INTRODUCTION
By virtue of its location in the circulatory system, the liver acts as an essential barrier to prevent the systemic dissemination of potentially deadly pathogens and toxic xenobiotics that enter the portal circulation from the gastrointestinal tract. To carry out this function, the liver is home to an extensive network of resident and patrolling immune cells that target and kill pathogens. Further, hepatocytes, the primary functional cell type of the liver, express a battery of xenobiotic metabolizing enzymes that detoxify potentially harmful chemicals and target them for excretion. While the liver is highly efficient at preventing systemic exposure to toxic substances, it is prone to injury from reactive metabolites generated from the biotransformation of xenobiotics. To counter this, the liver has evolved a remarkable capacity for repair even after extensive injury. In cases of chronic injury, however, multiple cycles of injury and repair can ultimately lead to scar formation, also called fibrosis or in severe cases, cirrhosis. A cell type that is intimately involved in all aspects of liver injury and repair is the macrophage. This specialized cell of the immune system modifies its phenotype in response to local cues generated in the hepatic microenvironment. Through this action, macrophages can take on a number of diverse functions after liver injury, including killing and phagocytosing bacteria, producing cytokines that regulate recruitment and function of other immune cells, and/or producing tissue reparative growth factors. In the following review, we will discuss the role of macrophages in triggering the response to liver injury and highlight their role in various aspects of liver repair, particularly after acetaminophen (APAP)-induced liver injury. In addition, we will discuss the critical role of the fibrinolytic system in regulation of macrophage function after acute liver injury and during repair.
HEPATIC MACROPHAGES
Kupffer cells (KC), which are resident to the liver, are cells of the myeloid lineage normally present on the luminal side of the hepatic sinusoid. These cells detect, phagocytose, and degrade foreign materials, pathogens, and cellular debris that enter the liver through the portal circulation (for a comprehensive review of KC function[1]). KC arise from progenitor stem cells generated in the fetal yolk-sac early during development[2]. These cells migrate to the liver where they become fully functioning KC. During homeostasis or after toxin-induced liver injury, these cells are replenished through the local proliferation of mature KC[3]. Although the stimulus for local proliferation of KC is not fully known, studies suggest that colony stimulating factors may contribute to this process[4]. Under conditions where a substantial loss of KC occurs, such as after exposure to lethal irradiation, these cells can be replenished from bone marrow progenitors[5,6]. For instance, our recent studies determined that after lethal irradiation and bone marrow transplantation, approximately 40% of KC are replaced by macrophages originating from bone marrow which is consistent with findings by others[5,7]. During this process, monocytes are recruited from the circulation and take up residence within the hepatic sinusoids[8]. Overtime, local cues generated in the hepatic microenvironment reprograms these cells to become KC that are nearly indistinguishable from their predecessors. Recent studies suggest that this process requires Notch and transforming growth factor-β signals generated by sinusoidal endothelial cells and requires agonists of the liver X receptor[8].
In addition to KC, a second, distinct population of hepatic macrophages characterized by selective expression of Cx3cr1 was recently identified that resides proximal to the Glisson’s capsule[9]. Studies suggest that these macrophages provide a barrier against invasion of pathogens from the peritoneal cavity into the liver[9]. Remarkably, these macrophages appear to extend protrusions into the peritoneal cavity where they can sense and respond to bacteria[9]. Unlike KC, this macrophage population is replenished from circulating monocytes generated from myeloid progenitors in the bone marrow[3,9]. A similar population of Cx3cr1+ macrophages is also located proximal to blood vessels in the liver[3]. These macrophages may function as a last line of defense against dissemination of bacteria into the systemic vasculature. The mechanisms controlling recruitment and specialization of these macrophages remains to be investigated.
