©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 7, 2006; 12(5): 731-738
Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.731
Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.731
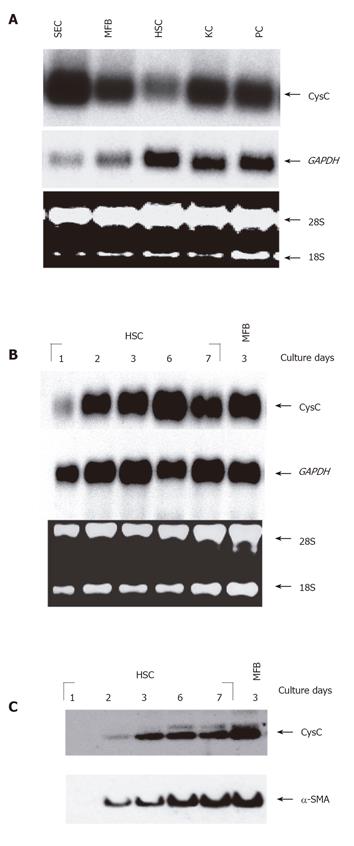
Figure 1 CysC mRNA expression in different rat liver cell types.
A: Equal amounts of total RNA samples (10 µg) isolated from cultured SECs, MFBs, HSCs, KCs, and PCs were analyzed by Northern blot. The blot was hybridized with a 32P-labeled probe specific for rat CysC; B: total RNA samples (5 µg/lane) of trans-differentiating HSCs/MFBs at indicated time intervals were analyzed for CysC expression by Northern blot analysis. As internal loading controls (A and B), the 18S and 28S rRNA as well as signals obtained after hybridization with a GAPDH-specific probe are shown. The blots were exposed for 4 (GAPDH) or 24 h (CysC), respectively; C: protein extracts (20 µg/lane) from primary rat HSCs cultured for 1 to 7 days or rat MFBs cultured for 3 days were analyzed for CysC expression by Western blotting under reducing conditions (50 mmol/L DTT) using antibody P-14 (sc-16989). To verify cellular trans-differentiation, the blot was stripped, and tested for α-SMA expression using a mouse monoclonal antibody (clone asm-1).
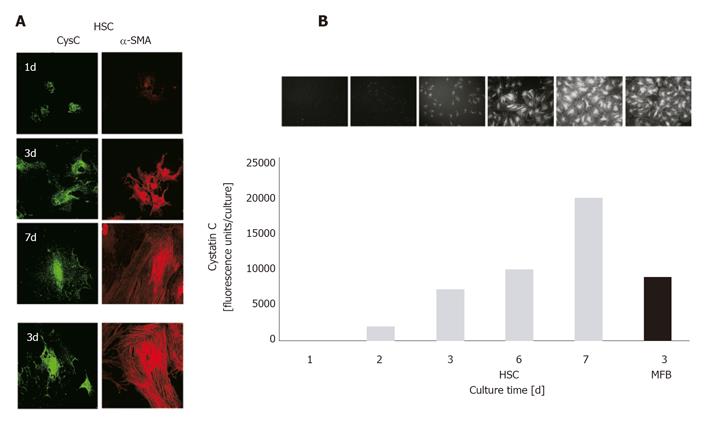
Figure 2 Confocal laser scanning microscopy and ELF assay for quantitative determination of CysC expression in HSCs and MFBs.
A: HSCs/MFBs were seeded on glass cover slides for indicated time intervals. After fixation, cells were permeabilized and incubated with primary antibodies directed against CysC or α-SMA. Primary antibodies were visualized with a FITC-labeled secondary antibody (CysC, green fluorescence) or a Cy3-labeled secondary antibody (α-SMA, red fluorescence). Original magnification was ×400. Negative controls, using normal IgGs instead of primary antibodies showed no staining (data not shown); B: HSCs/MFBs cultured in 96-well plates for indicated time intervals were fixed and stained for CysC. The staining was visualized by fluorescence microscopy (original magnification ×200). Fluorescence intensities were measured quantitatively using a 96-well fluorescence reader (excitation 365 nm, emission ≥515 nm).
