©The Author(s) 2019.
World J Gastroenterol. Aug 14, 2019; 25(30): 4222-4234
Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4222
Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4222
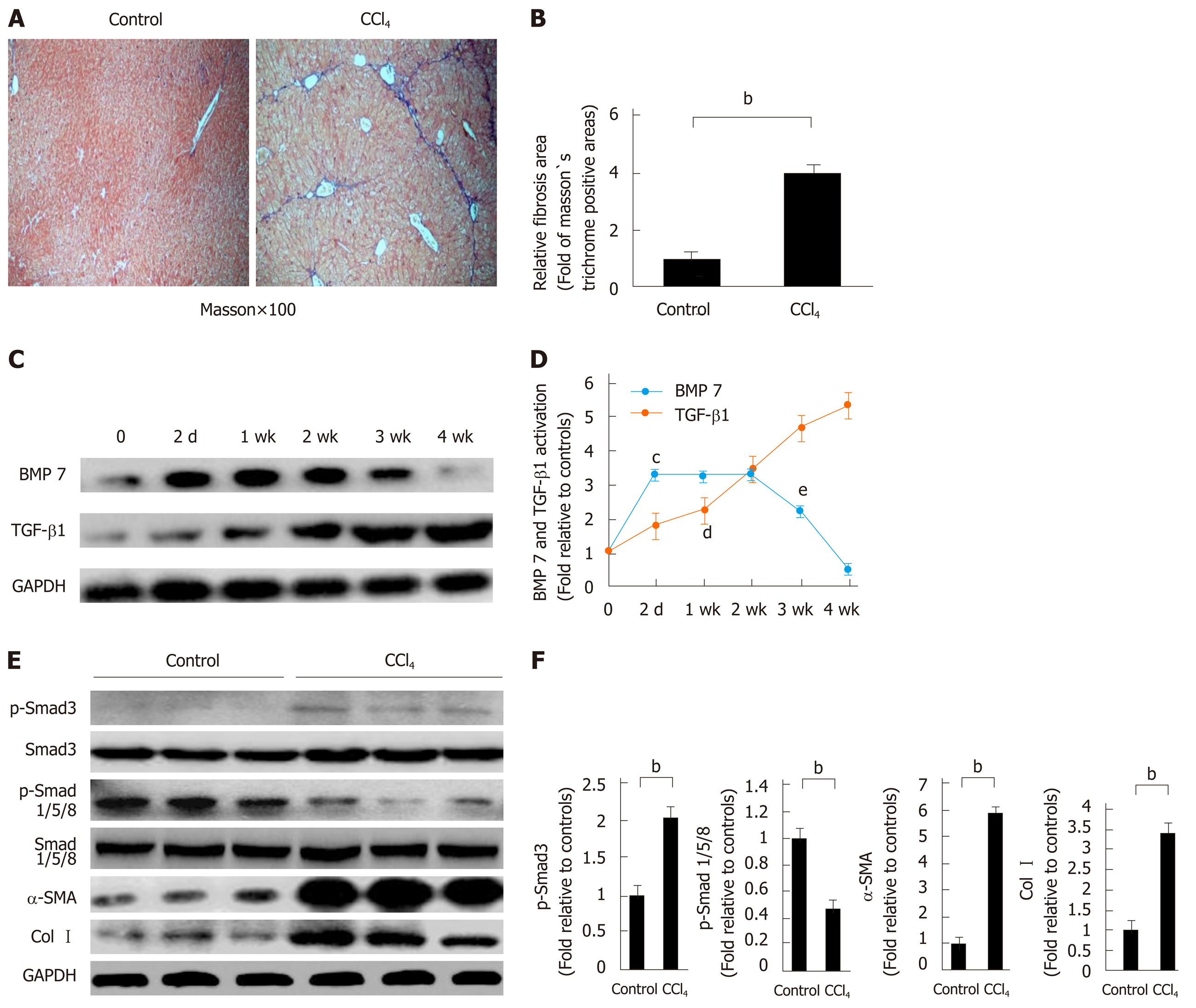
Figure 1 Decreased bone morphogenetic protein 7 and increased transforming growth factor-beta 1 in carbon tetrachloride-induced liver fibrotic mice.