MACROPHAGE FUNCTION AFTER ACUTE LIVER INJURY
Much of what we know regarding macrophage function in liver repair derives from studies investigating APAP-induced liver injury. APAP is a commonly used analgesic and antipyretic. Although APAP is considered safe at low, therapeutic doses (i.e., 4 g/d), APAP overdose, either accidental or intentional, results in approximately 56000 emergency room visits, 26000 hospitalizations and 458 deaths each year making it responsible for nearly 50% of all cases of acute liver failure (ALF) in the United States[10,11]. At low doses, APAP is rapidly metabolized by glucuronidation or sulfation in the liver and excreted into the urine by the kidneys. APAP can also be oxidized by cytochrome P450s to the hepatotoxic intermediate, N-acetyl-p-benzoquinone imine (NAPQI), however, at therapeutic doses, NAPQI is rapidly detoxified by glutathione. At toxic doses of APAP, glucuronidation and sulfation pathways become saturated, which shifts metabolism towards oxidation to NAPQI. High concentrations of NAPQI ultimately deplete cellular glutathione leading to the accumulation of NAPQI, which forms protein adducts stimulating oxidative stress, mitochondrial permeability transition, loss of ATP, and ultimately hepatocyte necrosis[12,13]. Within hours of hepatocyte injury, however, a robust reparative response is initiated[14]. During this process, hepatic macrophages become activated and release proinflammatory cytokines that stimulate the recruitment of various immune cell types[4]. Pro-mitogenic cytokines and growth factors are also released stimulating sinusoidal endothelial cell and hepatocyte proliferation. As cell proliferation proceeds, dead cell debris is removed from the liver, and anti-inflammatory cytokines are produced causing resolution of inflammation. Remarkably, within days of the initial insult, liver structure and function are fully restored.
CONTRIBUTION OF KC TO APAP-INDUCED LIVER INJURY AND REPAIR
Macrophages perform several key functions in the liver after APAP overdose, including production of immunomodulatory cytokines, phagocytosis of dead cell debris, and production of pro-mitogenic growth factors[15]. While it is well established that macrophages perform these critical functions, the importance of KC to these processes remains a matter of debate. Early studies indicated a pathogenic role for KC after APAP overdose. In these studies, treatment of mice with the macrophage inhibitor, gadolinium chloride, protected against APAP hepatotoxicity[16]. Subsequent studies indicated that inhibition of KC with gadolinium chloride prevented production of reactive oxygen species and peroxynitrite after APAP overdose, leading to reduced liver toxicity[17]. Accordingly, it was concluded that KC were critical for liver toxicity after APAP overdose[17]. More recent studies, however, which used clodronate-containing liposomes to fully deplete KC, demonstrated that KC depletion exacerbated hepatic necrosis at 8 and 24 h after an acutely toxic dose of APAP[18]. Further investigation revealed that KC depletion was associated with a reduction in the anti-inflammatory cytokine, IL-10[18]. Consistent with this finding, subsequent studies revealed that IL-10 knockout mice had increased liver toxicity and mortality after APAP overdose[19]. Based upon these findings, it was concluded that IL-10, released from KC, protected the liver from toxicity after APAP overdose. Although these studies demonstrate that KC are an important source of anti-inflammatory cytokines (e.g., IL-10), studies have also shown that KC are an important source of proinflammatory mediators after APAP overdose. In support of this, studies using a murine model of APAP-induced liver injury, demonstrated that KC release several proinflammatory cytokines, including IL-1β, tumor necrosis factor (TNF)-α, and Ccl2, by 6 h after APAP challenge[20,21]. Although KC appear important for early cytokine induction after APAP overdose, by 24 h after APAP treatment, the population of resident KC is substantially reduced by mechanisms that remain unclear[4,21]. A similar phenomenon, called the “macrophage disappearance reaction” occurs in other tissues after injury[22]. Although the importance of this to the pathogenesis of liver injury after APAP overdose is not known, KC numbers return to baseline levels by 72 h, through the local proliferation of mature KC[4]. One intriguing mechanism by which KC “disappear” after APAP overdose may be through pyroptosis. Pyroptosis is a form of necrotic cell death that occurs in macrophages exposed to pathogens. This form of cell death produces macrophage lysis resulting in the release of high concentrations of proinflammatory cytokines. The importance of this process to KC disappearance and cytokine induction after APAP overdose, however, remains to be investigated.
FUNCTION OF MONOCYTE-DERIVED MACROPHAGES IN LIVER REPAIR AFTER APAP-INDUCED LIVER INJURY
Studies have shown that a population of monocyte-derived macrophages, distinct from KC and other resident macrophages, rapidly infiltrate the liver after APAP overdose[23]. KC and monocyte-derived macrophages can be distinguished by flow cytometry based upon their level of expression of F4/80 and CD11b[4,23]. In APAP-treated mice, KC are identified as a CD11blow F4/80hi population whereas monocyte-derived macrophages are identified as a CD11bhi F4/80low population that transiently appears in the liver 12 h after APAP challenge[4,23]. These macrophages are likely distinct from resident monocyte-derived macrophages as they do not express Cx3cr1 at the onset of recruitment[4].
