Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7559
Peer-review started: May 6, 2016
First decision: June 20, 2016
Revised: July 11, 2016
Accepted: July 31, 2016
Article in press: July 31, 2016
Published online: September 7, 2016
Processing time: 122 Days and 0.1 Hours
To determine adiponectin expression in colonic tissue of murine colitis and systemic cytokine expression after melatonin treatments and sleep deprivation.
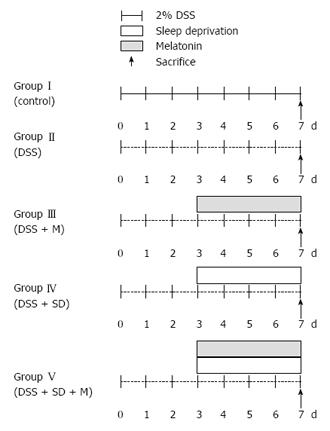
The following five groups of C57BL/6 mice were used in this study: (1) group I, control; (2) group II, 2% DSS induced colitis for 7 d; (3) group III, 2% DSS induced colitis and melatonin treatment; (4) group IV, 2% DSS induced colitis with sleep deprivation (SD) using specially designed and modified multiple platform water baths; and (5) group V, 2% DSS induced colitis with SD and melatonin treatment. Melatonin (10 mg/kg) or saline was intraperitoneally injected daily to mice for 4 d. The body weight was monitored daily. The degree of colitis was evaluated histologically after sacrificing the mice. Immunohistochemical staining and Western blot analysis was performed using anti-adiponectin antibody. After sampling by intracardiac punctures, levels of serum cytokines were measured by ELISA.
Sleep deprivation in water bath exacerbated DSS induced colitis and worsened weight loss. Melatonin injection not only alleviated the severity of mucosal injury, but also helped survival during stressful condition. The expression level of adiponectin in mucosa was decreased in colitis, with the lowest level observed in colitis combined with sleep deprivation. Melatonin injection significantly (P < 0.05) recovered the expression of adiponectin. The expression levels of IL-6 and IL-17 were increased in the serum of mice with DSS colitis but decreased after melatonin injection.
This study suggested that melatonin modulated adiponectin expression in colonic tissue and melatonin and adiponectin synergistically potentiated anti-inflammatory effects on colitis with sleep deprivation.
Core tip: We report this first study that melatonin and sleep deprivation are related to adiponectin expression in the colonic mucosa of murine colitis. C57BL/6 mice were feeding with 2% DSS for inducing colitis and using specially designed multiple platform water baths for sleep deprivation. Immuno-histochemical staining and Western blot analysis was performed using anti-adiponectin antibody. The expression level of adiponectin in mucosa was decreased in colitis, with the lowest level observed in colitis combined with sleep deprivation. Melatonin injection significantly (P < 0.05) recovered the expression of adiponectin. This study suggests that melatonin and adiponectin synergistically potentiate the anti-inflammatory effects in murine colitis.
- Citation: Kim TK, Park YS, Baik HW, Jun JH, Kim EK, Sull JW, Sung HJ, Choi JW, Chung SH, Gye MC, Lim JY, Kim JB, Kim SH. Melatonin modulates adiponectin expression on murine colitis with sleep deprivation. World J Gastroenterol 2016; 22(33): 7559-7568
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7559
Inflammatory bowel disease (IBD) is caused by multiple genetic, environmental, and host factors[1]. Environmental factors such as stress and sleep disturbance can affect the progression and relapse of patients with IBD[2,3]. Stress frequently influences sleep quality. IBD patients have significant sleep disturbance even during inactive state. Sleep disturbance might affect the quality of life and gastrointestinal symptoms. In addition, it can increase the risk of flare-up of IBD[4]. Inflammatory cytokines such as tumor necrosis factor, interleukin-1 (IL-1), and IL-6 are known as significant contributors to sleep disturbances. On the other hand, sleep disturbances can upregulate these inflammatory cytokines[5].
