Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 28, 2013; 19(28): 4511-4519
Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4511
Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4511
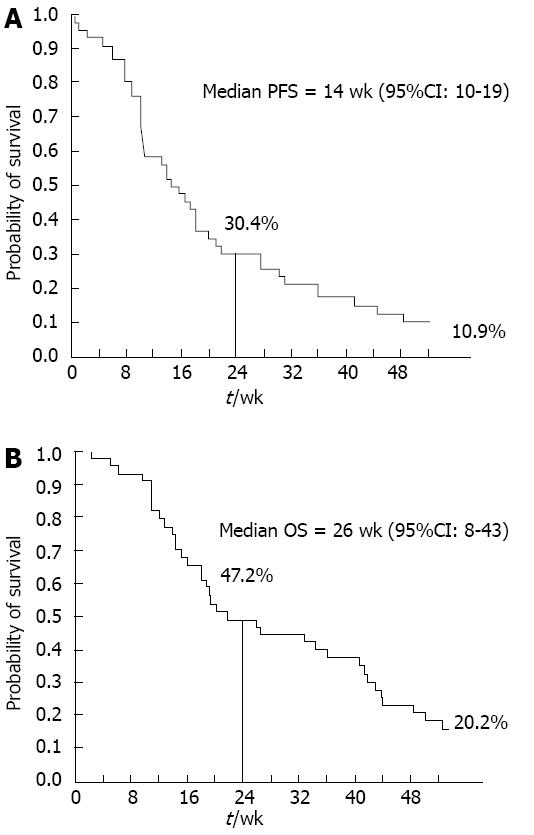
Figure 1 Kaplan-Meier analysis of progression-free survival and overall survival in the intent to treat population.
A: Progression-free survival (PFS); B: Overall survival (OS).
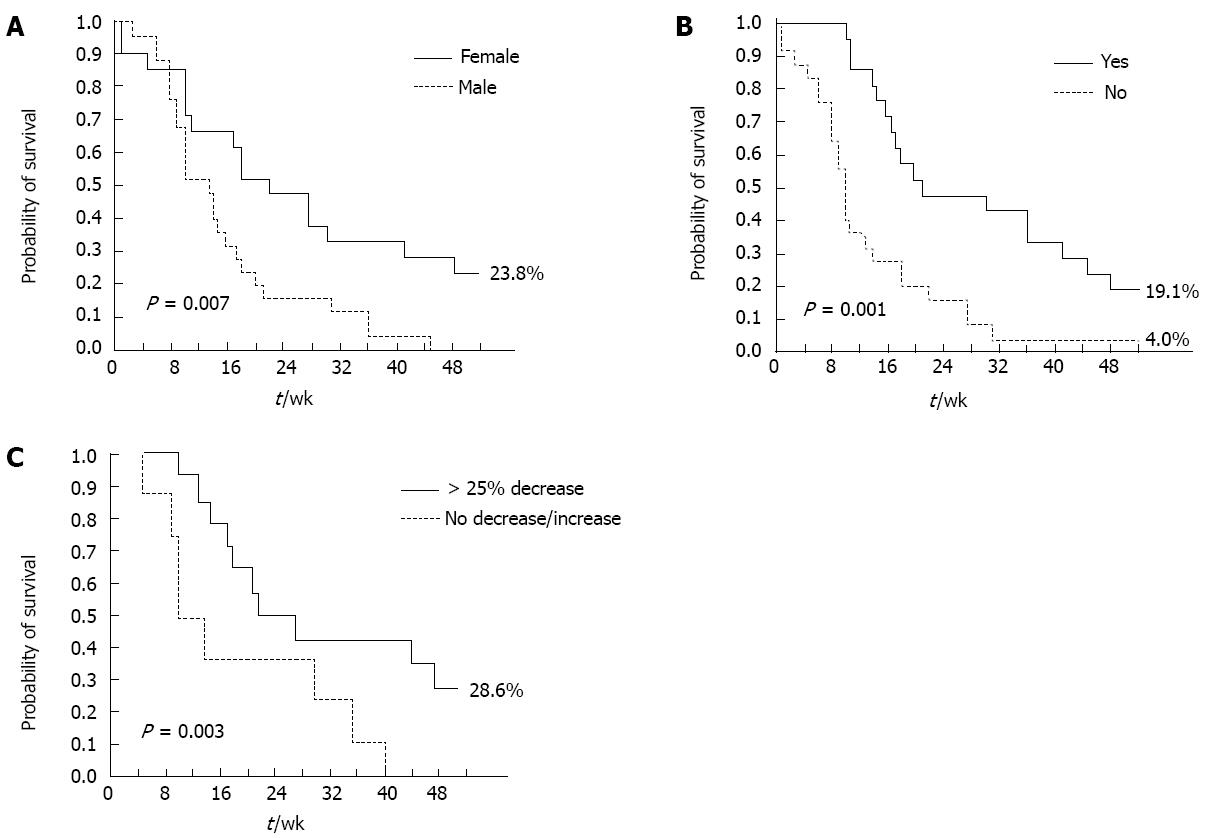
Figure 2 Kaplan-Meier analysis of independent progression-free survival predictors.