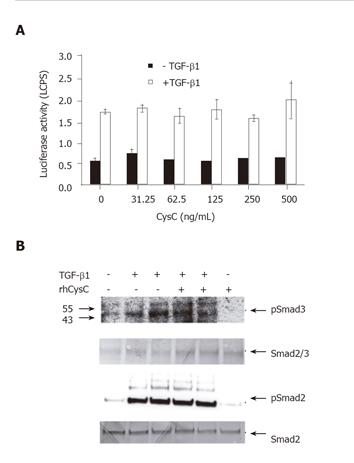
Figure 3 Effect of rhCysC on TGF-β1-induced reporter gene activity and Smad2/3 phosphorylation.
A: Cultured HSCs were infected with 50 MOI Ad-(CAGA)9-MLP-luciferase followed by 1 h incubation with the indicated doses of rhCysC prior to exposure with TGF-β1 (1 ng/mL) for 4 h or left untreated. The mean ± SD of the measured luciferase activities (n = 4) was given; B: phosphorylated Smad2 (pSmad2) and Smad3 (pSmad3) were detected by immunoblotting of whole cell protein lysates prepared after treatment of HSCs with or without 500 ng/mL rhCysC for 1 h followed by incubation with 1 or 5 ng/mL TGF-β1, respectively for an additional hour. The amount of total Smad2/3 or Smad3 was used as internal loading control.
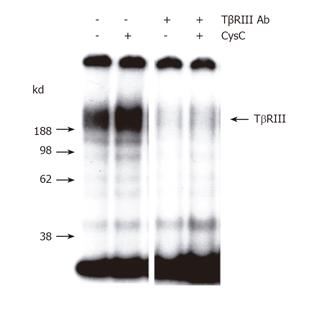
Figure 4 Effect of rhCysC on TGF-β1 binding to beta-glycan.
Cultured HSCs were incubated for 1 h with or without rhCysC or an antibody specific for the extracellular domain of beta-glycan followed by the application of [125I]-TGF-β1. The ligand was chemically cross-linked to the receptor and subsequently TβRIII was immunoprecipitated, separated by SDS-PAGE, and visualized by autoradiography. Control experiments including a 200-fold excess of unlabeled TGF-β1 showed no receptor labeling (data not shown).
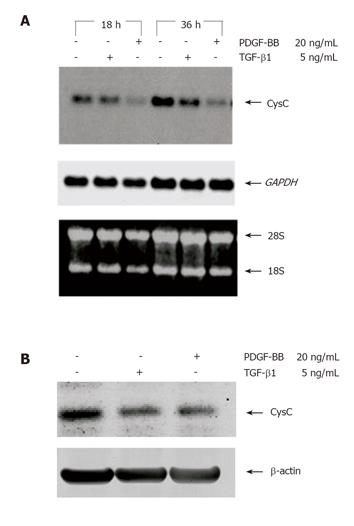
Figure 5 Effect of PDGF-BB and TGF-β1 on CysC expression in HSCs.
A: Cultured HSCs were serum starved to 2 mL/L FCS followed by treatment with or without PDGF-BB or TGF-β1 for 18 and 36 h, respectively. Total RNA (5 µg/lane) was isolated and analyzed by Northern blot for CysC expression. As internal loading controls, the 18S and 28S rRNA as well as signals obtained after hybridization with a GAPDH-specific probe were shown; B: for CysC protein expression, cells were treated with or without PDGF-BB or TGF-β1 for 72 h and protein lysates (20 µg/lane) were subjected to Western blot analysis using an antibody specific for CysC. The expression of β-actin was used as internal protein loading control.
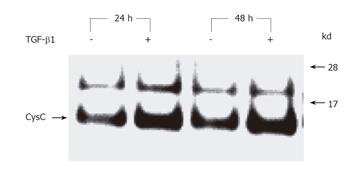
Figure 6 TGF-β1-induced CysC secretion in HSCs.
Serum-starved HSCs were treated with TGF-β1 (10 ng/mL) for 24 or 48 h and the cell-free supernatants were subjected to immunoprecipitation using goat antibody P-14 directed against CysC. Subsequently, the precipitates were analyzed for CysC expression by Western blotting using an independent primary rabbit antibody directed against CysC and visualized with a goat anti-rabbit IgG-HRP conjugate.
- Citation: Gressner AM, Lahme B, Meurer SK, Gressner O, Weiskirchen R. Variable expression of cystatin C in cultured trans-differentiating rat hepatic stellate cells. World J Gastroenterol 2006; 12(5): 731-738
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.731