©The Author(s) 2015.
World J Gastroenterol. Feb 14, 2015; 21(6): 1945-1955
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1945
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1945
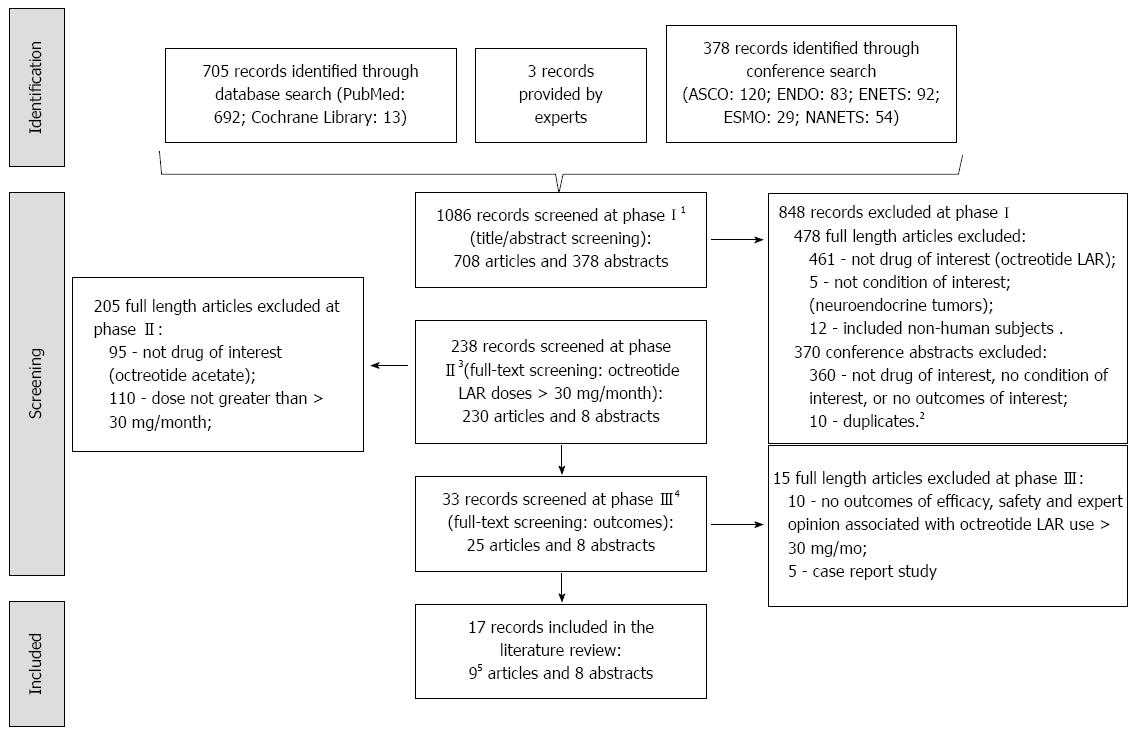
Figure 1 Record search and screening flow chart.
1Phase I: Title and abstract screen of all identified records through database search; screens for records that contain: octreotide LAR, neuroendocrine tumors, and human subjects. 2Duplicates include duplicate abstracts presented at different conferences and data reported in full-length articles included in this review. 3Phase II: Full-text screen of those records that passed phase I; screens for records containing use of octreotide LAR of doses greater than 30 mg/4 wk. 4Phase III: Full-text screen of those records that passed phase II; screen for records containing safety and effectiveness outcomes, and exclude records that are case reports. 5Two of the 10 included articles reported data on the same patients, hence only the most recent study (Woltering 2006) was retained in this review. American Society of Clinical Oncology (ASCO), Endocrine Society (ENDO), European Neuroendocrine Tumor Society (ENETS), European Society for Medical Oncology (ESMO), and North American Neuroendocrine Tumor Society (NANETS).
- Citation: Broder MS, Beenhouwer D, Strosberg JR, Neary MP, Cherepanov D. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: A systematic literature review. World J Gastroenterol 2015; 21(6): 1945-1955
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1945