©The Author(s) 2015.
World J Gastroenterol. Oct 14, 2015; 21(38): 10739-10748
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10739
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10739
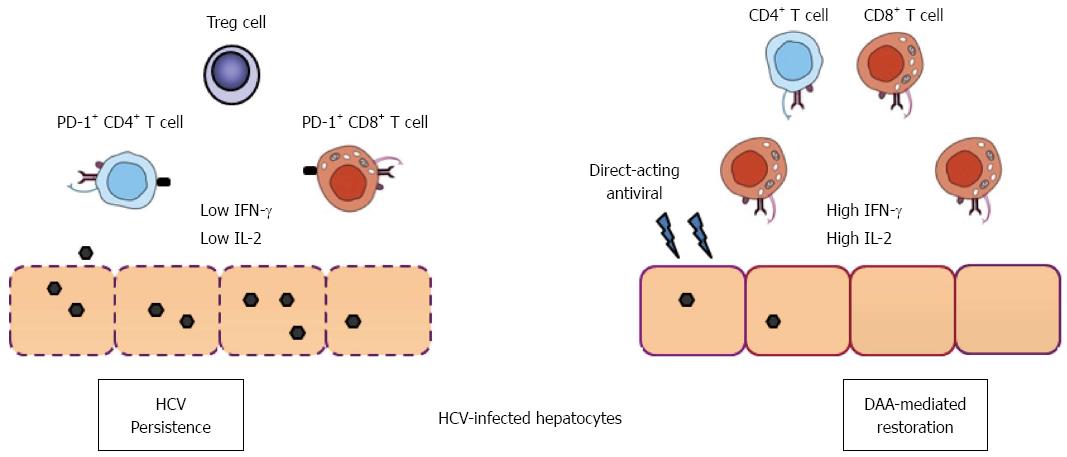
Figure 1 Direct-acting antiviral therapy and immune function restoration in hepatitis C virus infection.
In chronic hepatitis C infection, T cells having a narrow repertoire of TCRs mount a weak response to HCV antigens, and their effector functions are often impaired. Many CD8+ and CD4+ T cells express low levels of IFN-γ and IL-2 accompanied by up-regulation of PD-1 molecules in the liver. Development of T regulatory cells and compromised dendritic cell functions also contribute to T cell functional impairment. Recent data suggest that IFN-free direct-acting antivirals not only clear HCV in the majority of patients, but also result in the down-regulation of PD-1, leading to rapid restoration of virus-specific CD8+ T cell functions in patients[28]. HCV: Hepatitis C virus; IFN: Interferon; IL: Interleukin; PD-1: Program death-1; TCR: T cell receptor.
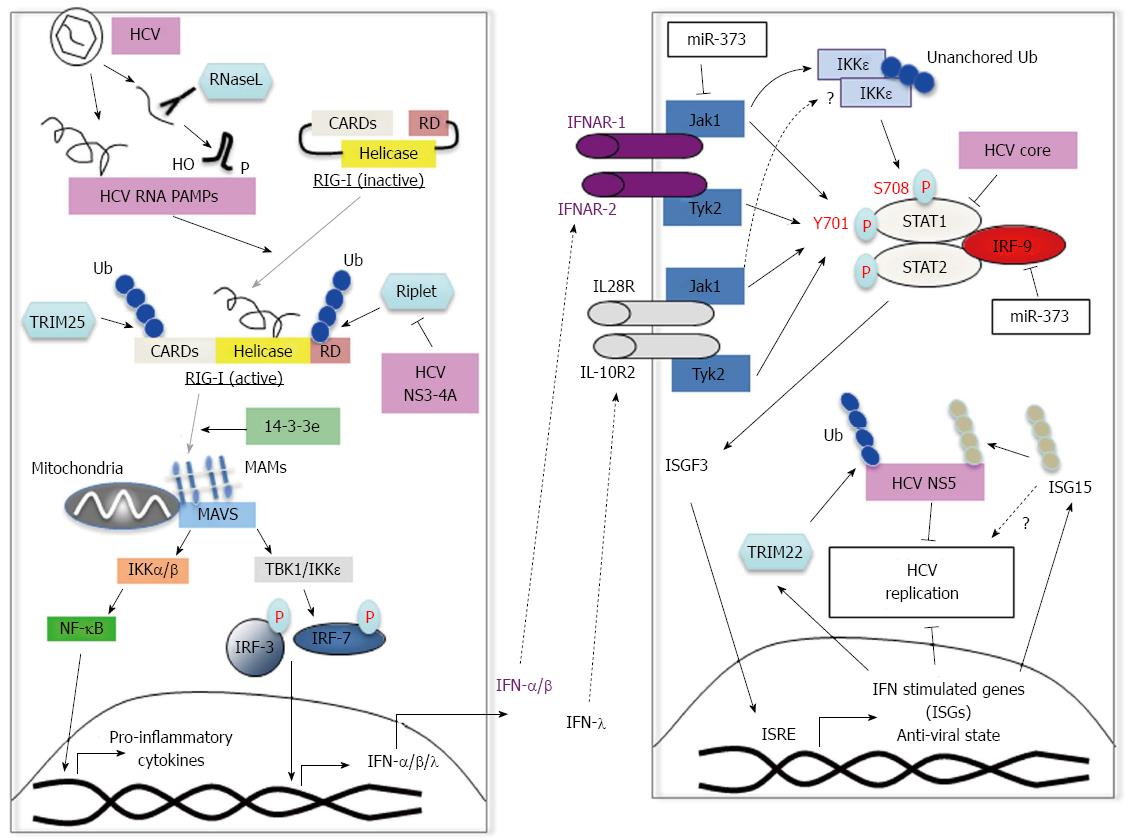
Figure 2 Innate immune response to hepatitis C virus and viral escape mechanisms.
A: RIG-I recognizes HCV PAMPs including HCV 5’-triphosphate RNA and small structured RNAs with 5'-hydroxyl (5'-OH) and 3'-monophosphoryl (3'-p) groups, which are cleaved by the host endoribonuclease RNase L. Binding of the HCV PAMP induces a conformational change in RIG-I and subsequent ubiquitination by TRIM25 and Riplet. RIG-I is then recruited to MAVS via mitochondria associated membranes (MAMs) and the chaperone protein 14-3-3e. Subsequently, TBK1 and IKKε phosphorylate IRF3 and IRF7 for IFN-I production as