Melatonin is secreted by pineal gland. It functions as a regulator of circadian rhythms and an antioxidant[6]. Melatonin levels in the gut are independent of pineal production. Pinealectomy has no influence on gut melatonin concentrations in rats[7]. At any time of the day or night, the gut contains at least 400 times more melatonin than that of the pineal gland, emphasizing the functional importance of melatonin in the gut[8]. The melatonin in GI tract has anti-inflammatory effect in experimental models of colitis in many previous reports[9-13]. Our previous study also shows that mRNA level of adiponectin is down regulated by sleep deprivation but up-regulated by melatonin based on microarrays and real-time PCR analysis of mice colon tissues[14]. Currently, the relationship between melatonin and adiponectin on colitis with sleep deprivation remains unknown. It is meaningful to evaluate the level of adiponectin expressed in colon tissue of mice by immunohistochemical staining and western blotting to understand the pathogenesis of inflammatory bowel disease. Therefore, the objective of this study was to investigate tissue expression of adiponectin in colitis with sleep deprivation and melatonin injection. The expression levels of cytokines in mouse during sleep deprivation with melatonin injection were also analyzed in this study.
A total of 30 eight-week-old C57BL male mice with body weight of 20-25 g were purchased from Samtako Inc. (Gyunggido, Korea). They were randomly assigned to the following five groups (6 mice per group): (1) Group I, control; (2) Group II, 2% Dextrose Sodium Sulfate(DSS) induced colitis for 7 d; (3) Group III, 2% DSS induced colitis and melatonin treatment; (4) Group IV, 2% DSS induced colitis with sleep deprivation (SD) (20 h wakening/d) using specially designed and modified multiple platform water baths; and (5) Group V, 2% DSS induced colitis with SD and melatonin (Figure 1). Temperature of 22-24 °C and humidity of 55%-60% were maintained with a 12-h light/dark cycle (lights on at 08:00). During the experiment period, mice had free access to food and water. Their body weights were measured daily. All procedures in experiments were conducted according to the Animal Care Guidelines of the National Institutes of Health and Korean Academy of Medical Sciences. The Institutional Review Board of Eulji Hospital at Eulji University College of Medicine approved the study protocol (Approval number: EUIACUC12-11).
2% DSS induced colitis model: For 7 d, mice were fed with 2% DSS (Sigma-Aldrich, Inc., United States) solution to induce colitis.
Partial sleep deprivation: On the 3rd day, all mice were moved to water bath. Water bath had either wide platforms for normal sleep or narrow platforms for sleep deprivation. Partial sleep deprivation of mice was achieved by using a modified multiple platform water bath specifically designed for sleep deprivation. In a water tank, 4 platforms and 2 mice were placed. Every mouse in the water bath could move from one platform to another by jumping. The water bath was filled with water for 4 cm from the base (enough to drown). When mice started to sleep, muscle atonia led mice to fall down into the water, waking up the mice. They would try to climb up the platform to avoid being drowned. Through all experiments, water was changed every day to clean water. Partial sleep deprivation of mice began from 2 PM to 10 AM (20 h) for 4 d. Mice had 4 h of sleep daily from 10 AM to 2 PM during the 4 d. After finishing the 4 d of partial sleep deprivation, mice were sacrificed for analysis.
Administration of melatonin: After induction of colitis with 2% DSS, melatonin was administered to mice intraperitoneally at a dose of 10 mg/kg for 4 d. Normal saline was administered to mice intraperitoneally as control.
Assessment of the severity of colitis: The severity of colitis was assessed by measuring weight loss and histological analysis. Colon tissues were fixed with 10% formaldehyde solution. Fixed tissues were dehydrated with alcohol and embedded with paraffin. Tissues were sliced into 4-μm thick sections. These sections were stained with hematoxylin and eosin to evaluate inflammatory change. The severity of inflammation in colon was determined, including loss of mucosal structure (score 0-3), crypt abscess (score 0-1), thickened muscle (score 0-3), cellular infiltration (score 0-3), and depletion of goblet cell (score 0-1). The final score for the severity of inflammation ranged from 0 to 11[15].
