©The Author(s) 2021.
World J Gastroenterol. Jun 28, 2021; 27(24): 3429-3439
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3429
Published online Jun 28, 2021. doi: 10.3748/wjg.v27.i24.3429
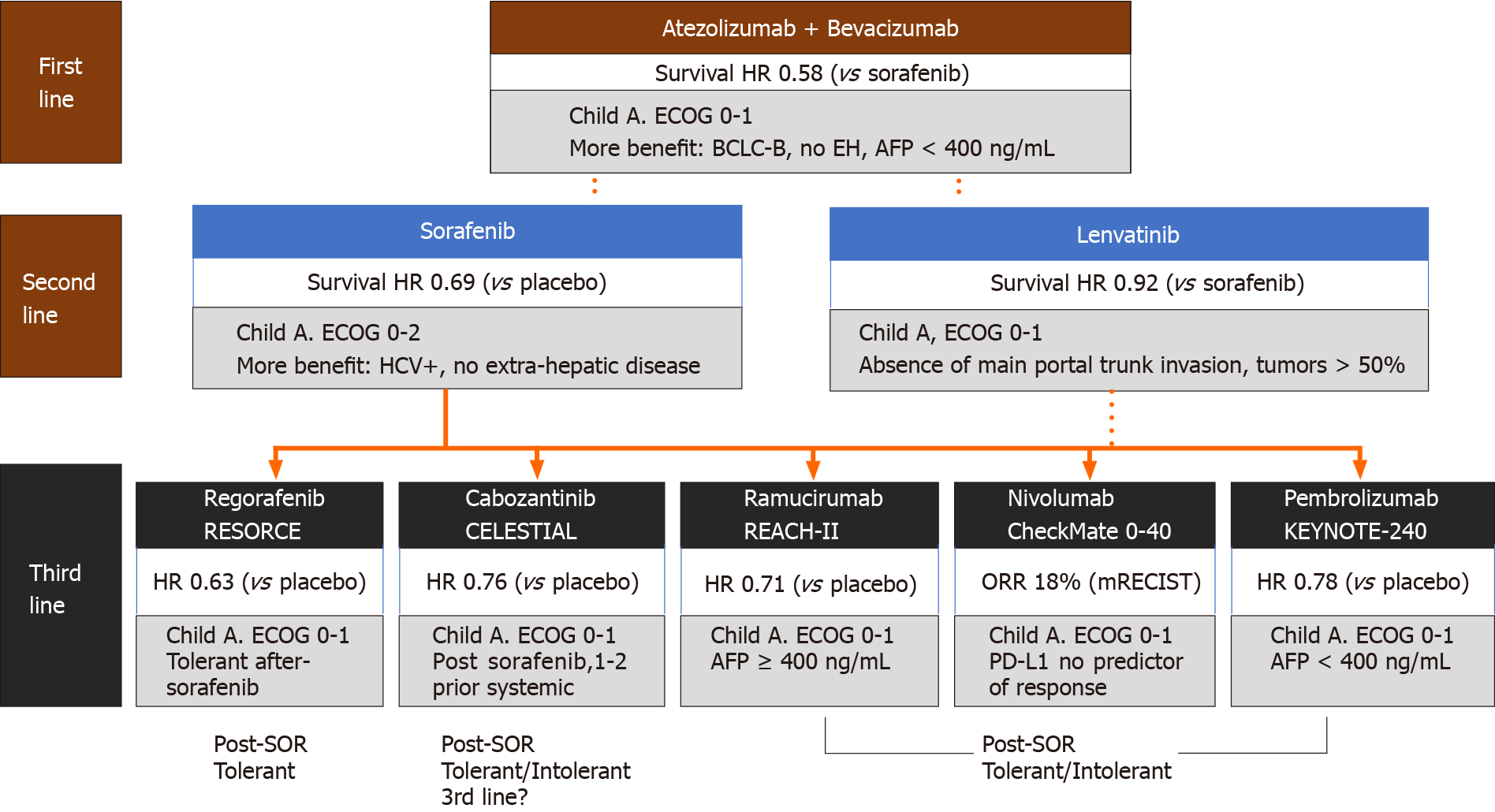
Figure 1 Systemic therapy for advanced hepatocellular carcinoma.
