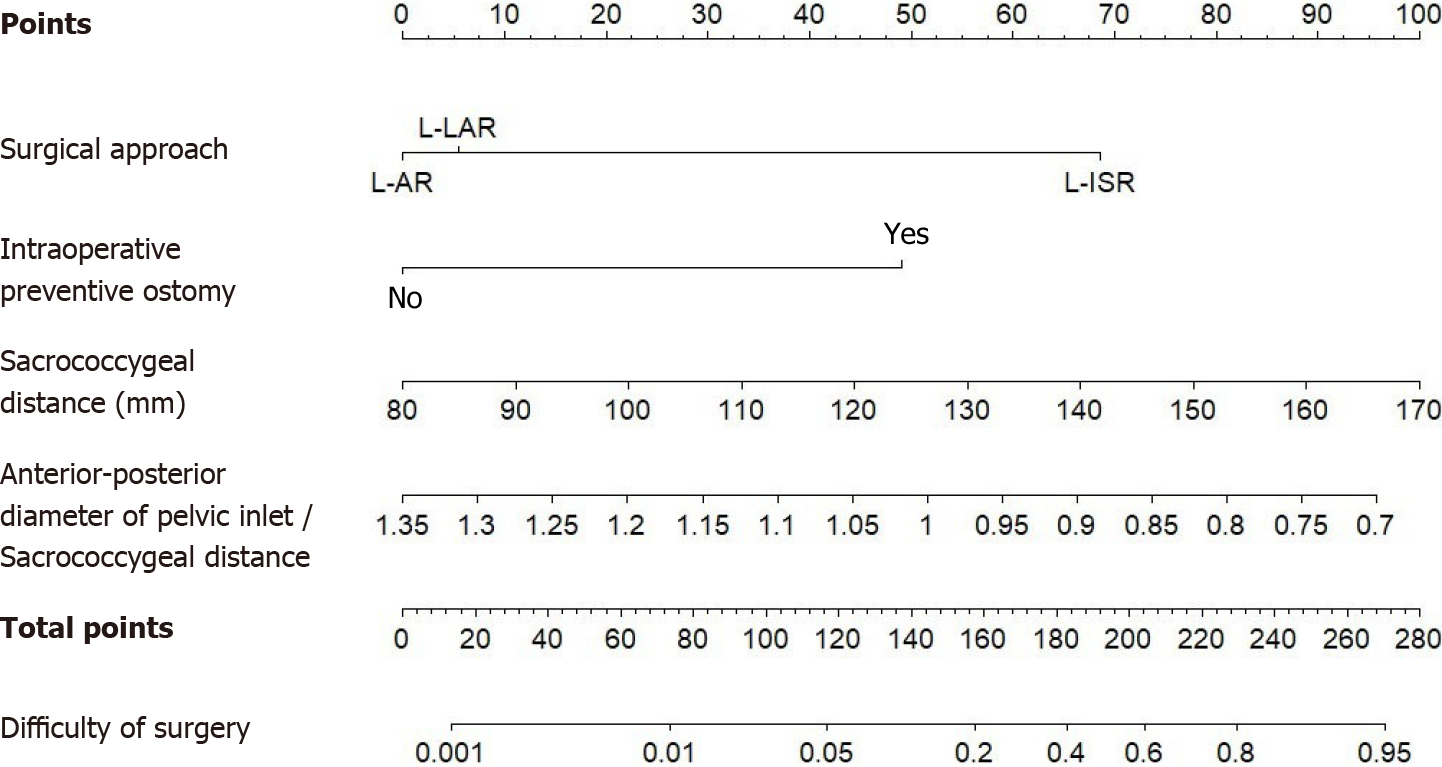
Based on the results of the multivariate logistic analysis, a nomogram clinical prediction model was constructed to predict the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer (Figure 6). The risk factors included the surgical approach, intraoperative preventive ostomy, and sacrococcygeal distance, while the protective factor was the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance.
The total score, obtained by summing up the individual scores assigned to each risk and protective factor, corresponds to the predicted probability of experiencing difficulty during laparoscopic radical sphincter-preserving surgery for rectal cancer. A higher total score indicates a greater likelihood of encountering difficulty during the procedure.