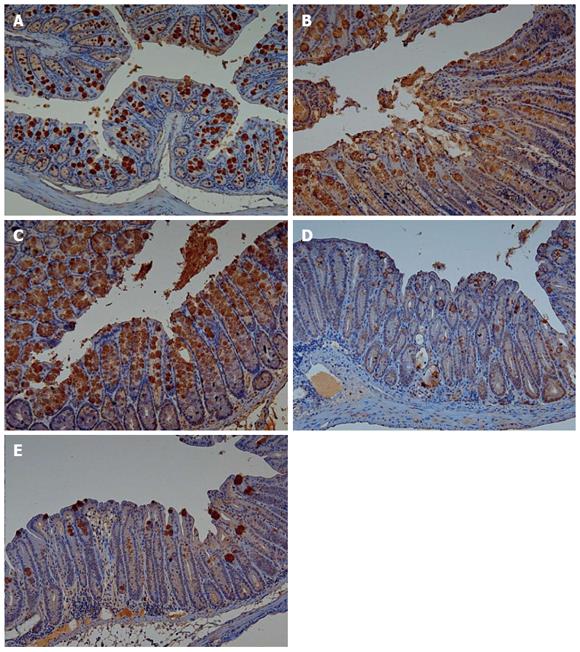
Immunohistochemical staining of adiponectin: Immunohistochemistry procedures were carried out using DAKO Autostainer plus (DAKO Cytomation, Carpinteria, CA, United States). Briefly, 4-μm sections of formalin-fixed and paraffin-embedded tissues were positioned onto poly-L-lysine slides. After deparaffinization and rehydration, antigen retrieval was performed using citrate buffer solution (pH 6.0) at 121 °C for 10 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min. The sections were then incubated with specific antibodies against Adiponectin (Abcam, Cambridge, United Kingdom, 1:500). Slides were then stained with 3,3′-diaminobenzidine and counterstained with hematoxylin. Immunoreactivity was determined based on the percentage of stained cells: 1 + for less than 10%, 2 + for 10%-50%, and 3 + for over 50%.
Quantification of adiponectin by western blot analysis: Tissues were homogenized in 10 volumes of extraction buffer (20 mmol/L Tris-HCl, pH 7.5, 1% Triton X-100) containing protease inhibitor cocktail (CompleteTM, Roche, Germany) on ice. Homogenates were centrifuged at 12000g for 1 h at 4 °C. The supernatant was collected and its protein concentration was determined using a commercial protein assay kit (Bio-Rad, CA). Protein samples were mixed with 2X sample buffer (Laemmli, 1970), boiled for 5 min, and cooled at room temperature. After briefly spinning, clear supernatants were resolved on 8% SDS-polyacrylamide gels. Proteins were electrophoretically transferred to nitrocellulose membranes (GE Healthcare, United Kingdom) and blocked with Tris-buffered saline (TBS) containing 1% bovine serum albumin overnight at 4 °C. After rinsing three times with TBS/0.1% Tween 20 (TBST) for 10 min each, protein blots were incubated with rabbit anti-adiponectin polyclonal antibody (ab62551, abcam, United Kingdom) diluted 1:1000 in TBS for 2 h at room temperature. After rinsing with TBST three times for 10 min each, the blots were incubated with peroxidase-labeled goat anti-rabbit IgG (Invitrogen, OR, United States) diluted 1:1000 in TBST for 1 h. The blots were washed with TBST for 10 min followed by washing with TBS for 10 min. Signals of protein bands were detected using an ECL kit (GE Healthcare).
Measurement of serum cytokines: Blood were collected by intracardiac sampling from the experimental mice just before sacrificing. Serum was prepared to measure cytokine levels. Levels of IL-17 (Ray Bio ELISA Kit Mouse IL-17; RayBiotech, Norcross, GA, United States), IL-6 (Ray Bio ELISA Kit Mouse IL-6; RayBiotech), and TNF-α (Ray Bio ELISA KitMouse TNF-alpha; RayBiotech) were evaluated using commercially available kits following the manufacturer’s instructions.