Note: First and second-line options may be presented as first-line options in parallel. Exclusion criteria in the REFLECT trial shown for lenvatinib[5]. AFP: Alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; EH: Extrahepatic; HCV: Hepatitis C virus; HR: Hazard ratio; ORR: Overall response rate; PD-L1: Programmed death ligand 1; SOR: Sorafenib.
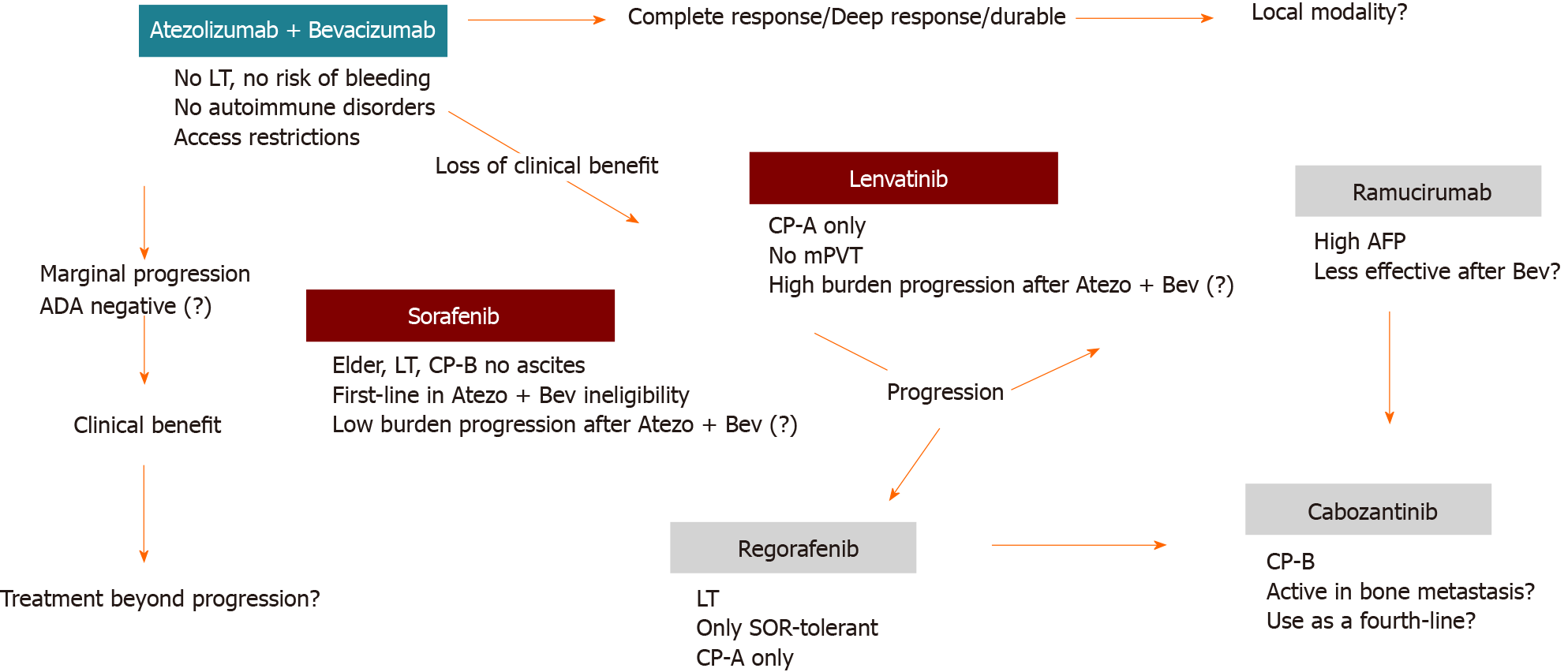
Figure 2 Sequencing systemic therapies in real-world setting.
Note: These recommendations should be individualized for each patient. ADA: Anti-drug antibodies; AFP: Alpha-fetoprotein; Atezo + Bev: Atezolizumab + bevacizumab; CP: Child-Pugh; LT: Liver transplantation; mPVT: Main portal vein thrombosis; SOR: Sorafenib.
- Citation: Piñero F, da Fonseca LG. Trial eligibility in advanced hepatocellular carcinoma: Does it support clinical practice in underrepresented subgroups? World J Gastroenterol 2021; 27(24): 3429-3439
- URL: https://www.wjgnet.com/1007-9327/full/v27/i24/3429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i24.3429