Mice were challenged by intraperitoneal injections of carbon tetrachloride (CCl4) or control vehicle (Control, corn oil as the vehicle) as described in Materials and Methods. The severity of liver fibrosis was evaluated by using the measures, including A: Masson’s trichrome staining of fibrotic areas (by collagen deposition, 100×); B: Morphometric analysis of fibrotic lesions in the liver; C: Western blot analysis of expression of BMP7 and TGF-β1. At the indicated time points after CCl4 injection (2 d, 1 wk, 2 wk, 3 wk, and 4 wk), equivalent amount of whole liver detergent lysates were blotted for detecting BMP7 and transforming growth factor-beta 1 (TGF-β1). The level of BMP7 protein expression first rose and then fell, which significantly decreased at 4 wk, while TGF-β1 expression showed a gradual upward trend; D: Trend chart of BMP7 and TGF-β1 protein expression in liver-injured mouse model induced by CCl4. cP < 0.01 or dP < 0.01 compared to time point 0, eP < 0.05 compared to time point 2 wk; E: Western blot analysis of expression of p-Smad3, p-Smad1/5/8, α-SMA, and Col I. At the time point 4 wk, equivalent amount of whole liver detergent lysates were blotted for detecting p-Smad3, p-Smad1/5/8, α-SMA (a marker for myofibroblast differentiation), and Col I. Each lane represents one individual mouse. GAPDH was used as a loading control; and F: Densitometry analysis of the expression of p-Smad3, p-Smad1/5/8, α-SMA, and