well as IFN-λ, and pro-inflammatory cytokines via NF-κB activation. The NS3-4A of HCV inhibits Riplet-dependent activation of RIG-I; B: IFN-I and IFN-λ are recognized by the IFNA receptor and IL28/IL-10 receptor respectively. Both trigger downstream signaling through phosphorylation of STAT1 and STAT2. Together, STAT1, STAT2 and IRF9 form the ISGF3 complex, which translocates to the nucleus for induction of antiviral ISGs. The tyrosine kinases JAK1 and TYK2, phosphorylate tyrosine 701 (Y701) on STAT1. In addition, phosphorylation of S708 on STAT1 by the IKKε kinase (activated by unanchored polyubiquitin chains) is also required for ISG induction. It is currently unknown whether IFN-λ stimulation also results in IKKε activation. miR-373 is up-regulated in HCV-infected cells and inhibits JAK1 and IRF9 function resulting in reduced STAT1 phosphorylation. TRIM22, which is induced by IFN-I, inhibits HCV replication probably by a mechanism involving ubiquitination of viral NS5. ISG15, another IFN-I inducible protein can inhibit HCV replication by ISGylation of viral NS5 rendering unstable. ISG15 has also been proposed to have pro-viral roles. The core protein of HCV associates with STAT1 and promotes its degradation. RIG-I: Retinoic acid inducible gene; HCV: Hepatitis C virus; PAMP: Pathogen-associated molecular pattern; IFN: Interferon; IL: Interleukin; ISGF: Interferon-stimulated gene factor; STAT: Signal transducers and activators of transcription; NF-κB: Nuclear factor-kappa B; IKK: Inhibitor of NF-κB kinase.
- Citation: Sun J, Rajsbaum R, Yi M. Immune and non-immune responses to hepatitis C virus infection. World J Gastroenterol 2015; 21(38): 10739-10748
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10739