All data were represented as mean ± SD. Statistical Package for Social Sciences software (SPSS; Korean version 18.0) was used for all statistical analyses. One way analysis of variance (ANOVA) was used to calculate the statistical significance. Significance was considered when P value was less than 0.05.
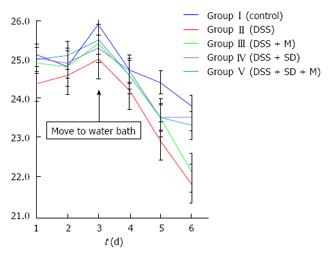
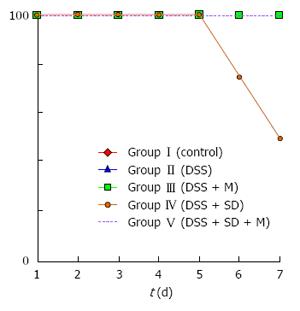
On the 3rd d, mice were moved to water bath. From that time, the body weight of all mice started to decrease due to stressful water bath condition. The body weight of 2% DSS induced colitis group was significantly (P = 0.005) lower than that of the control group (Figure 2). Sleep deprivation induced severe weight loss. Half of mice were dead during sleep deprivation (Figure 3). Melatonin failed to significantly recover the weight loss, but it could help all mice survive during sleep deprivation.
In histological analysis, the colon of 2% DSS induced colitis mice showed edema and infiltration of inflammatory cells into the mucosa compared to that of the control group. Melatonin treatment reduced inflammation in the colon of 2% DSS induced colitis in group III. The number of infiltrating cells and mucosal injury such as ulcer or necrosis were increased by sleep deprivation in the colon of 2% DSS induced colitis mice. Melatonin treatment also diminished the inflammation and erosion in the colon of 2% DSS induced colitis mice with sleep deprivation. The microscopic inflammatory score of the colitis group (Group II) was 6.5 ± 0.6. It was significantly (P = 0.003) decreased to 4.4 ± 0.8 by melatonin treatment (Group III). However, it was significantly (P = 0.018) increased to 8.5 ± 0.5 by sleep deprivation (Group IV). The histologic score of 8.5 ± 0.5 of 2%DSS induced colitis mice with sleep deprivation (group IV) was decreased (P = 0.067) by melatonin treatment (group V, score of 7.0 ± 1.0). Changes of inflammatory score in all groups were statistically significant (P < 0.01).
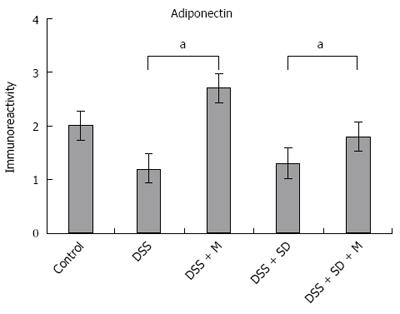
Adiponectin expression in colonic mucosa was determined by immunohistochemical staining (Figure 4). Colitis mice and colitis mice with sleep deprivation showed decreased adiponectin immunoreactivity compared to mice of the control group. In melatonin injection group, mice showed significant recovery in adiponectin immunoreactivity (Figure 5).
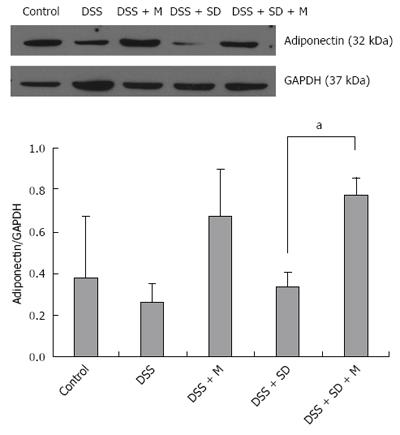
Based on western blot analysis, the expression levels of adiponectin were decreased in the colitis group and the colitis with sleep deprivation group compared to that in the control group (Figure 6). In the group of colitis with sleep deprivation, adiponectin expression was significantly (P < 0.05) recovered by melatonin injection (Figure 6). These results are consistent with results from immunohistochemical staining.
