©The Author(s) 2016.
World J Gastroenterol. Sep 7, 2016; 22(33): 7569-7578
Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7569
Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7569
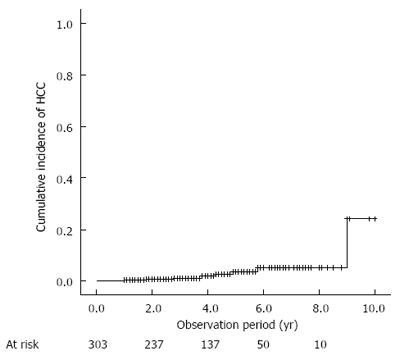
Figure 1 Cumulative incidence of hepatocellular carcinoma development after sustained virological response.
HCC: Hepatocellular carcinoma.
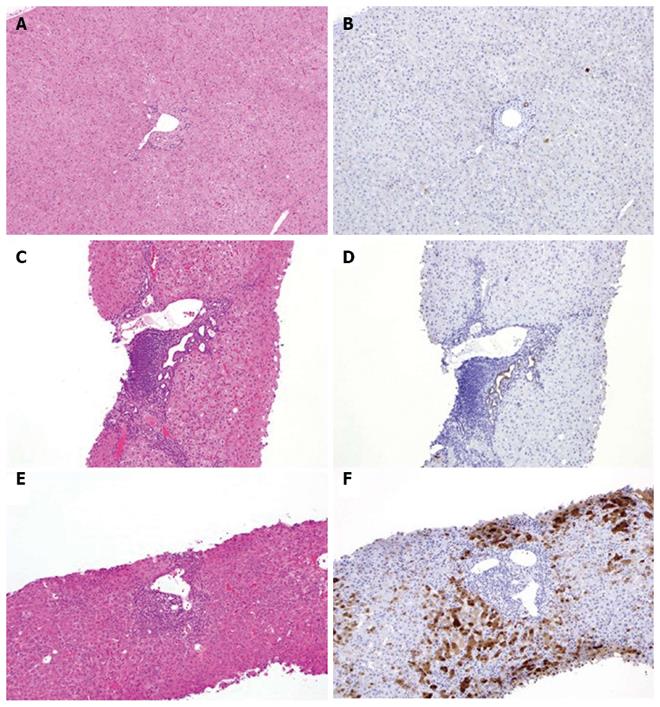
Figure 2 Representative AKR1B10 immunohistochemical staining of specimens.
Normal liver tissue (A, B) and tissue from patients with chronic hepatitis C (C-F). Hematoxylin and eosin staining (A, C, E) and AKR1B10 immunostaining (B, D, F). Positive control, bile-duct epithelium; original magnification × 40.
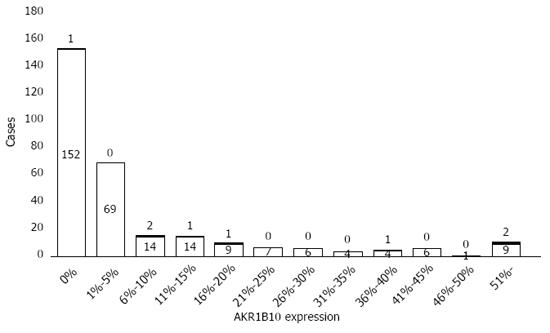
Figure 3 Distribution of AKR1B10 expression levels in the study cohort.
Filled and blank patterns indicate patients with and without hepatocellular carcinoma development, respectively.
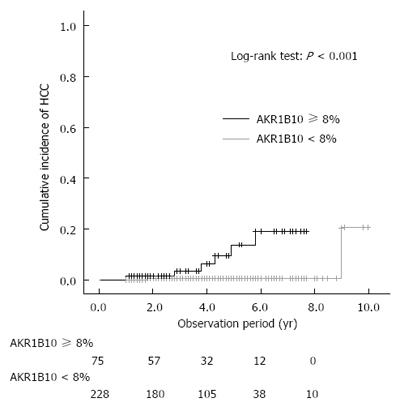
Figure 4 Cumulative incidence of hepatocellular carcinoma development after sustained virological response, shown according to AKR1B10 expression level.
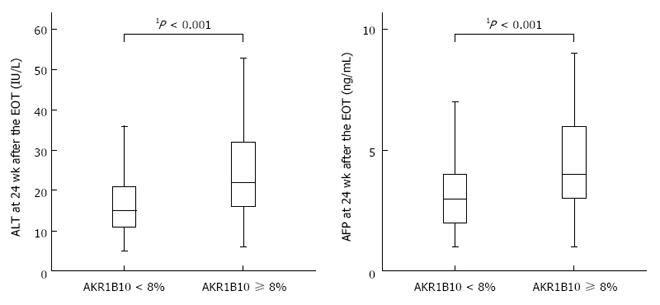
Figure 5 Relationships between baseline AKR1B10 expression levels and alanine aminotransferase and α-fetoprotein levels after sustained virological response.
1Mann-Whitney U test. EOT: End of treatment.
- Citation: Murata A, Genda T, Ichida T, Amano N, Sato S, Tsuzura H, Sato S, Narita Y, Kanemitsu Y, Shimada Y, Hirano K, Iijima K, Wada R, Nagahara A, Watanabe S. Pretreatment AKR1B10 expression predicts the risk of hepatocellular carcinoma development after hepatitis C virus eradication. World J Gastroenterol 2016; 22(33): 7569-7578
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7569