Col I. Data are pooled and represented as the mean ± SE, n = 8 animals per group. bP < 0.01. BMP7: Bone morphogenetic protein 7; p-Smad3: Phosphorylated Smad3; CCl4: Carbon tetrachloride; TGF-β1: Transforming growth factor-beta 1; Col I: Collagen I; α-SMA: Alpha-smooth muscle actin.
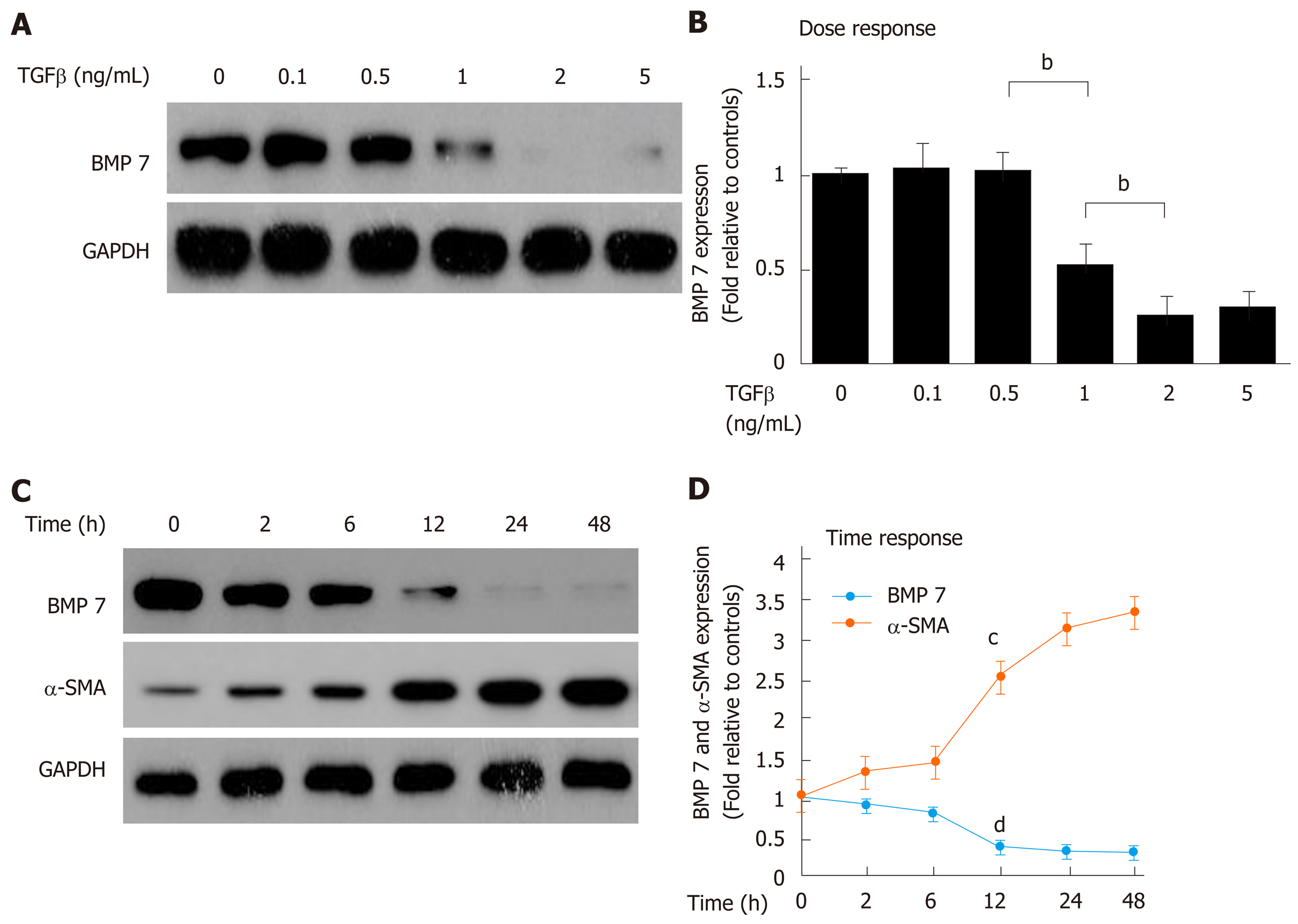
Figure 2 Transforming growth factor-beta 1 inhibits bone morphogenetic protein 7 in a time- and dose-dependent manner.
A: Western blot analysis of expression of BMP7. Primary mouse HSCs were serum starved for 20 h, treated with TGF-β1 at the indicated dose (ng/mL) for 24 h, and lysed. Equivalent amount of whole cell detergent lysates were blotted with the indicated antibodies for detecting BMP7 and GAPDH. B: Densitometry analysis of BMP7 at the indicated TGF-β1 dose. C: Western blot analysis of expression of BMP7 and α-SMA. Primary mouse HSCs were serum starved for 20 h, treated with TGF-β1 (2 ng/mL) for the indicated time periods (h), and lysed. Equivalent amount of whole cell detergent lysates were blotted with the indicated antibodies. D: Densitometry analysis of the association of BMP7 and α-SMA at the indicated time in response to TGF-β1 stimulation. Data are pooled from at least three independent experiments and represented as the mean ± SE. cP < 0.01 or dP < 0.01 compared to time point 0. BMP7: Bone morphogenetic protein 7; TGF-β: Transforming growth factor-beta; α-SMA: Alpha-smooth muscle actin.
