©The Author(s) 2020.
World J Gastroenterol. Apr 28, 2020; 26(16): 1888-1900
Published online Apr 28, 2020. doi: 10.3748/wjg.v26.i16.1888
Published online Apr 28, 2020. doi: 10.3748/wjg.v26.i16.1888
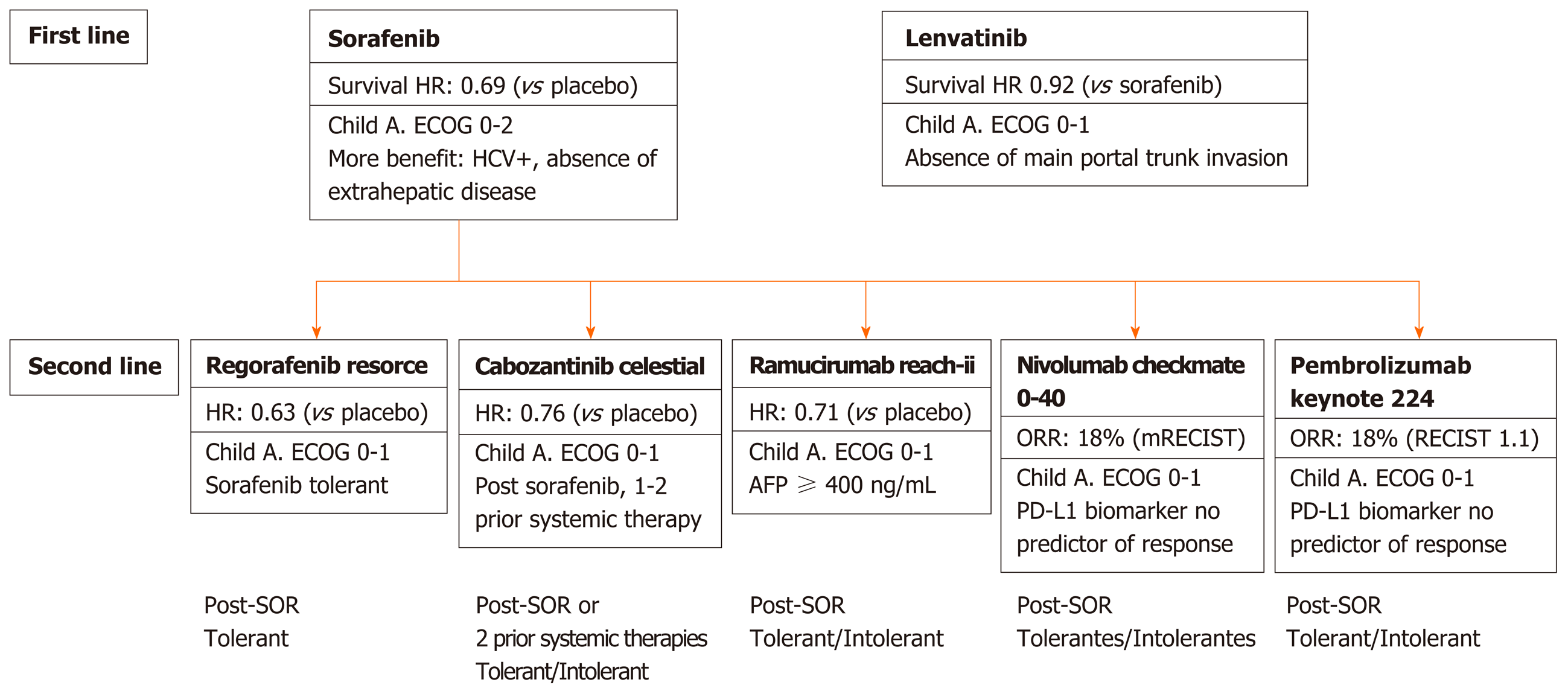
Figure 1 First and second line therapies for advanced hepatocellular carcinoma.