Several studies have demonstrated that monocyte-derived macrophages are recruited to the liver after injury by the chemokine, chemokine (C-C motif) ligand 2 (Ccl2), also called monocyte chemoattractant protein-1. This chemokine stimulates chemotaxis of monocytes by activating the C-C chemokine receptor type 2 (Ccr2)[24]. After APAP overdose, hepatic expression of Ccl2 is increased in hepatocytes and KC by 12 h after administration[21]. This is soon followed by the accumulation of Ccr2-positive monocyte-derived macrophages. A role for Ccl2 in the recruitment of monocyte-derived macrophages to the liver after APAP overdose was confirmed by showing that monocyte-derived macrophage numbers were substantially reduced in the livers of Ccr2 knockout mice[21,23]. Interestingly, although similar levels of injury were observed in wild-type and Ccr2 knockout mice following APAP challenge, there was a failure to clear necrotic cells from the livers, indicating an important role for monocyte-derived macrophages in the phagocytic removal of dead cells[23]. Recently, it was reported that infusion of alternatively-activated macrophages into APAP treated mice enhances phagocytic clearance of dead cell debris, an approach that may be very valuable therapeutically in APAP overdose patients.
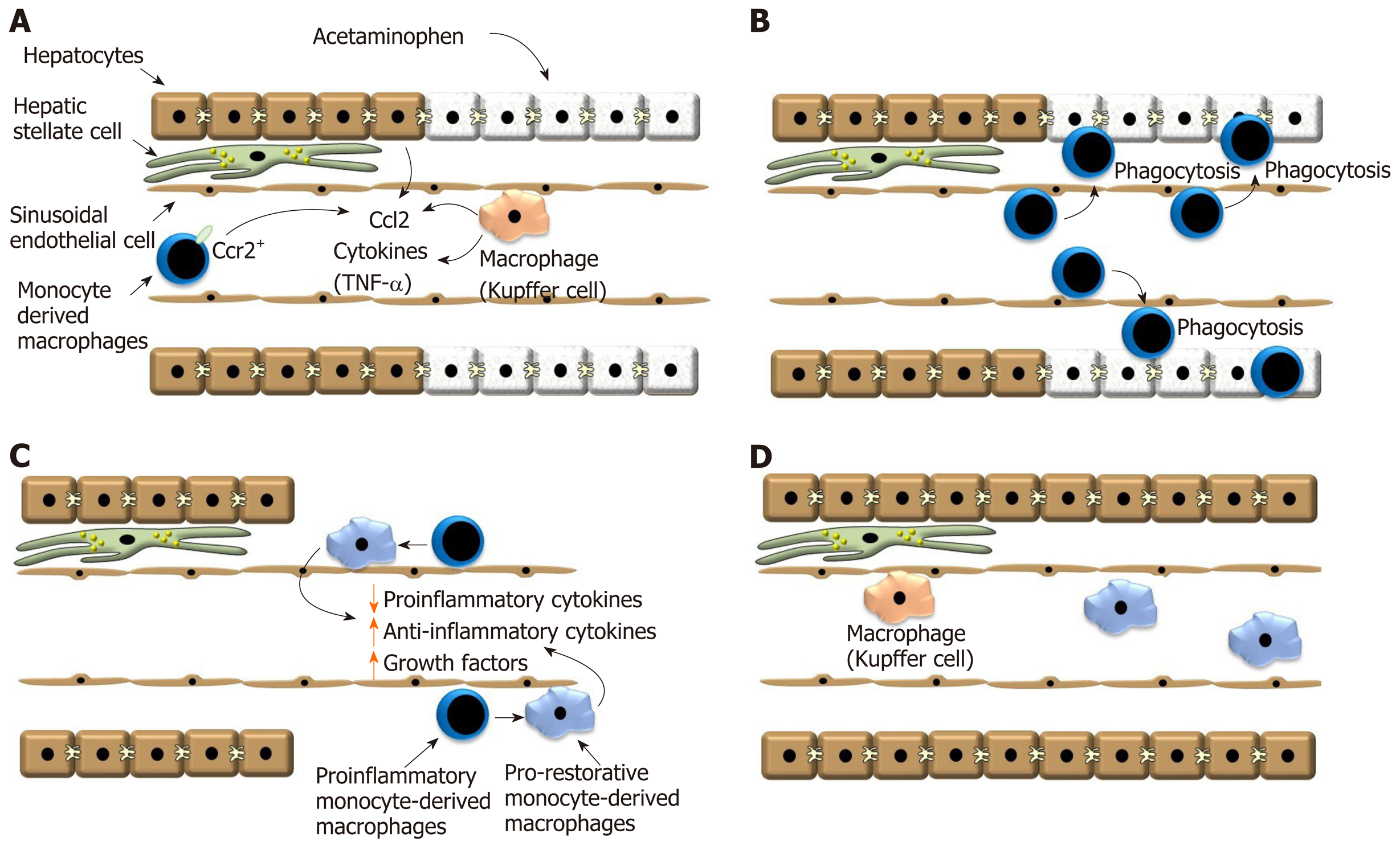
Further studies identifying macrophage subsets in the livers of mice following APAP challenge have demonstrated the dynamic presence of three distinct macrophage subsets[4]. In these studies, Ly6C and the chemokine (C-X3-C motif) receptor 1 (Cx3cr1) were used to characterize different macrophage subsets. KC, which are Ly6Clo Cx3cr1-, were significantly reduced at 24 h after APAP challenge (i.e., macrophage disappearance reaction), while there was a dramatic increase in Ly6Chi Cx3cr1+ macrophages that were recruited to the liver in a Ccr2- and M-CSF-dependent manner[4]. By 72 h, the dominant macrophage population in the liver was Ly6Clo Cx3cr1+, which was distinct from the KC population (Ly6Clo Cx3cr1-). Adoptive transfer experiments using green fluorescent protein- (GFP)-labeled monocytes determined that the infiltrating Ly6Chi Cx3cr1- macrophages ultimately gave rise to the Ly6CloCx3cr1+ macrophage subset[4]. Molecular profiling using microarray analysis revealed that the Ly6Chi