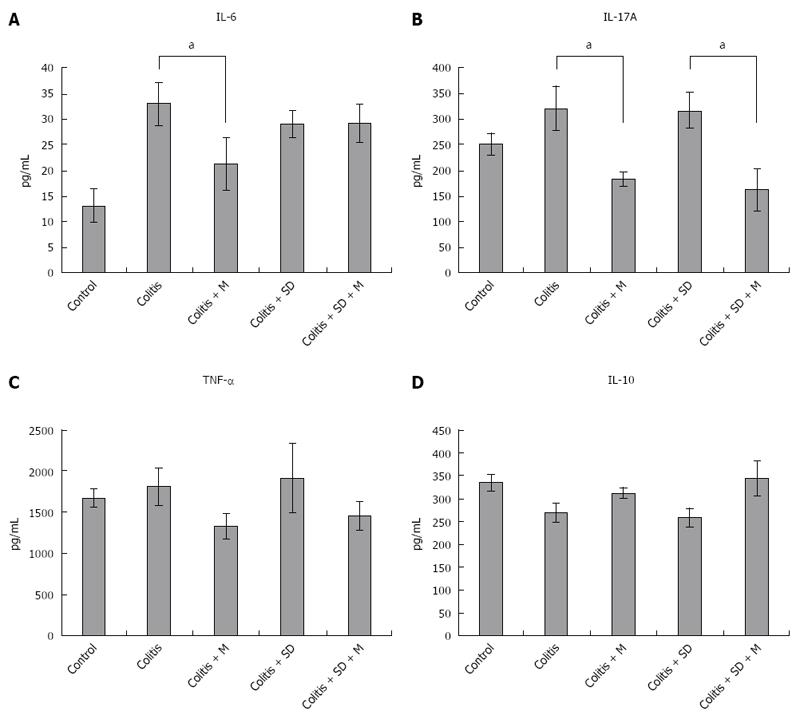
The levels of pro-inflammatory cytokines such as IL-6, IL-17, and TNF-α in the serum of mice were increased in DSS colitis group and colitis with sleep deprivation group compared to those of the control group. Their levels were decreased after melatonin injection. Even in the control group, the levels of these pro-inflammatory cytokines were relatively higher level than that in the melatonin treatment group. This could be due to the fact that all mice were moved to water bath at the 3rd experimental day when control mice got stressed out due to their fear of water. The expression levels of IL-6, IL-17, and TNF-α were significantly (P < 0.05) decreased after melatonin treatment (Figure 7). The level of anti-inflammatory cytokine IL-10 was lower in colitis. However, it had a tendency of increase after melatonin injection, indicating that melatonin treatment might have systemic anti-inflammatory properties.
Melatonin plays various important roles in the GI tract. As a physiological antagonist to serotonin, it decreases peristalsis and stimulates the secretion of mucosal bicarbonate mediated by MT2 receptor. An important receptor independent action of melatonin in the GI tract is that it is a free radical scavenger. The preventive role of melatonin against ulcer formation for healing has been well established[16]. Previous studies have suggested that melatonin administration is beneficial for IBD via its antioxidant, anti-apoptotic, and anti-inflammatory properties.
In this study, pathologic examination showed that melatonin could attenuate inflammation and decrease the severity of inflammation under sleep deprivation (Table 1). During our experiment, moving to water bath was stressful to mouse. Control mice also lost weight in the water bath. The sleep deprivation group showed aggravated weight loss and half of this group could not survive until the end of experiments. However, melatonin injection could help the survival of mice under sleep deprivation.
| Control | DSS | DSS+M | DSS+SD | DSS+SD+M | P value | |
| Microscopic scores | 0 ± 0 | 6.5 ± 0.6 | 4.4 ± 0.8 | 8.5 ± 0.5 | 7.2 ± 1.0 | < 0.01 |
In our previous study, we found the genetic expression of adiponectin after melatonin injection with sleep deprivation using microarray and PCR[14]. Here, we tried to confirm the tissue expression of adiponectin after melatonin injection through immunohistochemical staining (Figure 4) and Western blotting (Figure 6). Our results showed that adiponectin expression was decreased in both colitis and colitis with sleep deprivation condition. However, melatonin injection significantly recovered the adiponectin expression in colon tissues, in consistent with genetic change that we found earlier.
