Copyright
©The Author(s) 2019.
World J Gastroenterol. Aug 28, 2019; 25(32): 4715-4726
Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4715
Published online Aug 28, 2019. doi: 10.3748/wjg.v25.i32.4715
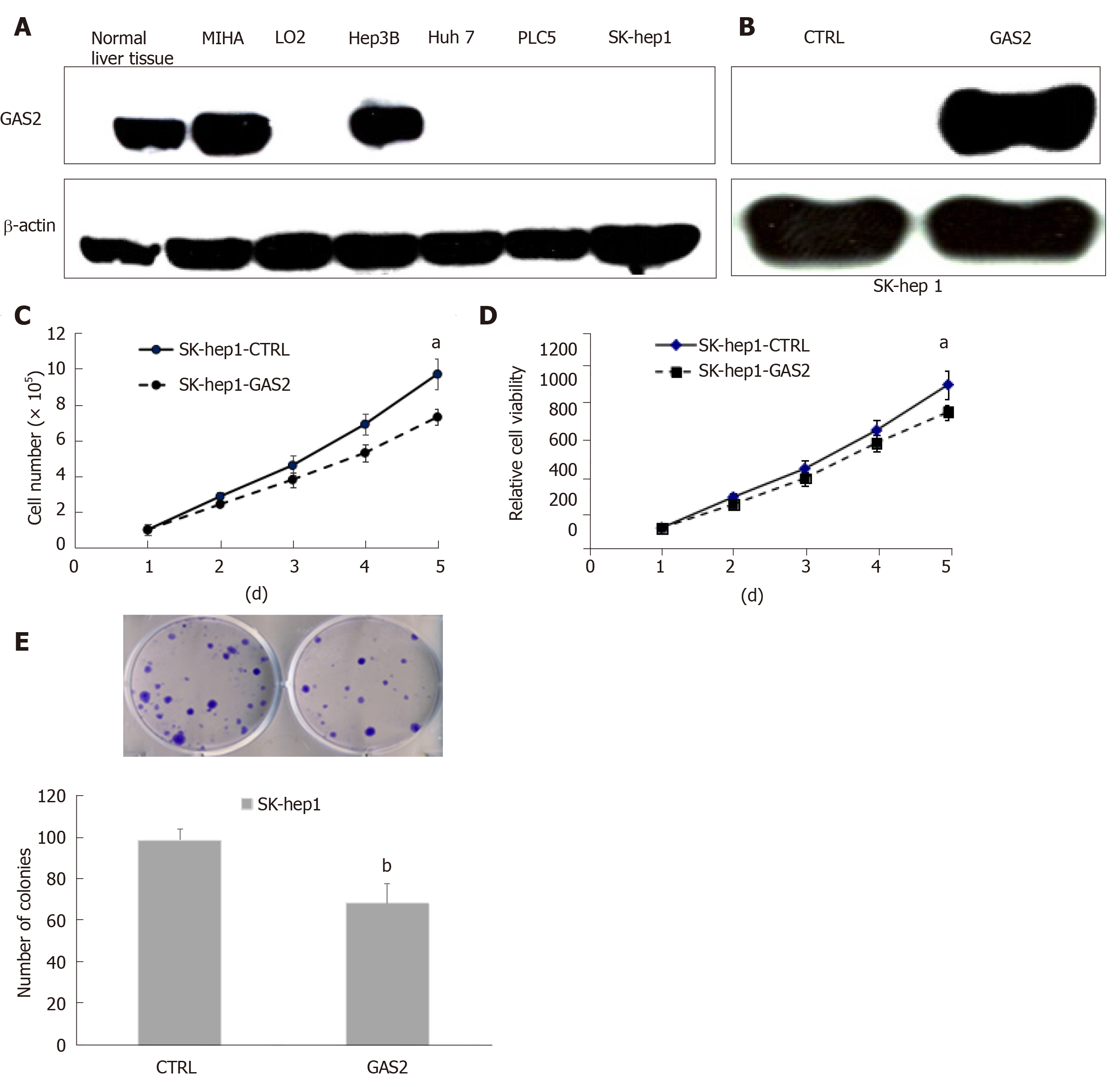
Figure 1 GAS2 exerts tumor-suppressive functions in HCC cells.