RECIST: Response Evaluation Criteria for Solid Tumors; ECOG: Eastern Cooperative Oncology Group; SOR: Sorafenib.
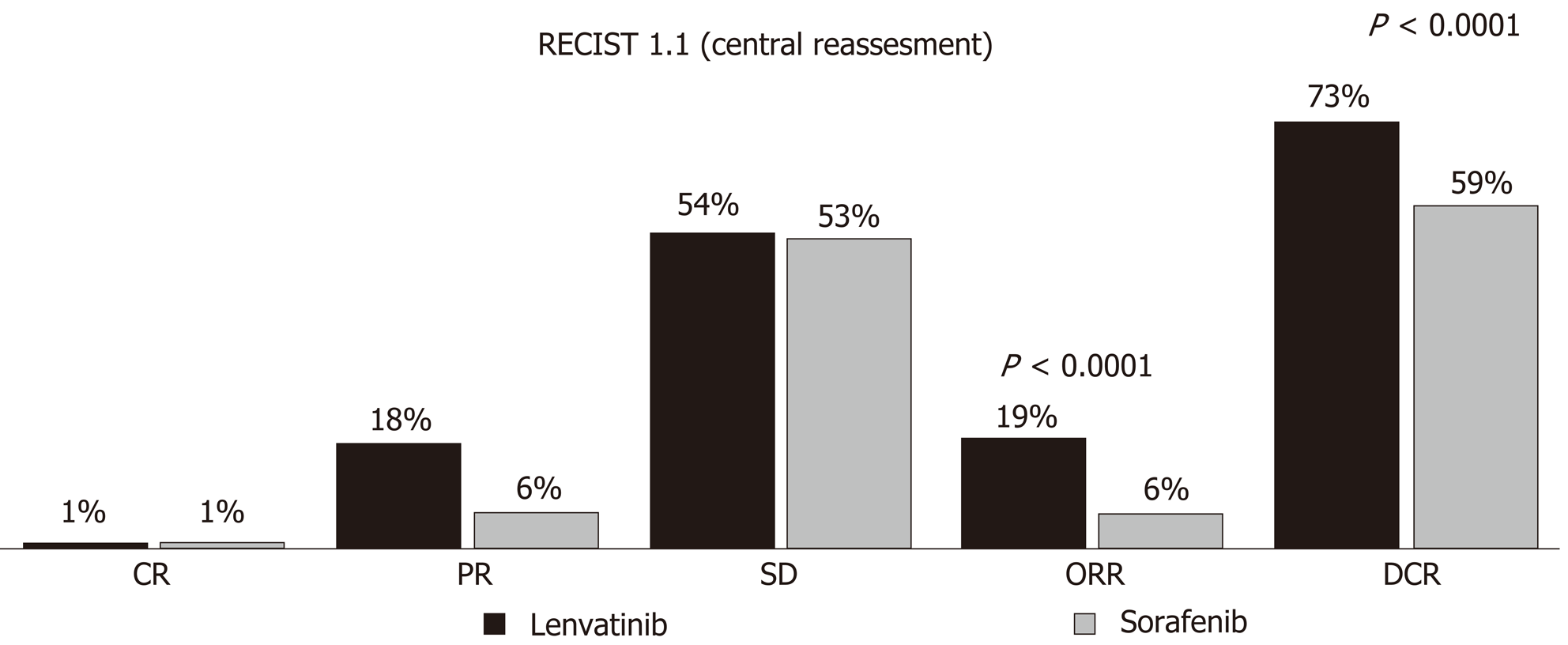
Figure 2 Radiological tumor response between sorafenib and lenvatinib according to RECIST 1.
1 criteria, reported in the REFLECT trial. RECIST: Response Evaluation Criteria for Solid Tumors; ORR: Objective response rate.