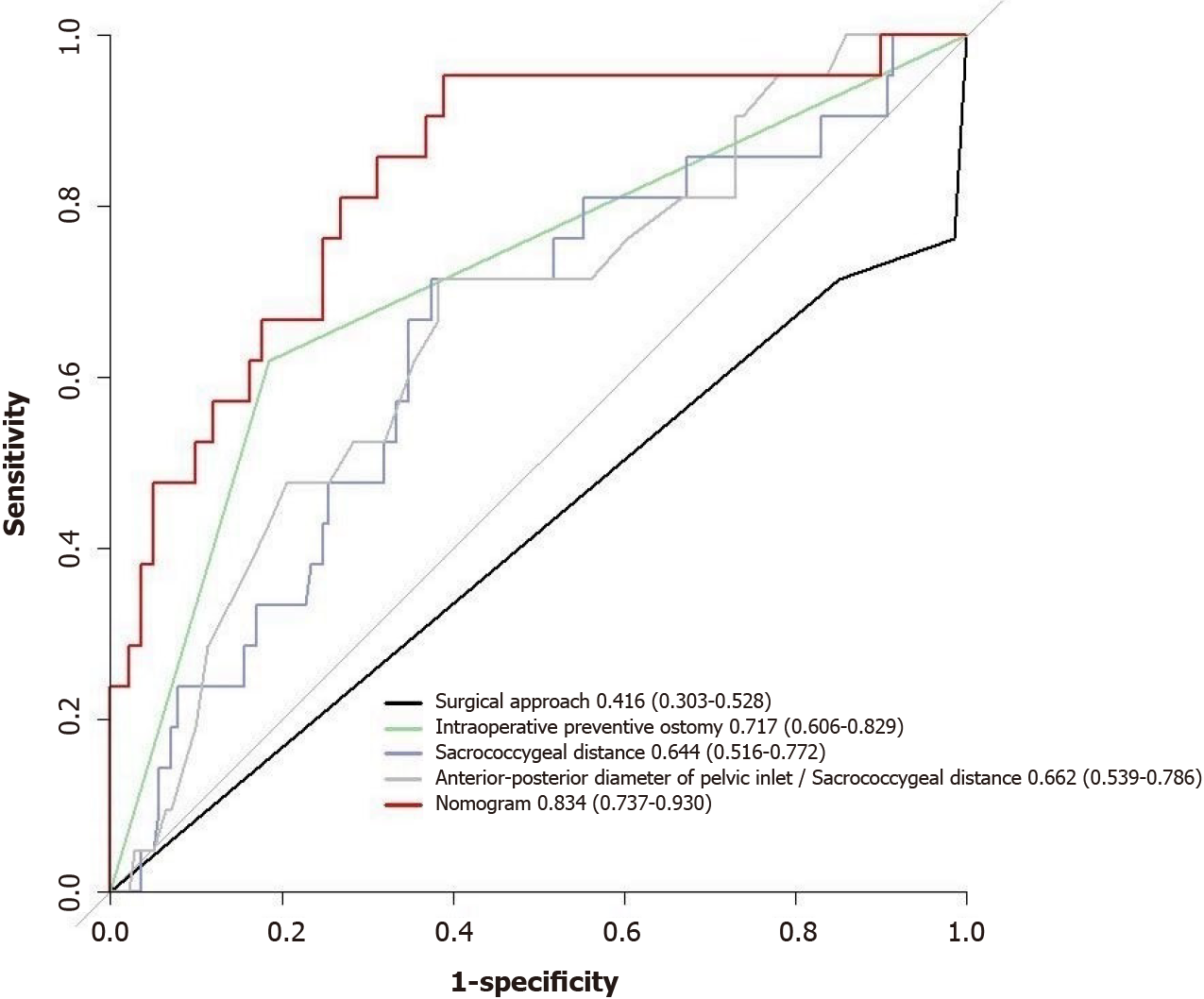
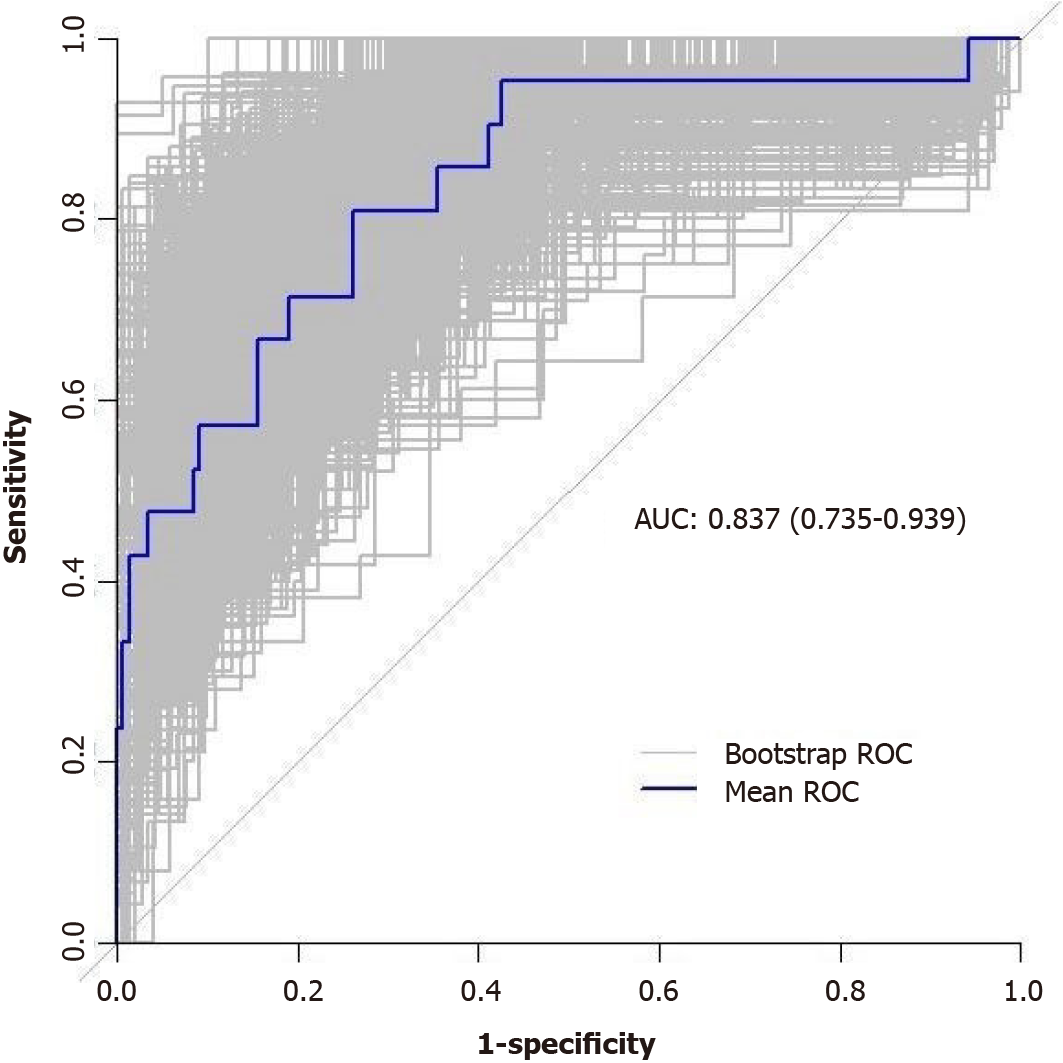
The performance of the nomogram prediction model was assessed using the receiver operating characteristic (ROC) curve. The ROC curve evaluates the predictive ability of various risk factors. The results showed that different risk factors had different areas under the ROC curve (AUC) when predicting the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer. The AUC values for the individual risk factors were as follows: (1) Surgical approach: AUC = 0.416 (95%CI: 