Cx3cr1- macrophages expressed high levels of proinflammatory genes, indicating an M1-like phenotype, while the Ly6CloCx3cr1+ macrophages expressed high levels of pro-restorative and anti-inflammatory genes, indicating an M2-like phenotype[4]. The gene expression profile of Ly6CloCx3cr1+ macrophages was distinct from that of KC which demonstrated variable expression of pro-restorative genes. Collectively, these findings indicate that Ly6Chi CX3CR1- proinflammatory macrophages rapidly accumulate in the liver after APAP overdose (Figure 1). These cells traffic into the necrotic foci where they phagocytose dead cell debris and switch phenotype to Ly6CloCx3cr1+ pro-restorative macrophages. Consistent with this, it was recently reported that phagocytosis of neutrophils by macrophages triggers macrophage phenotype switching after APAP overdose. Once these cells switch phenotype, they produce pro-repair growth factors and anti-inflammatory cytokines that trigger the transition from the inflammatory phase of liver injury to the reparative phase. While the mechanism by which monocyte-derived macrophages are recruited to the liver is well established, the mechanisms controlling the intrahepatic trafficking, phagocytosis and phenotype switching by these cells remains poorly understood. Our recent studies, however, indicate that the enzyme plasmin, a component of fibrinolysis, may be important for these processes.
Figure 1 Ly6Chi CX3CR1- proinflammatory macrophages rapidly accumulate in the liver after acetaminophen overdose.
A: After exposure to a hepatotoxicant, such as acetaminophen, hepatocyte necrosis triggers release of Ccl2 by hepatocytes and Kupffer cells. Ccl2 recruits Ccr2 expressing monocytes to the liver that ultimately become macrophages; B: Monocyte-derived macrophages traffic into the necrotic lesions where they phagocytose dead cell debris; C: Monocyte-derived macrophages then transition from a proinflammatory phenotype into a pro-reparative phenotype. This process decreases synthesis of proinflammatory cytokines, increases synthesis of anti-inflammatory cytokines and pro-reparative growth factors; D: Proliferation of hepatic cells ultimately results in the restoration of the hepatic structure. Ccl2: Chemokine, chemokine ligand 2; Ccr2: C-C chemokine receptor type 2; TNF-α: Tumor necrosis factor.