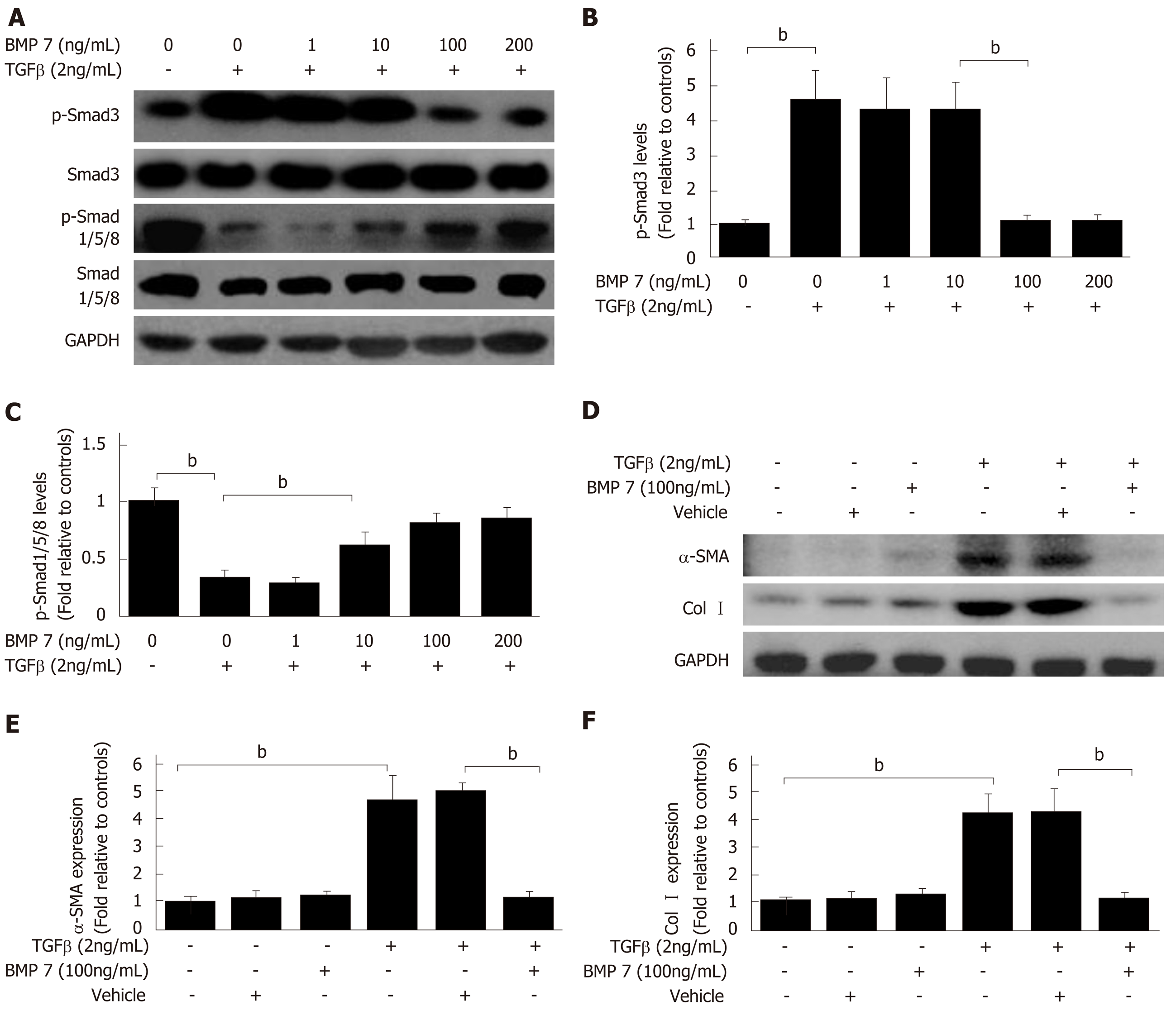
Figure 3 Bone morphogenetic protein 7 inhibits hepatic stellate cell activation, myofibroblast differentiation, and collagen formation induced by transforming growth factor-beta 1.
A: Western blot analysis of expression of p-Smad3 and p-Smad1/5/8. Serum starved (for 20 h) primary mouse HSCs were treated with transforming growth factor-beta 1 (TGF-β1) (2 ng/mL) followed by exogenous BMP7 at the indicated dose or Vehicle for 24 h, and lysed. Equivalent amount of whole cell detergent lysates were blotted with the indicated antibodies. B and C: Densitometry analysis of the effect of exogenous BMP7 on phosphorylation of Smad3 (p-Smad3) and phosphorylation of Smad1/5/8 (p-Smad1/5/8) at the indicated dose. D: Serum starved (for 20 h) primary mouse HSCs were treated with TGF-β1 (2 ng/mL) followed by exogenous BMP7 (100 ng/mL) or Vehicle for 24 h, and lysed. Equivalent amount of whole cell detergent lysates were blotted with the indicated antibodies. E: Densitometry analysis of the effect of exogenous BMP7 on HSCs myofibroblast differentiation and collagen expression evaluated by alpha-smooth muscle actin (α-SMA) and collagen I (Col I) expression (normalized to GAPDH) in panel D. Data are pooled from at least three independent experiments and represented as the mean ± SE. bP < 0.01. BMP7: Bone morphogenetic protein 7; p-Smad3: Phosphorylated Smad3; p-Smad1/5/8: Phosphorylated Smad1/5/8; TGF-β1: Transforming growth factor-beta 1; Col I: Collagen I.