0.303-0.528); (2) Intraoperative preventive ostomy: AUC = 0.717 (95%CI: 0.606-0.829); (3) Sacrococcygeal distance: AUC = 0.644 (95%CI: 0.516-0.772); and (4) Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance: AUC = 0.662 (95%CI: 0.539-0.786). On the other hand, the nomogram prediction model showed an AUC of 0.834 (95%CI: 0.737-0.930), indicating better predictive performance compared to individual risk or protective factors (Figures 7 and 8).
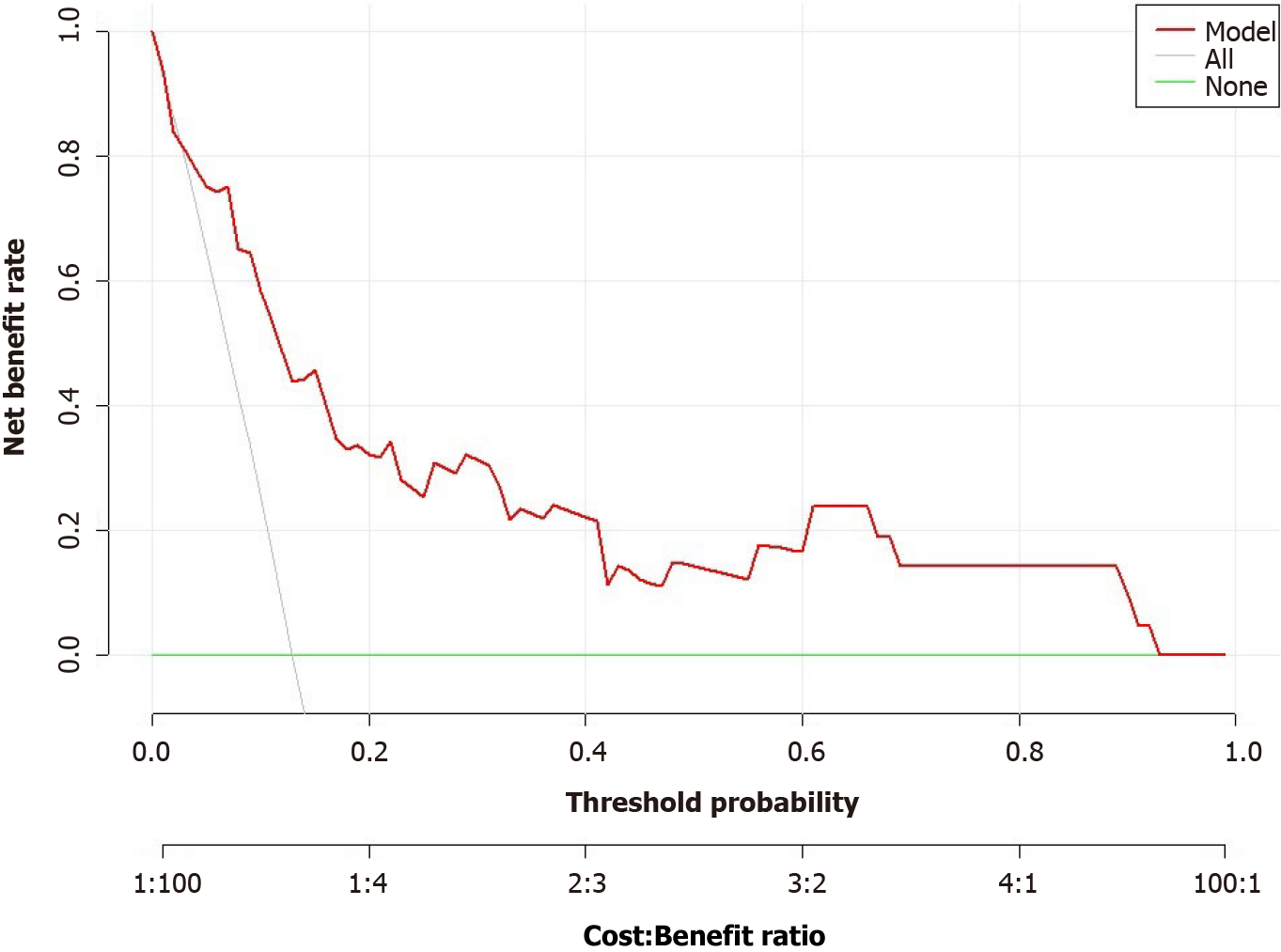
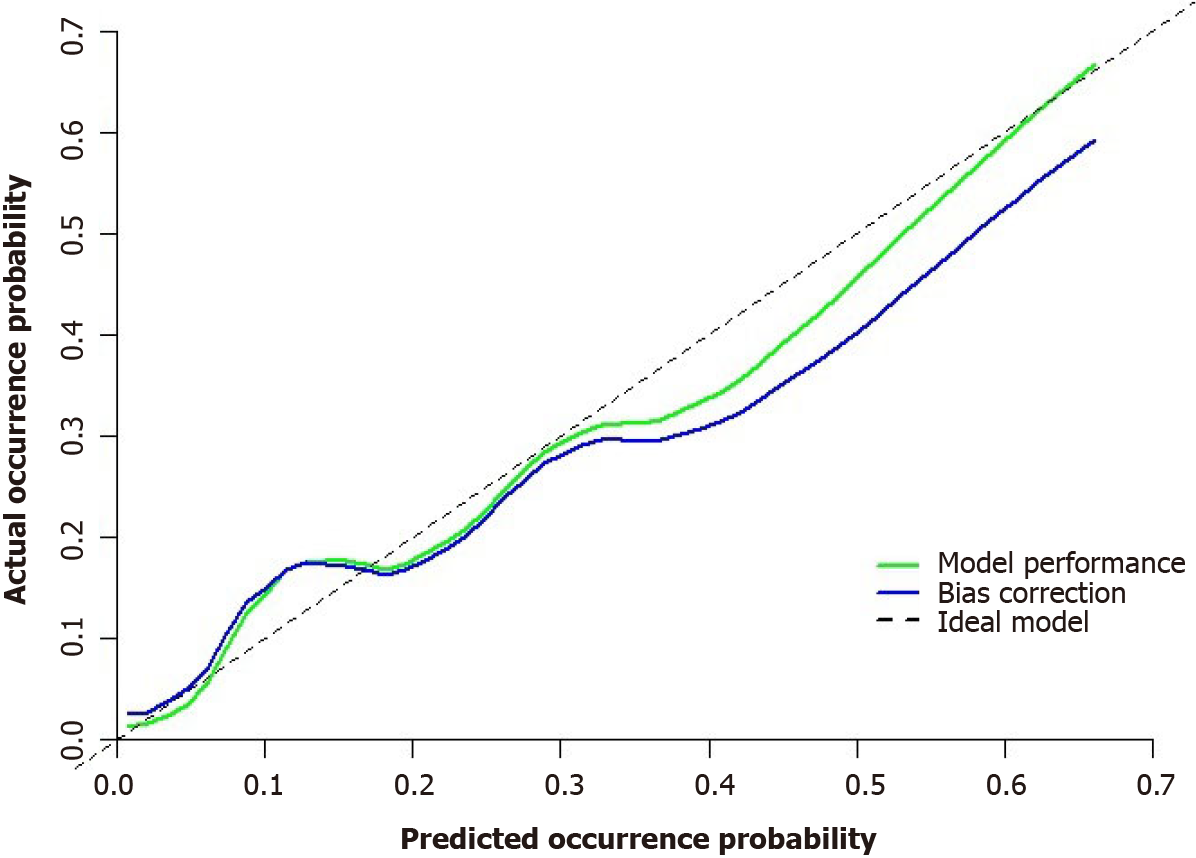
The model’s predictive ability was further validated through Bootstrap internal sampling validation. After 1000 repetitions of sampling, the internally validated C-index of the model was 0.837, suggesting good discriminative ability (Figure 9). The calibration curve demonstrated a good consistency between the predicted and actual results (Figure 10).
To assess the goodness-of-fit of the nomogram prediction model, the Hosmer-Lemeshow test was conducted. The test result showed a chi-square value of 11.797 and a P value of 0.161, indicating that the model’s fit was good (P value > 0.05).