REGULATION OF HEPATIC MACROPHAGE FUNCTION BY COMPONENTS OF FIBRINOLYSIS
Plasminogen, the zymogen form of the proteolytic enzyme plasmin, is a 90 kDa plasma glycoprotein that is produced in the liver and circulates in the blood[25]. This protein is converted to its active form, plasmin, through proteolytic cleavage by either tissue-type plasminogen activator or urokinase-type plasminogen activator[26]. Plasmin is a serine protease that is most well-known for its ability to degrade fibrin clots. Several other plasmin substrates have been identified, however, including coagulation proteins, components of the complement system, extracellular matrix proteins and several matrix metalloproteinases (for review, see[27]). Similar to other proteases, such as thrombin, plasmin can activate intracellular signaling pathways through activation of one of several putative plasmin receptors[28]. One of these receptors, annexin A2/S100A10, is a heterotetrameric complex composed of two molecules of annexin A2 and two molecules of S100A10. Studies have shown that plasmin stimulates production of proinflammatory cytokines by human monocyte-derived macrophages through this receptor by a mechanism that requires activation of mitogen-activated protein kinases and nuclear factor-κB (NF-κB)[29,30]. Another putative plasmin receptor is the G protein-coupled receptor, protease-activated receptor-1 (PAR-1). PAR-1 activation by a variety of proteases produces a tethered ligand that binds to the receptor and activates signaling[31,32]. Interestingly, treatment of mice with selective PAR-1 antagonists was shown to prevent plasmin-mediated migration of leukocytes into the pleural cavity, an effect that was dependent upon mitogen-activated protein kinases- and NF-κB-dependent release of Ccl2[33]. In addition to these receptors, several other putative plasmin receptors have been identified that stimulate signaling in macrophages, including enolase-1, histone H2B, and Plg-Rkt[28,34-36].
Several studies indicate that plasmin is a key regulator of monocyte and macrophage function in the liver after injury. For example, plasminogen deficiency was shown to impair recruitment of macrophages to the liver after a stab injury[37,38]. Others have demonstrated that phagocytic clearance of antibody labeled erythrocytes by KC was substantially reduced in plasminogen knockout mice indicating an important role for plasmin in regulation of phagocytosis[39]. Consistent with this finding, Bezerra and colleagues demonstrated that deficiency in plasminogen prevented clearance of dead hepatocytes after treatment with a hepatotoxic dose of carbon tetrachloride[40]. Because these studies indicate a key role for plasmin in regulation of hepatic macrophages, we recently evaluated the role of plasmin in regulation of macrophage function after APAP overdose.