A: Western blot analysis of GAS2 expression in liver and HCC cell lines. β-actin was used as the loading control; B: GAS2 transfected in SK-hep1 cells was identified by western blotting. β-actin was used as the loading control; C: Cell counting (aP < 0.05 vs control); D: Cell viability (aP < 0.05 vs control); E: Anchorage-dependent colony formation (bP < 0.01 vs control). GAS2: Growth arrest-specific gene 2.
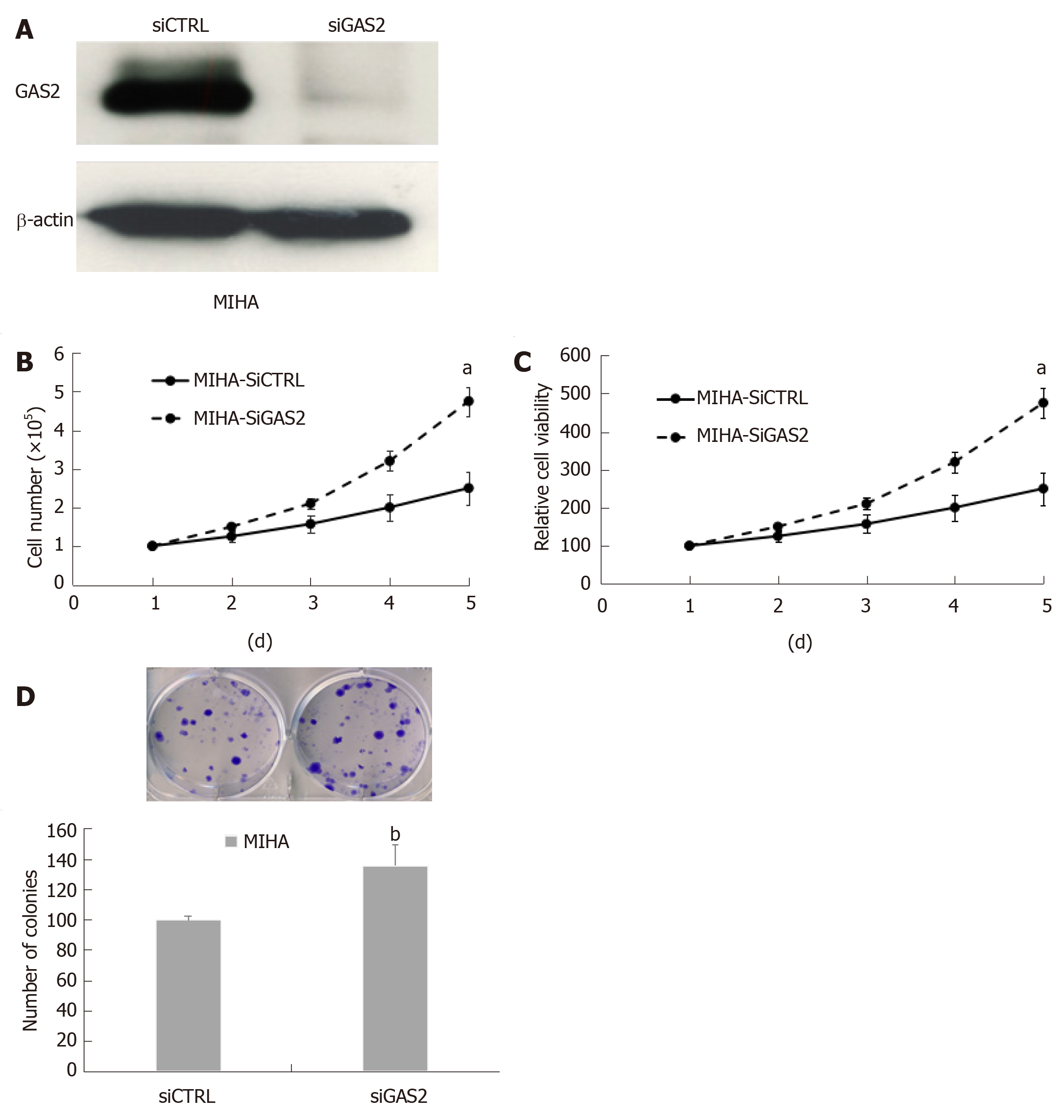
Figure 2 Effect of knockdown on endogenous GAS2 in MIHA cells.