Colorectal cancer stands as one of the most common malignant tumors affecting the digestive system. According to the latest global cancer statistics, colorectal cancer holds the third position in overall incidence among malignant tumors worldwide and is the second leading cause of death from malignancies[12]. Since the first work was published by Heald et al[13] in 1982, TME has been accepted as a core concept as a cure for rectal cancer[14,15]. The widespread adoption of TME principles has significantly reduced the local recurrence rate of rectal cancer[16]. In recent years, advancements in surgical staplers and the application of neoadjuvant chemoradiotherapy have increased the rates of sphincter preservation in mid-low rectal cancer, especially in low rectal cancer. However, despite these advancements, a considerable number of rectal cancer patients still encounter challenge with failed sphincter-saving procedures[17,18]. This challenge arises due to the deep location of mid-low rectal tumors within the narrow pelvic cavity and their close anatomical relationship with surrounding organs. Factors such as limited pelvic space and a thickened mesorectum make achieving optimal visualization during surgery difficult, thereby increasing the complexity of the surgical procedure. Particularly for patients with characteristics indicative of a “difficult pelvis”, such as male patients, an enlarged prostate, obesity, and narrow pelvic structures[19,20], preserving the anal sphincter during surgery often presents challenges. Therefore, finding solutions to address the surgical dilemma faced by rectal cancer patients with a “difficult pelvis” has become an urgent requirement for colorectal surgeons[21].
In recent years, there has been a growing focus on utilizing pelvic measurements for the preoperative assessment of rectal cancer both domestically and internationally. Previous studies[22-25] primarily concentrated on comparing pelvic dimensions between males and females and assessing the impact of individual measurements and angles on surgical limitations. Some studies employed two-dimensional MRI pelvic measurements to examine the correlation between pelvic dimensions and the difficulty of rectal cancer surgery. However, routine preoperative MRI encounters limitations such as a 5 mm slice thickness, prolonged imaging time, and unclear, blurry images after 3D reconstruction, hindering its widespread clinical implementation. Conversely, CT thin-slice scanning with a thickness of 1mm or less enables the generation of clear and visually realistic images after 3D reconstruction. Therefore, CT-based 3D pelvic reconstruction holds the potential to accurately evaluate the pelvic surgical space in patients with rectal cancer.
Sun et al[26] explored using MRI-based artificial intelligence to generally assess laparoscopic rectal surgery difficulty. However, no existing research specifically details the optimal approach to predict the technical difficulty associated with performing laparoscopic sphincter-preserving radical resection, the particular procedure for rectal cancer addressed by our work. A recent systemic review by Przedlacka et al[27] concluded that 3D modelling technology demonstrates potential utilization in multiple aspects of rectal cancer surgery. However, as this review predominantly included feasibility or pilot studies, it indicates the relative novelty of applying 3D modelling technology to rectal cancer surgery. The authors found the limitations of the paper suggest further research is needed to fully evaluate the benefits and limitations of this technology in clinical practice. Limited research has been conducted on the application of CT-based 3D pelvic measurements in rectal cancer surgery, especially concerning laparoscopic procedures. In the context of open rectal cancer surgery, Gu et al[28] observed that CT measurement parameters, such as the shortest diameter of the pubic symphysis to the coccyx, sacrococcygeal arch length, and sacrum curvature, impact open sphincter-preserving surgery. Another study by Yan et al[29] found that a wider and shallower pelvis correlated with reduced surgical duration and blood loss in open TME surgery. Conversely, a deeper, narrower pelvis with greater sacrum curvature increased surgical difficulty. Furthermore, the ratio of the anterior-posterior diameter to the sacrococcygeal distance predicted the difficulty of TME surgery. Zur Hausen et al[5] identified a shorter obstetric conjugate diameter (the shortest distance from the sacral promontory to the upper margin of the pubic symphysis) as a risk factor for incomplete TME with inadequate mesorectal excision in open surgery. In our previous research, we proposed that BMI, tumor height, and maximum tumor diameter can predict the difficulty of open surgery for mid-low rectal cancer. Additionally, beyond relevant clinicopathological parameters, a wider and shallower pelvis with smaller curvature is associated with shorter surgical duration and reduced intraoperative bleeding. Conversely, a narrower, deeper pelvis with greater curvature increases the surgical difficulty[30].
The findings of Targarona et al[31] indicate that the local anatomical and pathological characteristics of the pelvis directly impact the surgical outcome of laparoscopic rectal surgery. Specifically, the lower pelvic diameter emerges as an independent predictor of intermediate openings, surgical duration, and postoperative complication rates. Wang et al’s[32] study revealed significant associations between the transverse diameter of the pelvis (including the transverse entrance diameter, acetabular spacing, interacetabular spine diameter, and inter-sciatic tuberosity diameter), anterior-posterior diameter of pelvic outlet and the angle of the inferior border of symphysis pubis to the superior border of symphysis pubis to the sacral promontory with surgical duration. Akiyoshi et al[33] identified the anterior-posterior diameter of pelvic outlet as an independent predictor of surgical duration for laparoscopic TME, with a larger anterior-posterior diameter of pelvic outlet associated with shorter surgical duration. Shimada et al[6] discovered that the transverse diameter of the pelvic inlet was significantly linked to surgical duration in patients undergoing open or laparoscopic anterior rectal resection. A corrected multivariable analysis of operative-related factors indicated that the ratio of the anterior-posterior diameter of pelvic inlet to the transverse diameter was the sole independent risk factor for prolonged surgical duration. This suggests that the shape of the pelvic inlet may be useful in predicting surgical duration. In contrast, a study by Ogiso et al[34] found no significant correlation between CT measurement of pelvic diameter and surgical duration in L-AR for rectal cancer. This led to the suggestion that a narrow pelvis was not a contraindication to this type of surgery. Although, it’s vital to appreciate that their research did a limited sampling, including only 50 patients, and measured only five bony pelvic parameters: the anterior-posterior and transverse diameters of pelvic inlet and outlet, and sacrococcygeal distance.
