©The Author(s) 2021.
World J Gastroenterol. Aug 28, 2021; 27(32): 5362-5375
Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5362
Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5362
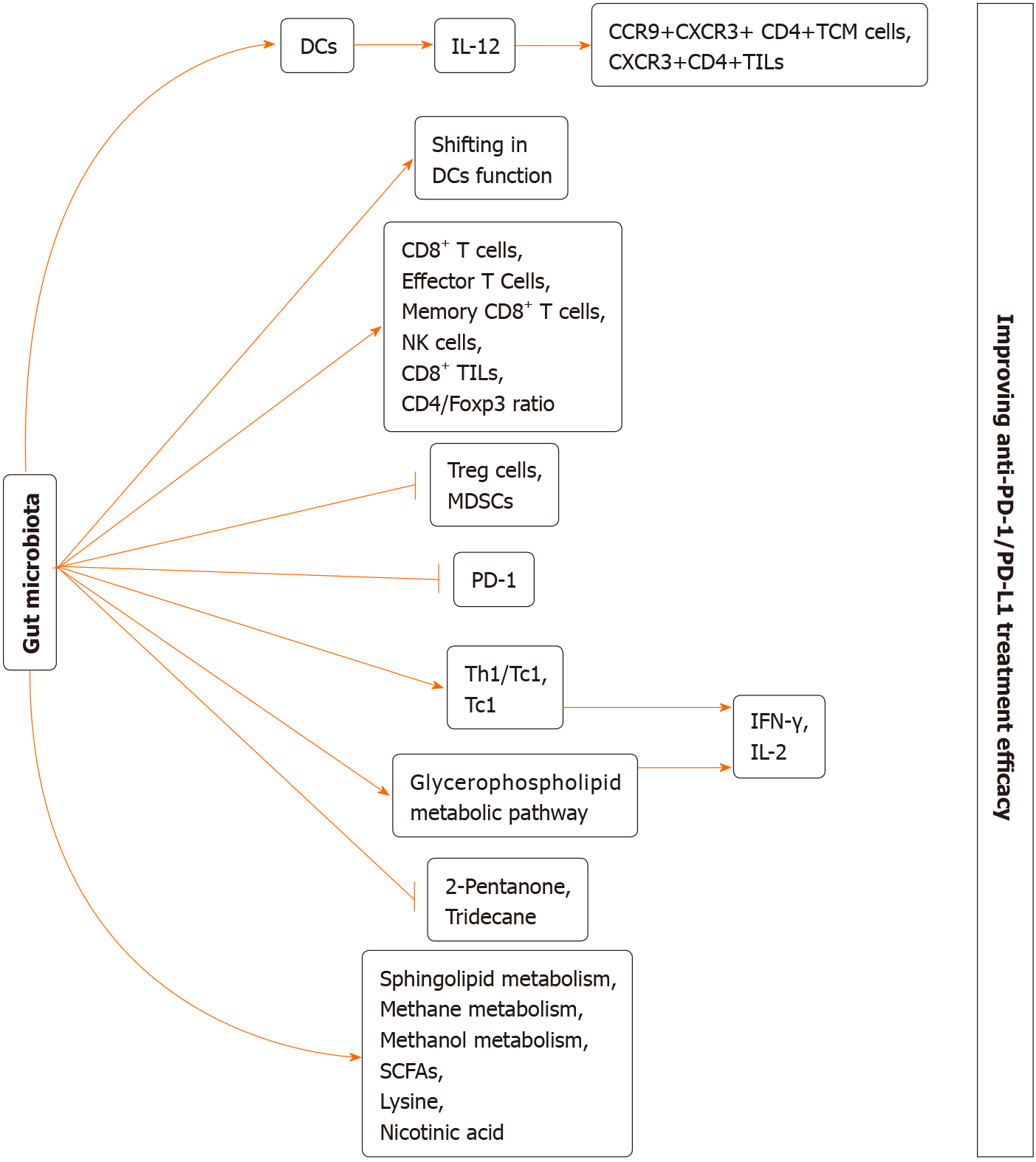
Figure 1 The potential mechanism of gut microbiome regulating anti-programmed cell death protein 1/programmed cell death protein ligand 1 treatment efficacy.
(1) Gut microbiota may increase interleukin (IL)-12 production by dendritic cells (DCs), thereby increasing the CCR9+CXCR3+CD4+ central memory T cells and CXCR3+CD4+ tumour-infiltrating lymphocytes (TILs); (2) Gut microbiota may shift in the function of DCs; (3) Gut microbiota may increase CD8+ T cells, effector T Cells, memory CD8+ T cells, natural killer cells, CD8+ TILs, and CD4/Foxp3 ratio; (4) Gut microbiota may decrease numbers of regulatory T cells and myeloid-derived suppressor cells; gut microbiota may downregulate programmed cell death protein 1 expression; (5) Gut microbiota may induce Th1/Tc1and Tc1 immune response, thereby increasing the interferon-γ and IL-2 secretion; (6) Gut microbiota may enhance glycerophospholipid metabolic pathway, thereby increasing the interferon-γ and IL-2 secretion; (7) Gut microbiota may decrease 2-pentanone and tridecane production; and (8) Gut microbiota may increase sphingolipid metabolism, methane metabolism, methanol metabolism, short chain fatty acids production, lysine production and nicotinic acid production. Altogether, all of these approaches may eventually improve anti-programmed cell death protein 1-1/programmed cell death protein ligand 1 treatment efficacy. DCs: Dendritic cells; TCM: Central memory T; Treg: Regulatory T; MDSCs: Myeloid-derived suppressor cells; SCFAs: Short chain fatty acids; PD-1: Programmed cell death protein 1; IFN-γ: Interferon.