A: Western blot analysis of siGAS2, β-actin was used as the loading control; B: Cell counting (aP < 0.05 vs control); C: Cell viability (aP < 0.05 vs control); D: Anchorage-dependent colony formation (bP < 0.01 vs control). GAS2: Growth arrest-specific gene 2.
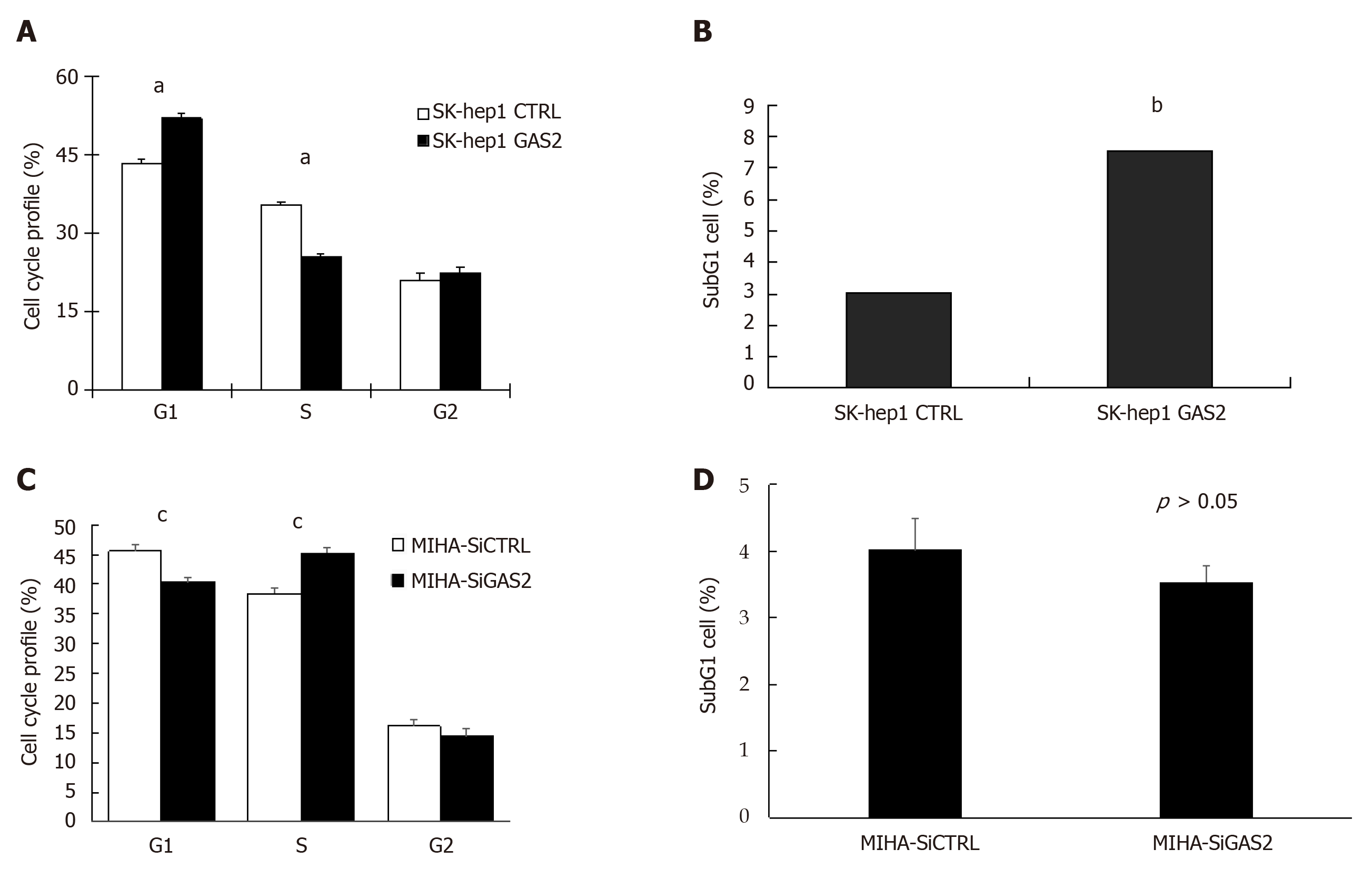
Figure 3 Effect of GAS2 on cell cycle progression.
SK-hep1 cells were transfected with pDEST40-GAS2 and pDEST40-CTRL plasmids followed by FACS analysis (FITC/PI). A: Cell populations in different fractions of the cell cycle phase were plotted (aP < 0.05 vs control); B: The cell population in subG1 phase was determined by flow cytometry (bP < 0.01 vs control). MIHA cells were transfected with siGAS2 and siCTRL followed by FACS analysis (FITC/PI); C: Cell populations in different