Currently, the indicators used to assess the difficulty of laparoscopic rectal cancer surgery include surgical duration, intraoperative blood loss, conversion to open surgery, postoperative complications (including anastomotic leakage), and postoperative pathological findings (including circumferential resection margin, quality of TME specimen, and total number of lymph nodes removed)[11,35-37]. Some studies have also defined the difficulty of surgery based on cutoff values derived from linear regression analysis related to the duration of pelvic clearance. For example, Kim et al[38] defined risk factors as the upper quartile cutoff values of sacral length and tumor size and the lower quartile cutoff values of sacral depth and interischial tuberosity diameter. Cases without any risk factors were classified as the easy group, those with 1-2 risk factors as the moderate group, and those with three or more risk factors as the difficult group. Moreover, surgical difficulty can be defined based on the surgeon’s assessment. This may involve situations like unplanned conversion from low anterior resection (LAR) to APR, seeking consultation with a second colorectal surgeon during the operation, employing modified surgical techniques for billing purposes (indicating cases with billing difficulty), or necessitating reoperation due to complications within the pelvic cavity[39].
To obtain a relatively comprehensive short-term outcome definition for assessing the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer, this study utilized the intraoperative and postoperative indicators proposed by Escal et al[9] and Yamamoto et al[10], modifying them for the definition criteria of the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer with suitable modifications. The modified definition criteria were introduced for the first time and applied to evaluate the surgical difficulty in patients with directly surgically radical resectable rectal cancer who did not undergo preoperative neoadjuvant therapy. The inclusion of both intraoperative and postoperative indicators in this definition is of great interest. It is relatively comprehensive in assessing surgical difficulty, as suboptimal surgical specimens and a complex postoperative course may elevate local recurrence rates and impact long-term patient survival[11,40]. In the present study, regarding perioperative outcomes, and postoperative pathological baseline characteristics, univariate analysis revealed significant associations (all P < 0.05) between the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer and surgical duration, intraoperative blood loss, postoperative complications (CD grade), duration of postoperative hospital stay, intraoperative use of trans-anal approach, and postoperative anal evacuation time. When assessing predictors associated with difficult laparoscopic rectal cancer surgery, univariate analysis demonstrated significant associations (all P < 0.05) with tumor height, surgical approach, intraoperative preventive ostomy, sacrococcygeal distance, anterior-posterior diameter of pelvic inlet/sacrococcygeal distance, and sacrococcygeal-pubic angle. However, other clinicopathological parameters, pelvic bony parameters, and soft tissue parameters did not show significant correlation with the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer (P > 0.05). The present study also indicated that the gender factor was not related to the difficulty of laparoscopic rectal cancer surgery, suggesting considerable variation and overlap of pelvic parameters between both sexes[24]. Probably, the correlation between pelvic parameters and the difficulty of laparoscopic rectal cancer surgery does not depend on the patient’s gender[6].
The current findings are valuable as they reveal a progressive increase in surgical difficulty during the transition in surgical approach from L-AR, L-LAR to L-ISR, particularly since L-ISR surgery requires the additional use of a tran-sanal incision to ensure the absence of cancer cells in the distal tumor margin. The use of intraoperative preventive ostomy is influenced not only by the proximity of the lower margin of the tumor to the anal margin but also by various complex factors such as unsatisfactory intraoperative anastomosis, the patient’s age, preoperative underlying comorbidities such as diabetes, poor nutritional status such as hypoalbuminemia, and preoperative neoadjuvant radiotherapy. Overall, patients with intraoperative use of preventive ostomy may experience greater surgical difficulty. This result aligns with Lee et al[41], who reported, through multivariable analysis, that intraoperative preventive ostomy is an independent predictor of operative time in patients undergoing radical resection (both open and laparoscopic and robotic LAR) for mid-low rectal cancer.
The sacrococcygeal distance reflects the depth of the pelvis in the vertical direction, while the anterior-posterior diameter of pelvic inlet reflects the width of the pelvis in the front-back direction. When the sacrococcygeal distance is larger, and the anterior-posterior diameter of pelvic inlet is smaller, the ratio of the anterior-posterior diameter of pelvic inlet to the sacrococcygeal distance will be smaller. This indicates that the pelvic space is deeper in the vertical direction and narrower in the front-back direction, suggesting increased surgical difficulty. This is especially relevant in laparoscopic surgery for low rectal cancer, where performing presacral dissection in a narrow, deep, concave pelvis can be challenging. The prominent sacral promontory and larger sacrococcygeal-pubic angle can hinder the view of the laparoscope. Conventional laparoscopes are rigid and cannot bend or fold, making exposure of deep pelvic structures relatively difficult. Although laparoscopic surgery provides the advantage of magnified visualization, surgical manipulation becomes more challenging for the surgeon[11,42]. Due to the risk of deviating from the correct dissection plane (the “Holy Plane”) and inadvertently injuring the presacral venous plexus, which can result in severe presacral bleeding, these technically challenging maneuvers should be performed by experienced colorectal surgeons. In cases where laparoscopic TME becomes difficult, tran-sanal TME can be considered an alternative approach. This can help reduce the surgical difficulty and preserve the patient’s anal sphincter function.