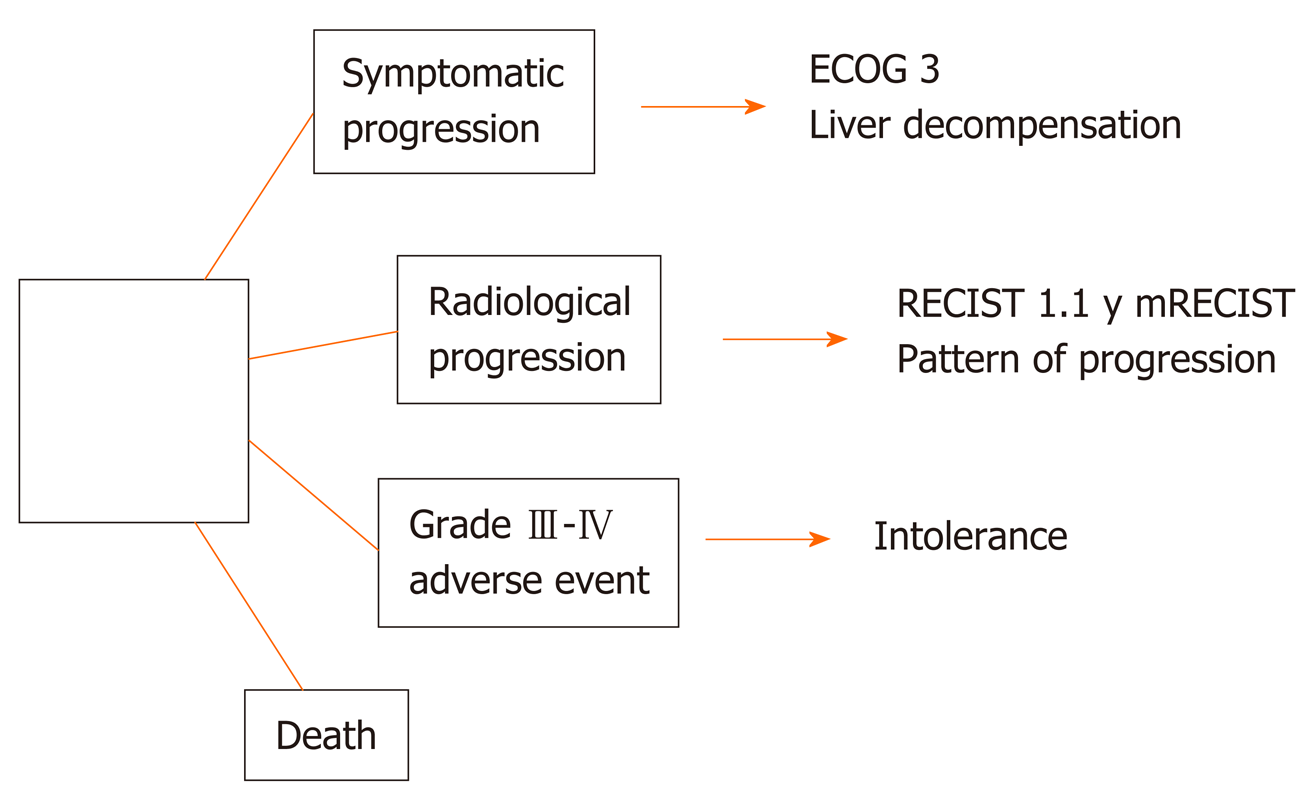
Figure 3 Clinical “stopping rules” of first and second line tyrosine kinase inhibitors.
RECIST: Response Evaluation Criteria for Solid Tumors; ECOG: Eastern Cooperative Oncology Group; RECIST: Response Evaluation Criteria for Solid Tumors criteria.