fractions of the cell cycle phase were plotted (cP < 0.05 vs control); D: Cell population in the subG1 phase was determined by flow cytometry. FACS: Fluorescence-activated cell sorting.
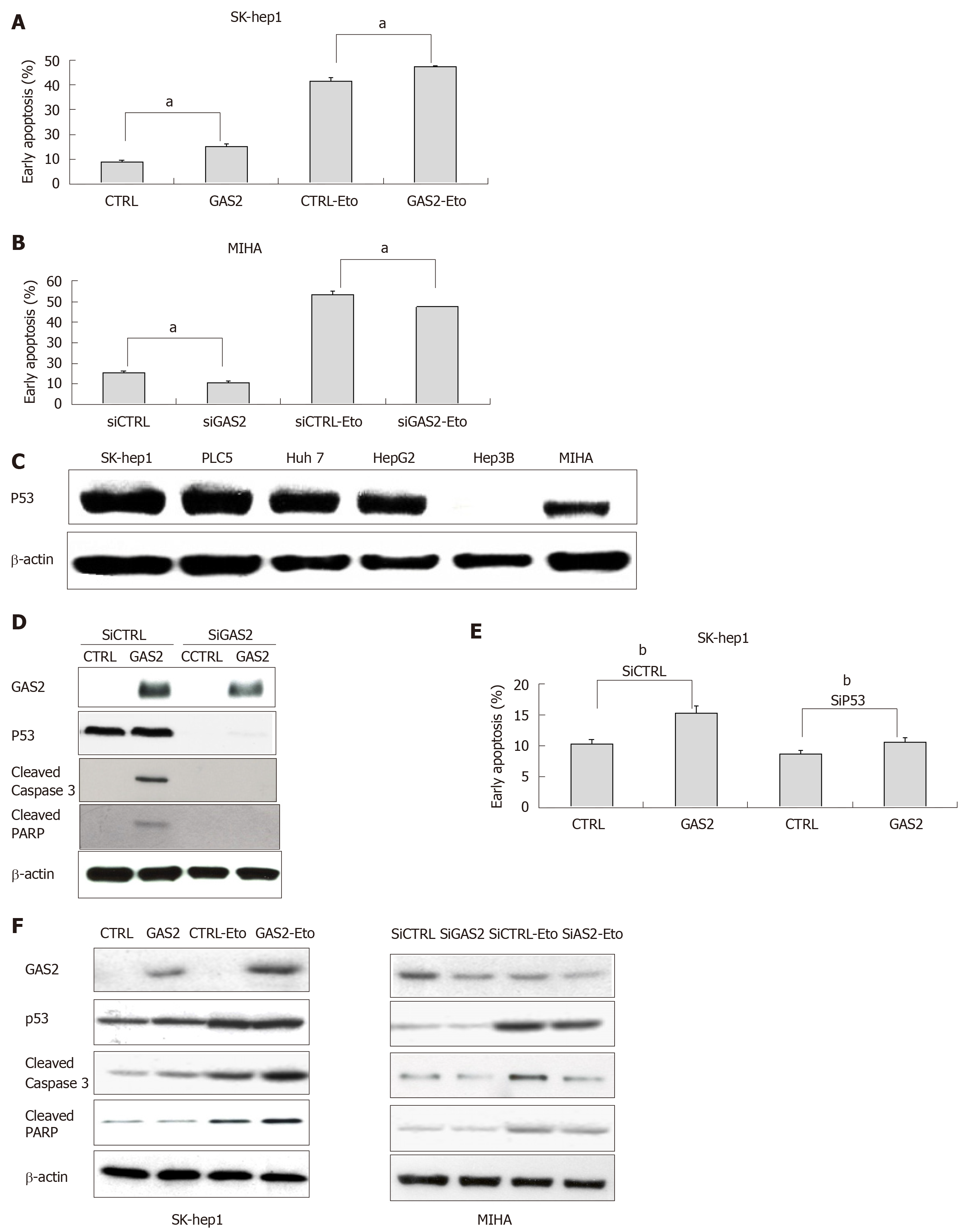
Figure 4 GAS2 inhibits HCC cell growth by increasing p53-mediated apoptosis.
A: Effect of GAS2 overexpression on apoptosis was determined by FACS using the Annexin V-APC apoptosis assay. A: The effect of 100 μM etoposide (Eto) in SK-Hep1 cells transfected with pDEST40-CTRL or pDEST40-GAS2 (aP < 0.05 vs control); (B) Effect of knocking down GAS2 in MIHA on apoptosis was determined by FACS using the Annexin V-APC apoptosis assay. The effect of 100 μM etoposide (Eto) in MIHA cells transfected with siCTRL or siGAS2 (aP < 0.05 vs control); C: Expression of p53 in hepatocytes and HCC cell lines was identified by western blotting, β-actin was used as loading control; D: siRNA-mediated knockdown of p53 (sip53) and overexpression of GAS2 in SK-Hep1 cells as well as the apoptosis markers such as cleaved caspase-3 and cleaved PARP were confirmed by western blotting; E: Effect of knocking down p53 (siP53) and overexpression of GAS2 in SK-Hep1 cells on apoptosis was determined by FACS using the Annexin V-APC apoptosis assay (bP < 0.01 vs control; mean values and SD from three replicate experiments); F: Cell apoptosis markers in the absence or presence of etoposide (Eto) in GAS2-overexpressing SK-Hep1 cells (left panel) or GAS2-ablated MIHA cells (right panel). FACS: Fluorescence-activated cell sorting; GAS2: growth arrest-specific gene 2.