Through univariate analysis and multivariable logistic regression analysis in this study, four parameters were identified as independent risk or protective factors for the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer: Surgical approach using L-ISR, intraoperative preventive ostomy, sacrococcygeal distance, and anterior-posterior diameter of pelvic inlet/sacrococcygeal distance. A nomogram model was constructed based on these factors to predict the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer. The nomogram in this study assigns scores to each level of the risk and protective factors (surgical approach, intraoperative preventive ostomy, sacrococcygeal distance, anterior-posterior diameter of pelvic inlet/sacrococcygeal distance) based on their impact on the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer. The scores for each risk and protective factor are summed to obtain a total score, and the probability of an individual undergoing a high-difficulty laparoscopic radical sphincter-preserving surgery for rectal cancer is calculated using a functional transformation relationship. AUC value for nomogram was 0.834 (95%CI: 0.737-0.930). After repeated sampling, bootstrap internal validation of the nomogram model yielded a C-index of 0.837. The calibrating line demonstrated good correlation among predicted and actual results.
However, this study has certain limitations. It is a single-center retrospective observational study, leading to restricted representativeness. Despite meticulous data selection, both selection bias and information bias remain inevitable. The retrospective nature of the study prevented the further breakdown of surgical duration into pelvic dissection time, hindering a more accurate prediction of the difficulty of TME for rectal cancer. Notably, cases undergoing neoadjuvant therapy were excluded due to their limited number. Subsequent studies should focus on increase the inclusion of cases with neoadjuvant therapy and integrating measurements from MRI to more thoroughly assess the impact of pelvic bone parameters and soft tissue parameters on the difficulty of laparoscopic rectal cancer surgery. Additionally, our constructed nomogram requires further validation at other hospitals.
Our study revealed that the surgical approach using L-ISR, intraoperative preventive ostomy, and sacrococcygeal distance are identified as independent risk factors influencing the difficulty of laparoscopic radical sphincter-preserving surgery for rectal cancer. Conversely, the anterior-posterior diameter of pelvic inlet/sacrococcygeal distance acts as a protective factor. The constructed nomogram incorporates these four parameters, allowing for preoperative prediction of the difficulty associated with laparoscopic radical sphincter-preserving surgery for rectal cancer.
| 1. | Veenhof AA, Engel AF, van der Peet DL, Sietses C, Meijerink WJ, de Lange-de Klerk ES, Cuesta MA. Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis. 2008;23:469-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Bertani E, Chiappa A, Della Vigna P, Radice D, Papis D, Cossu L, Biffi R, Bianchi PP, Luca F, Andreoni B. The Impact of pelvimetry on anastomotic leakage in a consecutive series of open, laparoscopic and robotic low anterior resections with total mesorectal excision for rectal cancer. Hepatogastroenterology. 2014;61:1574-1581. [PubMed] |
| 3. | Kaufmann D, Lauscher JC, Gröne J, Zur Hausen G, Kreis ME, Hamm B, Niehues SM. CT-based measurement of the inner pelvic volume. Acta Radiol. 2017;58:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Curtis NJ, Thomas C, Dennison G, Ockrim JB, Conti JA, Dalton R, Allison AS, Francis NK. Factors Predicting Operative Difficulty of Laparoscopic Total Mesorectal Excision. Dis Colon Rectum. 2019;62:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Zur Hausen G, Gröne J, Kaufmann D, Niehues SM, Aschenbrenner K, Stroux A, Hamm B, Kreis ME, Lauscher JC. Influence of pelvic volume on surgical outcome after low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Shimada T, Tsuruta M, Hasegawa H, Okabayashi K, Ishida T, Asada Y, Suzumura H, Kitagawa Y. Pelvic inlet shape measured by three-dimensional pelvimetry is a predictor of the operative time in the anterior resection of rectal cancer. Surg Today. 2018;48:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26131] [Article Influence: 1187.8] [Reference Citation Analysis (2)] |
| 8. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York (NY): Springer; 2017. |
| 9. | Escal L, Nougaret S, Guiu B, Bertrand MM, de Forges H, Tetreau R, Thézenas S, Rouanet P. MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg. 2018;105:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Yamamoto T, Kawada K, Kiyasu Y, Itatani Y, Mizuno R, Hida K, Sakai Y. Prediction of surgical difficulty in minimally invasive surgery for rectal cancer by use of MRI pelvimetry. BJS Open. 2020;4:666-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 11. | Sun Y, Chen J, Ye C, Lin H, Lu X, Huang Y, Chi P. Pelvimetric and Nutritional Factors Predicting Surgical Difficulty in Laparoscopic Resection for Rectal Cancer Following Preoperative Chemoradiotherapy. World J Surg. 2021;45:2261-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 13. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1972] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 14. | National Comprehensive Cancer Network. NCCN Guidelines, Rectal Cancer. [cited 4 April 2022]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461. |