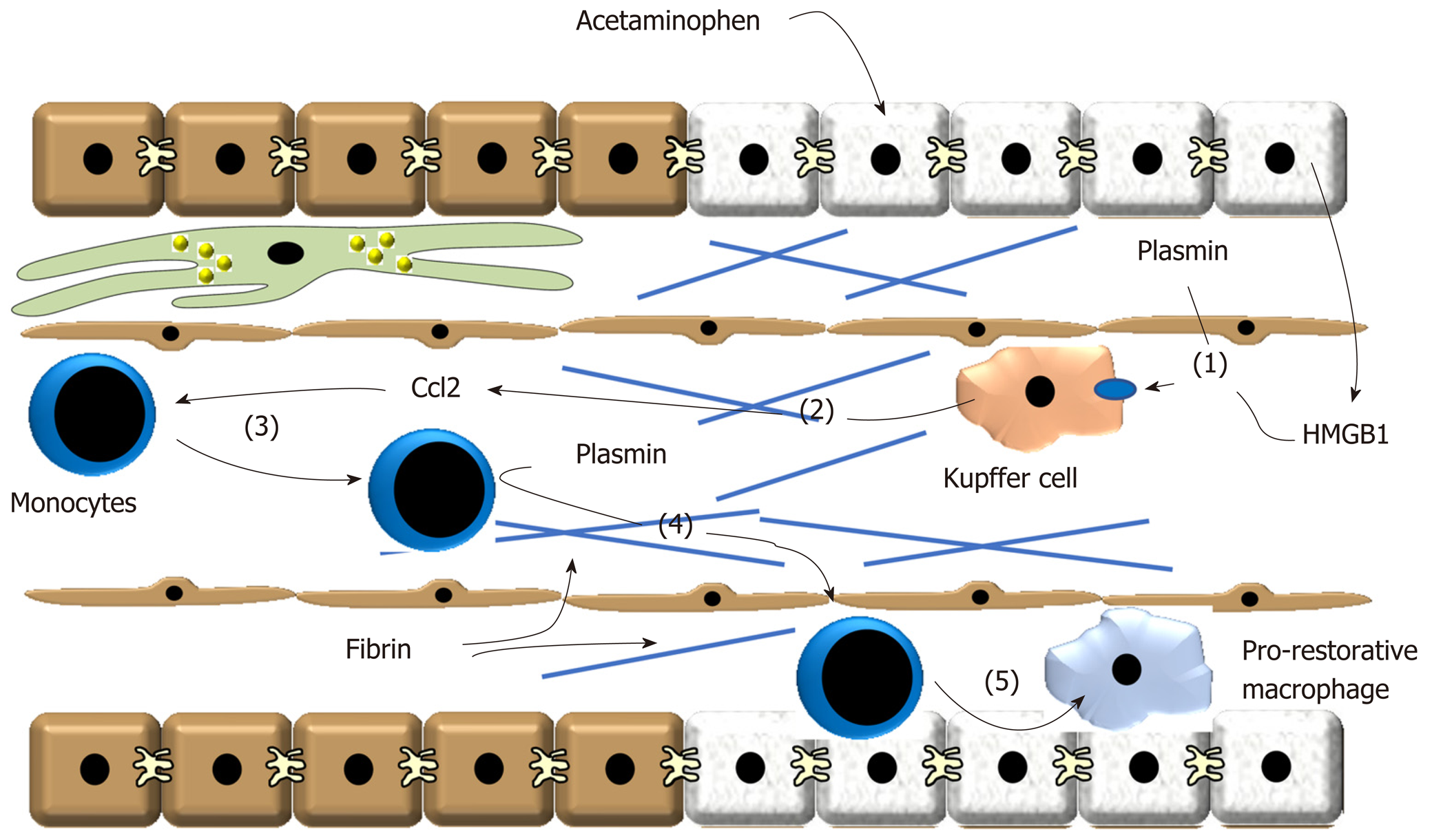
In mice treated with APAP, plasmin activity is increased in the liver by 6 h after treatment[41]. Interestingly, inhibition of plasmin activity with tranexamic acid prevents detachment of sinusoidal endothelial cells and subsequent sinusoidal hemorrhaging[41]. To examine the impact of plasmin generation on macrophage activation, we similarly treated mice with APAP followed by treatment with tranexamic acid. These studies revealed that inhibition of plasmin activity prevented early upregulation of several proinflammatory cytokines, including TNF-α, Ccl2 and the neutrophil chemokines Cxcl1 and Cxcl2[42]. Because KC contribute to early induction of proinflammatory cytokines, we evaluated whether plasmin directly stimulates these cells to produce cytokines[42]. Similar to in vivo, treatment of these cells with plasmin increased expression of TNF-α, Ccl2, Cxcl1 and Cxcl2 consistent with the hypothesis that plasmin directly activates KC (Figure 2)[42]. It has been proposed that the damage-associated molecular pattern molecule, high-mobility group B1 (HMGB1) protein is released from damaged hepatocytes and triggers KC cytokine release through a toll-like receptor 4- and receptor for advanced glycation end products-dependent mechanism[43]. In support of this, hepatocyte-specific deletion of HMGB1 was shown to reduce cytokine release after APAP overdose[43]. Surprisingly, though, we found that treatment of KC with recombinant HMGB1, at concentrations above those detected in the blood after APAP overdose, had no effect on proinflammatory cytokine synthesis in KC[42]. Remarkably, though, HMGB1 synergistically enhanced upregulation of cytokines in these cells[42]. This suggested that fibrinolysis, in the face of ongoing liver injury (i.e., HMGB1 release) produces a more robust inflammatory response. Our studies showed further that upregulation of proinflammatory cytokines by plasmin, and the synergistic enhancement by HMGB1, occurred by an NF-kB-dependent mechanism. Interestingly, though, unlike previous studies, upregulation of proinflammatory cytokines by plasmin did not require either annexin A2 or PAR-1 suggesting that another plasmin receptor may be important for this process in liver[42].
Figure 2 Treatment of these cells with plasmin increased expression of tumor necrosis factor-α, Ccl2, Cxcl1 and Cxcl2 consistent with the hypothesis that plasmin directly activates Kupffer cells.