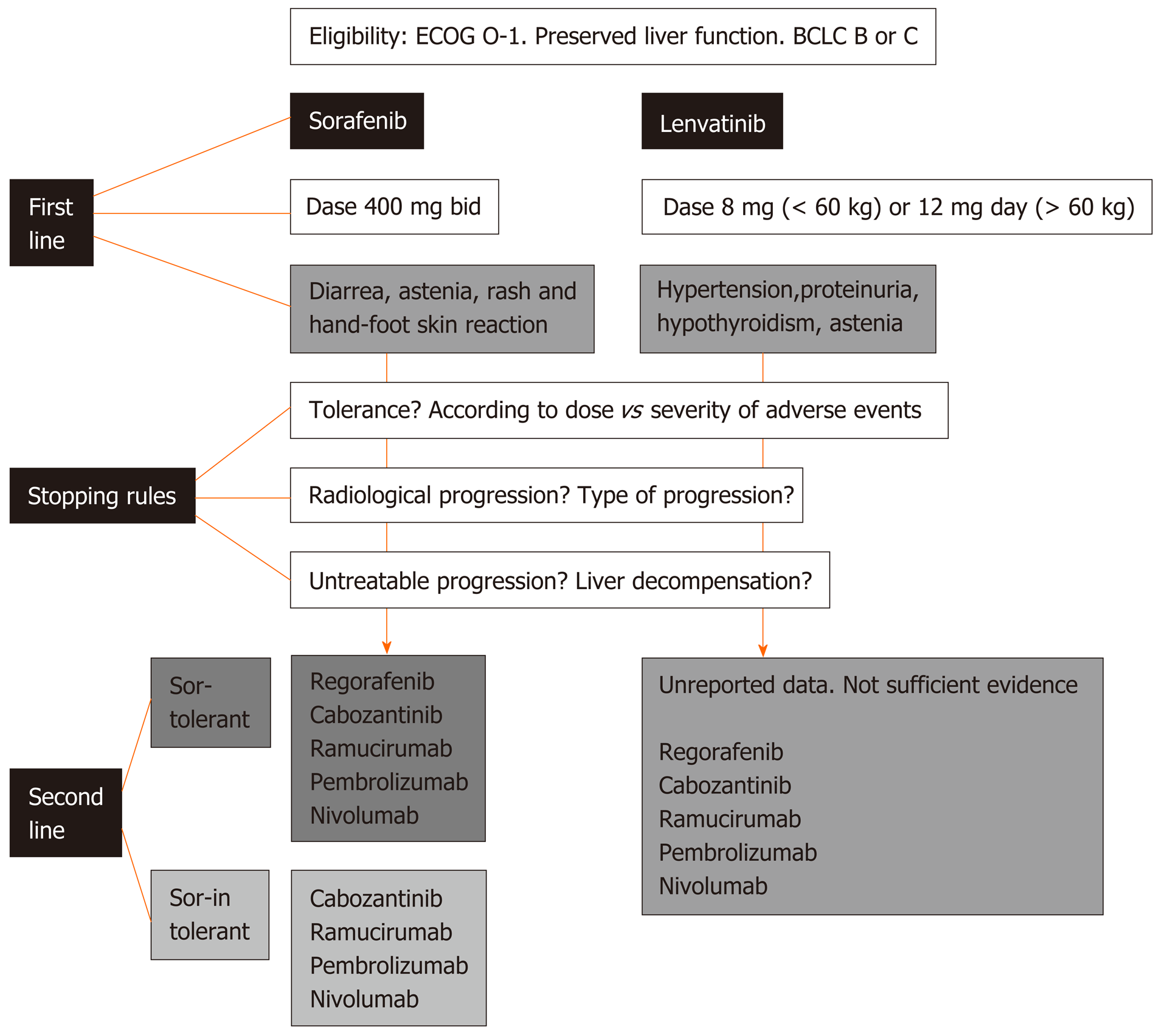
Figure 4 Flow chart for clinical-decision making processes of first and second line systemic treatment for advanced hepatocellular carcinoma.
BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group.
- Citation: Piñero F, Silva M, Iavarone M. Sequencing of systemic treatment for hepatocellular carcinoma: Second line competitors. World J Gastroenterol 2020; 26(16): 1888-1900
- URL: https://www.wjgnet.com/1007-9327/full/v26/i16/1888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i16.1888