| 15. | Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | Dorudi S, Steele RJ, McArdle CS. Surgery for colorectal cancer. Br Med Bull. 2002;64:101-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Law WL, Chu KW. Impact of total mesorectal excision on the results of surgery of distal rectal cancer. Br J Surg. 2001;88:1607-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O; Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Sun Z, Hou WY, Liu JJ, Xue HD, Xu PR, Wu B, Lin GL, Xu L, Lu JY, Xiao Y. Predictive value of MRI pelvic measurements for "difficult pelvis" during total mesorectal excision. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Wu A, He G, Wang L, Dong Q, Liu X, Li Y, Leng J, Zhang X, Sun T, Zhang Y, Yao Y. Short-term outcome of transanal total mesorectal excision for male low rectal cancer patients with "difficult pelvis" : a single center report from Peking University Cancer Hospital. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:646-653. [PubMed] [DOI] [Full Text] |
| 22. | Salerno G, Daniels IR, Brown G, Norman AR, Moran BJ, Heald RJ. Variations in pelvic dimensions do not predict the risk of circumferential resection margin (CRM) involvement in rectal cancer. World J Surg. 2007;31:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ, Sagar PM, Burke D. MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis. 2005;7:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Salerno G, Daniels IR, Brown G, Heald RJ, Moran BJ. Magnetic resonance imaging pelvimetry in 186 patients with rectal cancer confirms an overlap in pelvic size between males and females. Colorectal Dis. 2006;8:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc. 2010;24:2974-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Sun Z, Hou W, Liu W, Liu J, Li K, Wu B, Lin G, Xue H, Pan J, Xiao Y. Establishment of Surgical Difficulty Grading System and Application of MRI-Based Artificial Intelligence to Stratify Difficulty in Laparoscopic Rectal Surgery. Bioengineering (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Przedlacka A, Pellino G, Fletcher J, Bello F, Tekkis PP, Kontovounisios C. Current and future role of three-dimensional modelling technology in rectal cancer surgery: A systematic review. World J Gastrointest Surg. 2021;13:1754-1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 28. | Gu J, Bo XF, Xiong CY, Wu AW, Zhang XP, Li M, An Q, Fang J, Li J, Zhang X, Wang HY, Gao F, You WC. Defining pelvic factors in sphincter-preservation of low rectal cancer with a three-dimensional digital model of pelvis. Dis Colon Rectum. 2006;49:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Yan HH, Lou Z, Sheng J, Zhang W, Fu CG, Meng RG. Computed tomography pelvimetry as a predictor of technical difficulty in total mesorectal excision. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:846-850. [PubMed] [DOI] [Full Text] |
| 30. | Zhou XC, Su M, Hu KQ, Su YF, Ye YH, Huang CQ, Yu ZL, Li XY, Zhou H, Ni YZ, Jiang YI, Lou Z. CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett. 2016;11:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008;247:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Wang C, Xiao Y, Qiu H, Yao J, Pan W. Factors affecting operating time in laparoscopic anterior resection of rectal cancer. World J Surg Oncol. 2014;12:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Ogiso S, Yamaguchi T, Hata H, Fukuda M, Ikai I, Yamato T, Sakai Y. Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: "narrow pelvis" is not a contraindication. Surg Endosc. 2011;25:1907-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Ferko A, Malý O, Örhalmi J, Dolejš J. CT/MRI pelvimetry as a useful tool when selecting patients with rectal cancer for transanal total mesorectal excision. Surg Endosc. 2016;30:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Chau J, Solomon J, Liberman AS, Charlebois P, Stein B, Lee L. Pelvic dimensions on preoperative imaging can identify poor-quality resections after laparoscopic low anterior resection for mid- and low rectal cancer. Surg Endosc. 2020;34:4609-4615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Hong JS, Brown KGM, Waller J, Young CJ, Solomon MJ. The role of MRI pelvimetry in predicting technical difficulty and outcomes of open and minimally invasive total mesorectal excision: a systematic review. Tech Coloproctol. 2020;24:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS, Cho HJ. Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech. 2011;21:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 39. | Iqbal A, Khan A, George TJ, Tan S, Qiu P, Yang K, Trevino J, Hughes S. Objective Preoperative Parameters Predict Difficult Pelvic Dissections and Clinical Outcomes. J Surg Res. 2018;232:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Sprenger T, Beißbarth T, Sauer R, Tschmelitsch J, Fietkau R, Liersch T, Hohenberger W, Staib L, Gaedcke J, Raab HR, Rödel C, Ghadimi M. Long-term prognostic impact of surgical complications in the German Rectal Cancer Trial CAO/ARO/AIO-94. Br J Surg. 2018;105:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Lee JM, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Prediction of transabdominal total mesorectal excision difficulty according to the angle of pelvic floor muscle. Surg Endosc. 2020;34:3043-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Ishihara S, Watanabe T, Fukushima Y, Akahane T, Horiuchi A, Shimada R, Nakamura K, Hayama T, Yamada H, Nozawa K, Matsuda K, Hashiguchi Y. Safety and factors contributing to the difficulty of laparoscopic surgery for rectal cancer treated with preoperative chemoradiotherapy. Tech Coloproctol. 2014;18:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Aydin S, Türkiye S-Editor: Lin C L-Editor: A P-Editor: Yuan YY