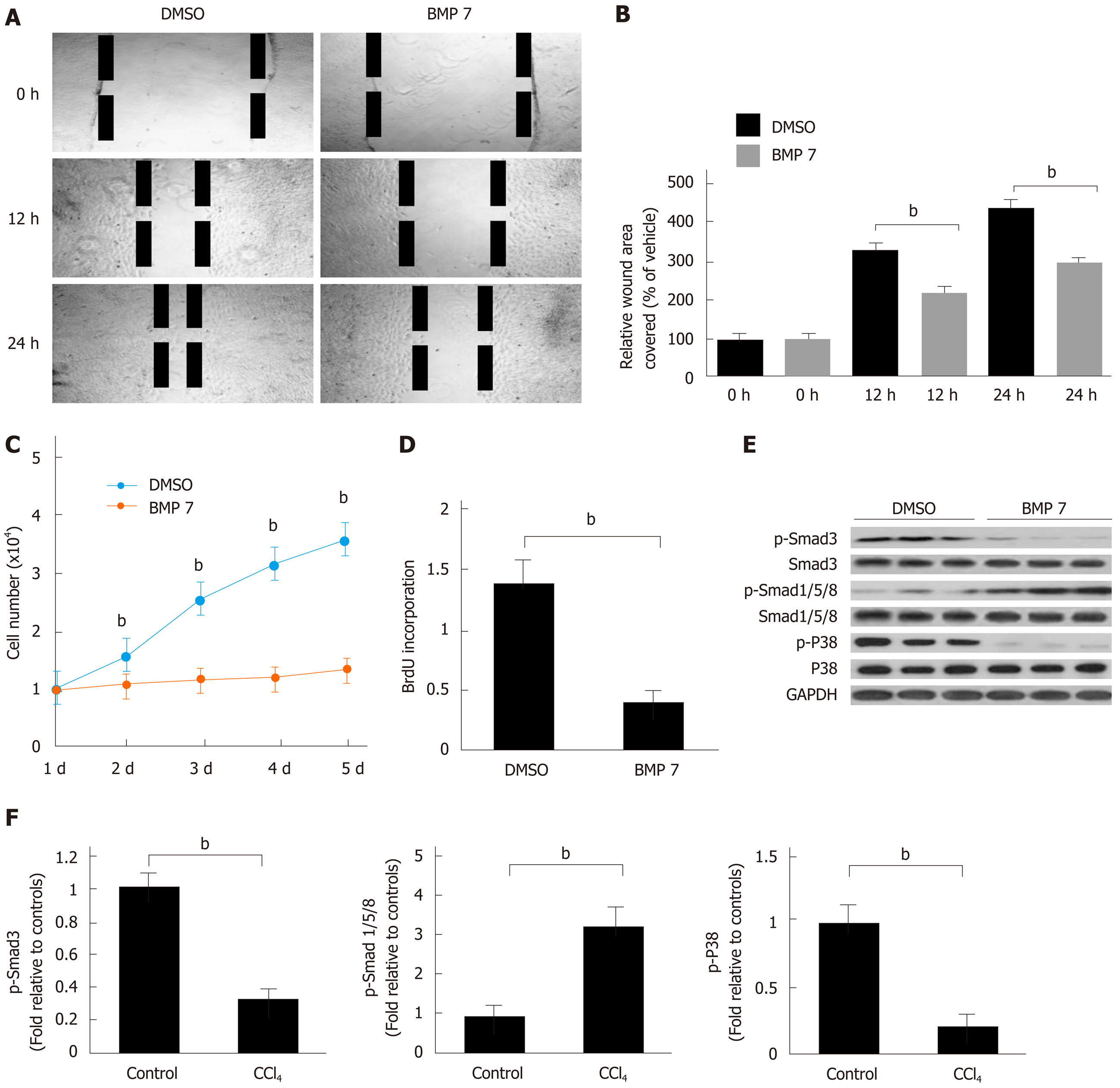
Figure 4 Bone morphogenetic protein 7 inhibits hepatic stellate cell migration and proliferation via promoting Smad1/5/8 and attenuating TGF-β1-Smad signaling.