A: Progression-free survival (PFS) by sex; B: PFS by skin rash; C: PFS by carbohydrate antigen 19-9 decrease.
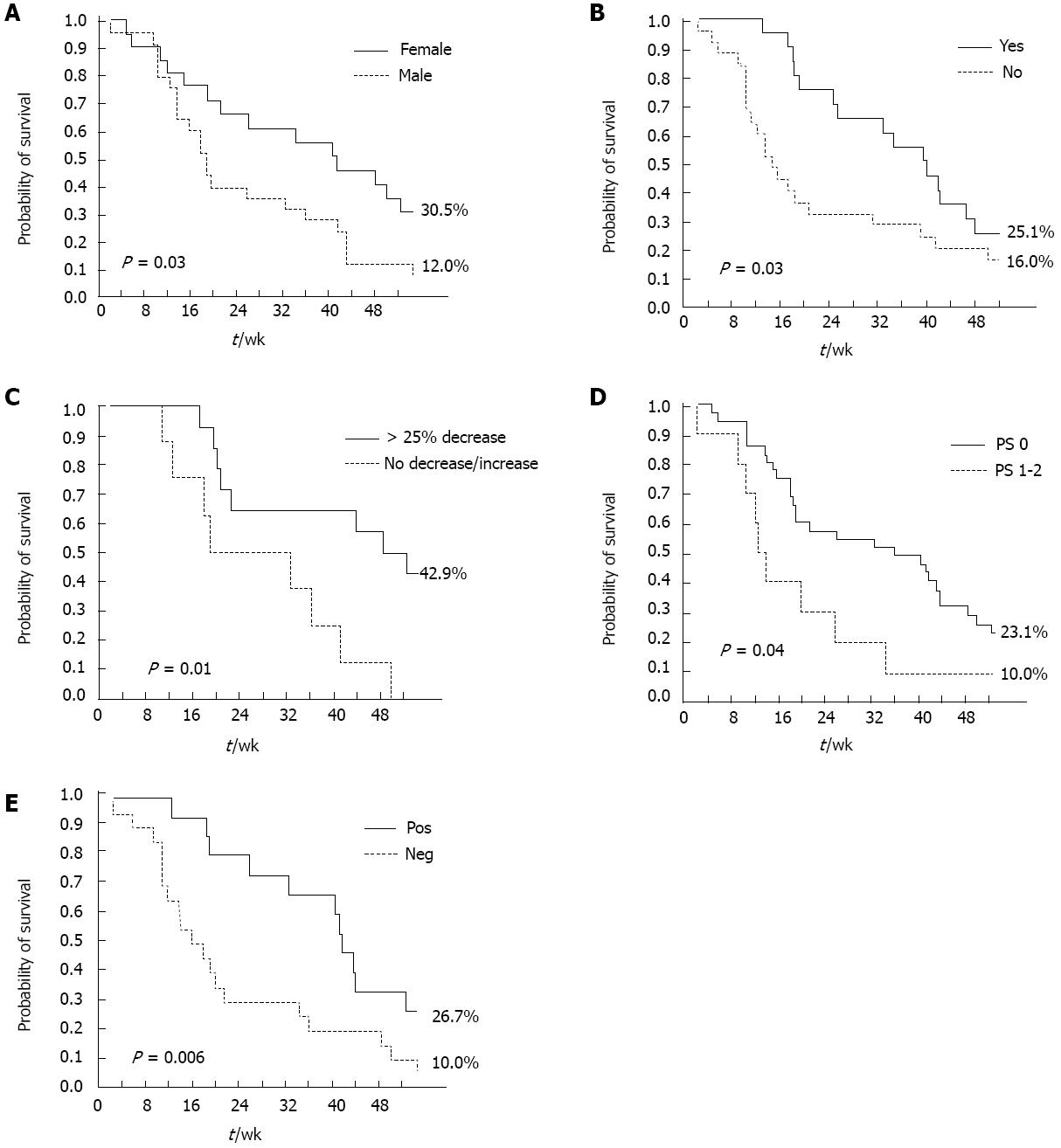
Figure 3 Kaplan-Meier analysis of independent overall survival predictors.
A: Overall survival (OS) by sex; B: OS by skin rash; C: OS by carbohydrate antigen 19-9 decrease; D: OS by Eastern Cooperative Oncology Group performance status (PS); E: OS by clinical benefit. Pos: Positive; Neg: Negative.
- Citation: Vaccaro V, Bria E, Sperduti I, Gelibter A, Moscetti L, Mansueto G, Ruggeri EM, Gamucci T, Cognetti F, Milella M. First-line erlotinib and fixed dose-rate gemcitabine for advanced pancreatic cancer. World J Gastroenterol 2013; 19(28): 4511-4519
- URL: https://www.wjgnet.com/1007-9327/full/v19/i28/4511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i28.4511