Adiponectin is mainly expressed by mature adipocytes. It circulates at high levels in the blood stream[17]. There are some reports about the anti-inflammatory effect of adiponectin. Adiponectin could inhibit the expression of NF-κB, TNF-α, IL-6, and IL-1b but induce the expression of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonists[18]. Adiponectin can also induce the production of IL-10 and IL-1Ra in human PBMC, macrophages, and DC while impairing the production of IFNγ in macrophages[19-21]. Overexpressed adiponectin in mice can decrease pro-inflammatory cytokines including cellular stress markers, TNF, IL-6, and IL-1b in mice[22,23]. Our study showed that mucosal expression of adiponectin was significantly decreased in DSS-induced colitis compared to that in the control. This result was consistent with other reports. Adiponectin mRNA level is reported to be decreased in inflamed colonic mucosa of DSS induced murine colitis[24]. Adiponectin can inhibit chemokine production in intestinal epithelial cells. It has been reported that adiponectin KO mice has much more severe colitis compared to wild type mice[25]. Recently, adiponectin expression in human tissue has been identified. In patients with ulcerative colitis, the mRNA expression of adiponectin in colonic mucosa is decreased[24]. Adiponectin concentration in mesenteric adipose tissue of CD patients has been found to be significantly lower in patients with internal fistula compared to that in patients without fistula[26].
Interaction of melatonin with adiponectin was first reported by Ríos-Lugo et al[18]. They showed that melatonin attenuated body weight increase and hyperglycemia, but increased mean plasma adiponectin in high fat-fed rats. The high-fat diet disrupted the normal 24-h pattern of adiponectin, which was counteracted by melatonin[18]. Direct effect of melatonin on adiponectin has not been clarified yet. An in vitro study using pre-adipocytes has shown an inhibitory effect of melatonin on adiponectin synthesis[18,27]. Another study has demonstrated that chronic melatonin administration to rats mainly affects the 24-h rhythm of adiponectin secretion[28]. A recent study has shown a significant increase in median plasma adiponectin levels and insulin sensitivity as early as 4 weeks after melatonin administration in cohort of patients with non-alcoholic steatohepatitis[29].
In this study, the expression level of adiponectin was lower in DSS-induced colitis than that in the control, with the lowest level in the sleep deprivation group. After melatonin injection, the expression level of adiponectin was significantly increased, even higher than that in the control. Based on these results, adiponectin expression in colonic tissue might be modulated by melatonin injection. These results are comparable to genetic expression of adiponectin after melatonin treatment in our previous study[14].
To understand the mechanism of inflammation, the levels of serum cytokines of mice were determined. Pro-inflammatory cytokines such as IL-6, IL-17A, and TNF-α were increased in colitis. As one of mechanisms to control inflammation, melatonin can modulate a variety of molecular targets, including NF-κB, cyclooxygenase-2, interleukin 17, matrix metalloproteinase-9, and connective tissue growth factor[30]. We also found that the levels of pro-inflammatory cytokines such as IL-6, IL-17A, and TNF-α were decreased while the level of IL-10 was increased after melatonin treatment (Figure 7). Therefore, adiponectin decreased levels of pro-inflammatory cytokines but increased level of IL-10 in macrophage and PBMC. Melatonin and adiponectin might have synergistic effect in these cytokine profiles. However, there was no significant cytokine change after sleep deprivation. This could be due to the fact that we could only check serum cytokine levels in survived mice. Dead mice might have higher pro-inflammatory cytokine levels than survived mice.