A: Exogenous BMP7 (100 ng/mL) reduced cell migration in a wound scratch heal assay. The scratch wounds were almost healed in the control but gaps remained in the BMP7 treated cells at 24 h. B: Data are pooled and shown as % of wound area covered by cells over the indicated hours, relative to that of uninfected wild type (WT) fibroblasts in dimethyl sulfoxide (DMSO) medium. C: Cell growth in the presence of BMP7 as measured by direct cell count. D: Cell proliferation measured by BrdU cell proliferation assay in the presence of BMP7. E: Western blot analysis of expression of p-Smad3, p-Smad1/5/8, and p-P38. HSCs were treated as in panel A and lysed at 24 h. Equivalent amount of whole cell detergent lysates were used for detection of phosphorylation of Smad3 (p-Smad3), phosphorylation of Smad1/5/8 (p-Smad1/5/8), and phosphorylation of P38 (p-P38). F: Densitometry analysis of the effect of BMP7 on p-Smad3, p-Smad1/5/8, and p-P38 activation at the indicated condition. Data are pooled from at least three independent experiments and represented as the mean ± SE. bP < 0.01. BMP7: Bone morphogenetic protein 7; p-Smad3: Phosphorylated Smad3; p-Smad1/5/8: Phosphorylated Smad1/5/8; TGF-β1: Transforming growth factor-beta 1; Col I: Collagen I; p-P38: Phosphorylated P38.
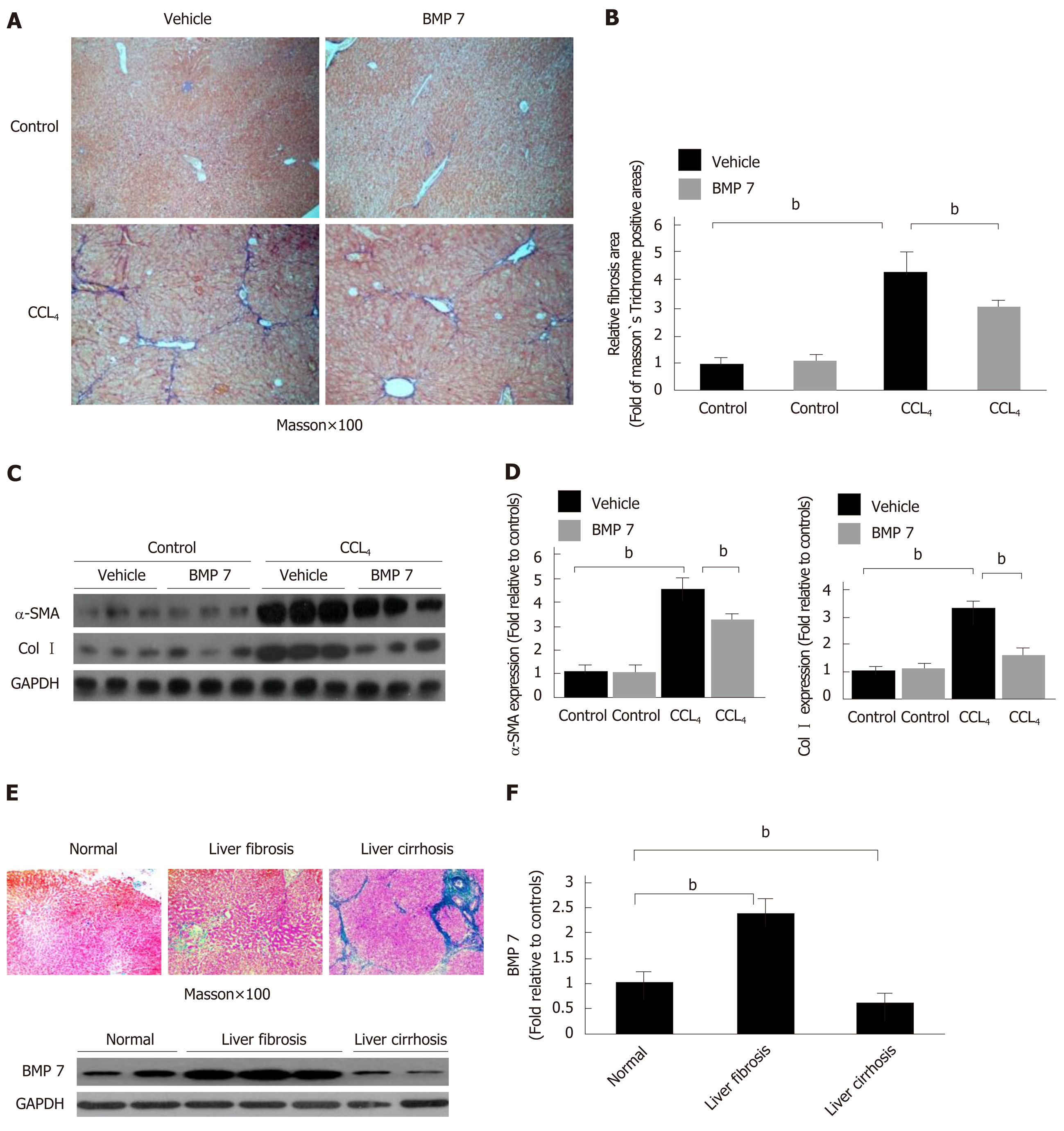
Figure 5 Exogenous bone morphogenetic protein 7 ameliorates liver fibrosis in carbon tetrachloride-induce mice model and bone morphogenetic protein 7 activation is abnormally changed in patient liver tissues.