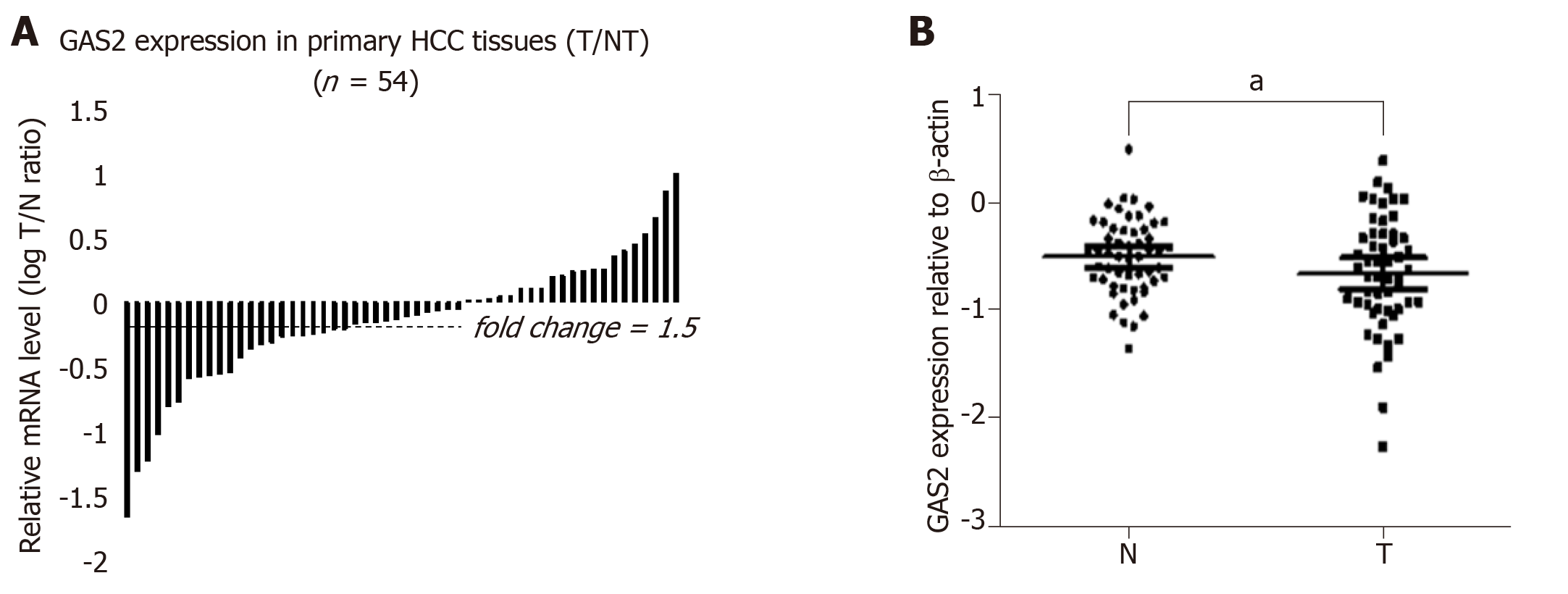
Figure 5 Expression of GAS2 in HCC specimens as determined by qPCR and western blotting.
A: Comparison of GAS2 mRNA expression in 54 paired tumor (T) and non-tumorous (N) tissues using PNN as an internal control. The bars (shown in log scale) illustrate the relative GAS2 mRNA level (T/N) in individual tissue pairs, of which negative and positive values respectively indicate downregulation and upregulation of GAS2 in HCC tumors. The differences of the T and N groups were statistically significant (P < 0.05 vs non-tumorous); B: Relative protein expression levels of GAS2 in 54 paired tumor (T) and non-tumorous (N) tissues using β-actin as an internal control. The average fold changes of GAS2 expression in tumor tissues were significantly lower than those in the paired non-tumor tissues (aP < 0.05 vs non-tumorous). GAS2: growth arrest-specific gene 2; HCC: Hepatocellular carcinoma.
- Citation: Zhu RX, Cheng ASL, Chan HLY, Yang DY, Seto WK. Growth arrest-specific gene 2 suppresses hepatocarcinogenesis by intervention of cell cycle and p53-dependent apoptosis. World J Gastroenterol 2019; 25(32): 4715-4726
- URL: https://www.wjgnet.com/1007-9327/full/v25/i32/4715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i32.4715