Our results showed that sleep deprivation group mice had more severe pathologic inflammation with worse survival rate, indicating that sleep is important for survival. Under the same condition, all melatonin injected mice survived during the experimental period (Figure 3). Although the precise mechanism on how melatonin helped the survival of mice is currently unknown, melatonin might be beneficial to overcome stressful conditions such as sleep deprivation for human.
Th17 cell lineage is important for pathogenesis of IBD. It produces important inflammatory cytokine IL-17. Our results also demonstrated that melatonin injection decreased IL-17 level. Th17 lineage-specific transcription factor RORα is a natural target of melatonin in T cells[31]. RORα, along with RORγ, can regulate Th17 cell differentiation[32], whereas down regulation of RORα expression is part of the typical Treg transcriptional signature[33]. A preliminary study has highlighted the in vivo inhibitory actions of melatonin on Treg cell generation in cancer patients[34]. It has been reported that in vivo administration of melatonin to mice subjected to experimental cancer has down regulation of CD4+CD25+ Treg cells and Foxp3 expression in tumor tissues[35]. These results suggest that melatonin may have adjuvant effect in inhibiting colitis associated cancer development in patients with IBD.
The levels of plasma melatonin in patients with ulcerative colitis (UC) have been reported to be significantly lower than those of healthy control[36]. They are higher in remission state than in active disease. Melatonin can also cause the disappearance of clinical symptoms of patients with UC. These symptoms are reported to have reappeared when melatonin consumption is stopped[37]. Adjuvant treatment with melatonin in UC patients is reported to keep remission for 12 mo with normal C- reactive protein ranges and high hemoglobin levels, suggesting that adjuvant melatonin therapy may help sustain remission of patients with UC[38]. However, more clinical trials should be performed to confirm the beneficial effect of melatonin on IBD, although basic studies strongly suggest such beneficial effect.
To the best of our knowledge, this is the first study to report that melatonin and sleep deprivation are related to adiponectin expression in colonic mucosa of DSS colitis. Results of this study were consistent with results of previous study about the genetic expression of adiponectin after melatonin and sleep deprivation.
In summary, we found that sleep deprivation aggravated inflammation and lowered survival rate. However, melatonin had a protective effect on inflammatory change and helped the survival of DSS-induced colitis of mice. In addition, melatonin modulated adiponectin expression in colitis mice with sleep deprivation. This study suggests that melatonin and adiponectin synergistically potentiate the anti-inflammatory effects in the colitis. Because severe colitis with sleep deprivation is a frequent condition of active IBD patients, melatonin is expected to be used for control of inflammation and sleep deprivation.
Melatonin is secreted by pineal gland. It functions as a regulator of circadian rhythms and an antioxidant. Melatonin levels in the gut are independent of pineal production. The gut contains at least 400 times more melatonin than the pineal gland, emphasizing the functional importance of melatonin in the gut. The melatonin in GI tract has anti-inflammatory effect in experimental models of colitis in many previous reports.
This previous study also shows that mRNA level of adiponectin is down regulated by sleep deprivation but up-regulated by melatonin based on microarrays and real-time PCR analysis of mice colon tissues
This is the first study that melatonin and sleep deprivation are related to adiponectin expression in the colonic mucosa of murine colitis being performed by immuno-histochemical staining and Western blot analysis. So, the authors confirmed previous genetic change of adiponectin on microarrays and real-time PCR analysis.
The expression level of adiponectin in mucosa was decreased in colitis, with the lowest level observed in colitis combined with sleep deprivation. Melatonin injection significantly recovered the expression of adiponectin. This study suggests that melatonin and adiponectin synergistically potentiate the anti-inflammatory effects in murine colitis.