Mice were challenged with carbon tetrachloride (CCl4) or corn oil (Control), followed by intraperitoneal injection of BMP7 (100 ng/g, three times per week for 4 wk) or 0.9% saline (Vehicle) as described in Materials and Methods. The severity of liver fibrosis was evaluated. A: Masson staining (100 ×). B: Morphometric analysis of fibrotic lesions in liver. C: Western blot analysis of expression of α-SMA and Col I. Equivalent amount of whole liver detergent lysates were blotted with the indicated antibodies. Each lane represents one individual mouse. GAPDH was used as a loading control. D: Densitometry analysis of the effect of BMP7 on myofibroblast differentiation (alpha-smooth muscle actin), extracellular matrix production (collagen I). Data are pooled and represented as the mean ± SE, n = 8 animals per group. bP < 0.01. E: Representative sections of Masson staining (100×) from paraffin-embedded sections of normal, fibrotic, and cirrhotic human liver tissues and Western blot analysis of expression of BMP7. Equivalent amount of whole liver detergent lysates were blotted with the BMP7 antibody. Each lane represents one individual person. GAPDH was used as a loading control. F: Densitometry analysis of BMP7 activation in indicated liver tissues. bP < 0.01. BMP7: Bone morphogenetic protein 7; Col I: Collagen I; α-SMA: Alpha-smooth muscle actin; CCl4: Carbon tetrachloride.
- Citation: Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, Deng KS, Wu YT, Mu M, Zhu JJ, Zeng JZ, Zhang BF, Wu X, Zhao XK, Li HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J Gastroenterol 2019; 25(30): 4222-4234
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4222