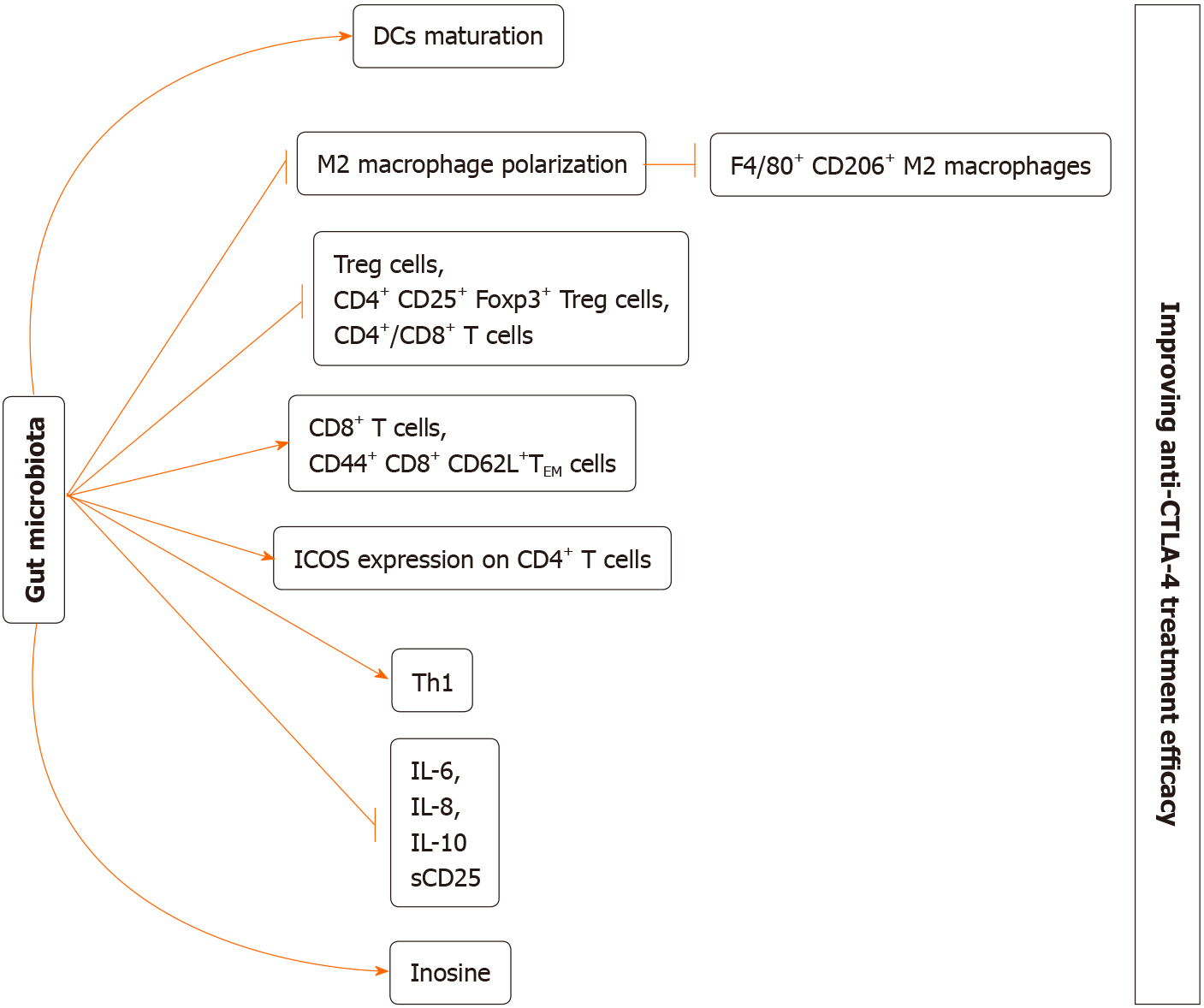
Figure 2 The potential mechanism of gut microbiome regulating anti-cytotoxic T-lymphocyte-associated protein 44 treatment efficacy.
(1) Gut microbiota may induce dendritic cells maturation; (2) Gut microbiota may inhibit the M2 polarization, thereby decreasing M2 macrophages (F4/80+CD206+); (3) Gut microbiota may decrease regulatory T cells, CD4+ CD25+ Foxp3+ regulatory T cells, and CD4+/CD8+ T cells; (4) Gut microbiota increase CD8+ T cells and CD44+CD8+CD62L+ effector memory T cells; (5) Gut microbiota may increase inducible T cell co-stimulator expression on CD4+ T cells; (6) Gut microbiota may induce T helper 1 immune response; (7) Gut microbiota may reduce interleukin (IL)-6; IL-8; IL-10, and sCD25 level; and (8) Gut microbiota may increase inosine production. Altogether, all of these approaches may eventually improve anti- cytotoxic T-lymphocyte-associated protein 4 treatment efficacy. DCs: Dendritic cells; Treg: Regulatory T; TEM: Effector memory T cells; ICOS: Inducible T cell co-stimulator; sCD25: Soluble CD25; IL: Interleukin.
- Citation: Kang YB, Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol 2021; 27(32): 5362-5375
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5362