(1) After acetaminophen overdose, plasmin is generated and high-mobility group B1 is released from dead hepatocytes. These synergize to stimulate release of (2) pro-inflammatory cytokines, including Ccl2, from Kupffer cells. (3) Ccl2 stimulates recruitment of proinflammatory monocytes that accumulate at the periphery of the necrotic lesion. (4) Plasmin generation stimulates degradation of fibrin and/or activate matrix metalloproteinases that facilitate trafficking of the monocytes into the injured region. (5) The monocytes phagocytose dead cells which contributes to their conversion of pro-restorative macrophages. Ccl2: Chemokine, chemokine ligand 2; HMGB1: High-mobility group B1.
As discussed, after APAP-induced liver injury, monocyte-derived macrophages are recruited to the liver. These cells accumulate in the necrotic foci where they phagocytose dead cell debris[44]. During this process, these cells switch phenotype from a proinflammatory macrophage to an anti-inflammatory, pro-restorative macrophage which decreases synthesis of proinflammatory cytokines and increases production of pro-repair growth factors[4]. Paradoxically, whereas inhibition of plasmin prevented early cytokine induction in KC, it also prevented termination of cytokine synthesis at later times after APAP overdose[42]. Further evaluation revealed that plasmin inhibition did not affect accumulation of monocyte-derived macrophages, however, it prevented trafficking of these cells into the necrotic lesions which prevented phagocytic removal of dead cells, similar to observations in carbon tetrachloride-treated mice (Figure 2)[40,42]. This resulted in a failure of proinflammatory monocyte-derived macrophages to transition to pro-restorative macrophages leading to a persistence of proinflammatory cytokine production[42]. While the mechanism by which plasmin stimulates monocyte-derived macrophage migration into necrotic foci remains unclear, it may have resulted from a failure to remove fibrin clots deposited in the lesions[45]. A recent study showed that plasmin is required for migration of macrophages into the peritoneal cavity, and that plasmin facilitates macrophage migration by removing fibrin that impedes their movement[46]. Further, plasmin can activate various matrix metalloproteinases, such as matrix metalloproteinase 9, that are critical for macrophage movement through extracellular matrix[27]. While these are possibilities, this remains to be determined in the liver. What also remains to be determined is the mechanism by which plasmin promotes macrophage phenotype switching during liver repair. While plasmin directly stimulates proinflammatory cytokine release from KC, we found that it did not directly stimulate proinflammatory monocyte-derived macrophages to transition to pro-restorative macrophages (B.L.C., unpublished observation). This suggests that plasmin facilitates this process by an indirect mechanism. One possibility is the failed removal of dead cell debris by these cells when plasmin is inhibited. Phagocytosis is a well-known stimulus for the conversion of proinflammatory macrophages into pro-restorative macrophages and as discussed earlier, phagocytosis of neutrophils appears to be important for macrophage phenotype switching after APAP overdose[47]. In further support of this, we found that ex vivo culture of monocyte-derived macrophages, isolated from the livers of APAP-treated mice, with necrotic hepatocytes terminates production of proinflammatory cytokines[42]. Therefore, plasmin may facilitate the migration of monocyte-derived macrophages into the necrotic lesions, thereby putting them in close proximity to dead cells debris, the key stimulus for phenotype switching.
CONCLUSION
In summary, macrophages play a key role in the response to liver injury. Depending upon the macrophage type, they can produce proinflammatory cytokines (i.e., KC), which recruit other immune cells, such as monocyte-derived macrophages that clear dead cell debris and terminate the proinflammatory response. A great deal remains to be determined regarding the mechanisms controlling these processes, however, and in particular, how plasmin contributes to their regulation. Elucidation of these mechanisms is important, because, studies have revealed that macrophage dysfunction is a key feature of ALF and that patients displaying features of macrophage dysfunction have the poorest outcome[48,49]. It is possible that disruption of macrophage function impairs liver repair in a subset of patients ultimately leading to liver failure and a poor outcome. Interestingly, studies have shown that blood plasminogen levels are greatly reduced in patients with severe acute liver injury. While this remains to be determined, it is possible that this reduces plasmin activity in the liver thereby causing macrophage dysfunction and a poor reparative response in ALF.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li J, Williams R S-Editor: Ma YJ L-Editor: A E-Editor: Zhang YL