No specific terminology are used in this paper
Using the DSS-induced colitis mice model, the authors studied the adiponectin expression in colonic tissue of murine colitis and cytokine expression in response to melatonin treatments and sleep deprivation. The results showed that sleep deprivation exacerbated DSS colitis, which can be alleviated by melatonin injection. The expression level of adiponectin in mucosa was decreased in colitis, with the lowest level observed in colitis combined with sleep deprivation.
| 1. | Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Niess JH, Mönnikes H, Dignass AU, Klapp BF, Arck PC. Review on the influence of stress on immune mediators, neuropeptides and hormones with relevance for inflammatory bowel disease. Digestion. 2002;65:131-140. [PubMed] |
| 4. | Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1748-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Ali T, Choe J, Awab A, Wagener TL, Orr WC. Sleep, immunity and inflammation in gastrointestinal disorders. World J Gastroenterol. 2013;19:9231-9239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (3)] |
| 6. | Marquez E, Sánchez-Fidalgo S, Calvo JR, la de Lastra CA, Motilva V. Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J Pineal Res. 2006;40:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Bubenik GA, Brown GM. Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of rats. Biol Signals. 1997;6:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011;17:3888-3898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Necefli A, Tulumoğlu B, Giriş M, Barbaros U, Gündüz M, Olgaç V, Güloğlu R, Toker G. The effect of melatonin on TNBS-induced colitis. Dig Dis Sci. 2006;51:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Li JH, Yu JP, Yu HG, Xu XM, Yu LL, Liu J, Luo HS. Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005;2005:185-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Akcan A, Kucuk C, Sozuer E, Esel D, Akyildiz H, Akgun H, Muhtaroglu S, Aritas Y. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J Gastroenterol. 2008;14:918-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Terranova ML, Ciccolo A, Caputi AP. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J Pineal Res. 2001;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Dong WG, Mei Q, Yu JP, Xu JM, Xiang L, Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J Gastroenterol. 2003;9:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Chung SH, Park YS, Kim OS, Kim JH, Baik HW, Hong YO, Kim SS, Shin JH, Jun JH, Jo Y. Melatonin attenuates dextran sodium sulfate induced colitis with sleep deprivation: possible mechanism by microarray analysis. Dig Dis Sci. 2014;59:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, Tahan V, Dorko K. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig Dis Sci. 2011;56:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 509] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 17. | Fajas L, Landsberg RL, Huss-Garcia Y, Sardet C, Lees JA, Auwerx J. E2Fs regulate adipocyte differentiation. Dev Cell. 2002;3:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (4)] |
| 18. | Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J Pineal Res. 2010;49:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1841] [Article Influence: 87.7] [Reference Citation Analysis (9)] |
| 20. | Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 629] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 21. | Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527-E533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 514] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23 Suppl 1:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Alonso-Vale MI, Andreotti S, Peres SB, Anhê GF, das Neves Borges-Silva C, Neto JC, Lima FB. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab. 2005;288:E805-E812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848-4856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Gonciarz M, Bielański W, Partyka R, Brzozowski T, Konturek PC, Eszyk J, Celiński K, Reiter RJ, Konturek SJ. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res. 2013;54:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Trivedi PP, Jena GB. Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig Dis Sci. 2013;58:3460-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Lardone PJ, Guerrero JM, Fernández-Santos JM, Rubio A, Martín-Lacave I, Carrillo-Vico A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J Pineal Res. 2011;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1380] [Cited by in RCA: 1361] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 33. | Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008;180:4785-4792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 34. | Vigoré L, Messina G, Brivio F, Fumagalli L, Rovelli F, DI Fede G, Lissoni P. Psychoneuroendocrine modulation of regulatory T lymphocyte system: in vivo and in vitro effects of the pineal immunomodulating hormone melatonin. In Vivo. 2010;24:787-789. [PubMed] |
| 35. | Liu H, Xu L, Wei JE, Xie MR, Wang SE, Zhou RX. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat Rec (Hoboken). 2011;294:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Chen M, Mei Q, Xu J, Lu C, Fang H, Liu X. Detection of melatonin and homocysteine simultaneously in ulcerative colitis. Clin Chim Acta. 2012;413:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Mann S. Melatonin for ulcerative colitis? Am J Gastroenterol. 2003;98:232-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J, Chojnacki J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol. 2011;62:327-334. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen JF S- Editor: Qi Y L- Editor: A E- Editor: Wang CH