INTRODUCTION
Gastric outlet obstruction (GOO) can be classified into mechanical obstruction—either from benign causes such as peptic ulcers, strictures, or tumors, or malignant causes like gastric, pancreatic, or duodenal cancers—and motility disorders, the most common being gastroparesis, often associated with diabetes, prior surgeries, or infiltrative diseases. Peptic ulcer disease was likely the most common cause of GOO before the identification of Helicobacter pylori and the invention of proton pump inhibitors. These advancements revolutionized the treatment of peptic disorders and resulted in their nearly complete disappearance as a cause of GOO. Today, malignant GOO has become more prevalent, with pancreatic cancer being the leading cause. Benign strictures are treated with endoscopic dilation techniques, but this approach can be risky and not long-lasting[1]. Placing temporary metal stents is another option; however, it carries risks and disadvantages. Some authors utilize lumen-apposing metallic stents (LAMS) for short benign strictures, demonstrating optimal results[2]. In most centers, malignant GOO cases are addressed by placing a permanent uncovered duodenal metal stent. The procedure is straightforward and safe, leading to symptom resolution in most instances. Major issues include stent-related complications, recurrent obstruction, and the need for re-interventions in up to a quarter of cases[3]. Surgical gastrojejunostomy (GJ) is a viable option, commonly performed for the durable and definitive relief of symptoms in benign and malignant GOO. This invasive procedure is associated with significant morbidity (13%-50%) despite showing similar outcomes to stenting concerning technical and clinical success, resuming oral intake, and length of hospital stay[4,5]. Most authors agree that surgical GJ offers superior long-term efficacy; however, periprocedural complications and delayed gastric emptying continue to pose significant challenges. Surgical GJ is justifiable when life expectancy exceeds three months[6]. Given the high morbidity associated with surgical bypass, endoscopists were intrigued for many years by the concept of creating gastrojejunoanastomosis endoscopically. The concept was introduced in 2002[7], but the first successful endoscopic ultrasound (EUS)-guided gastroenterostomy in a pig was reported more than 10 years later, in 2012[8]. Since then, a large amount of data has been collected, demonstrating that the procedure offers numerous advantages over surgical bypass and duodenal stenting, making it a viable first-line therapy in nearly every case of GOO. The indications have been broadened to include benign diseases, afferent loop syndrome, and other previously challenging conditions. Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) remains a complex and high-risk procedure, primarily conducted in tertiary referral centers. Many questions remain unanswered, particularly regarding long-term management and follow-up in benign cases, as well as the procedure's learning curve, training, and standardization. It remains unclear which technique is the best, who should perform the procedure, and how to manage intraprocedural complications. Improving safety is urgently needed. In this article, we will discuss the current role of EUS-GE in the management of GOO and provide data about available techniques, indications, results, comparisons with other modalities, adverse events, issues, and perspectives on future directions.
HISTORICAL DATA
The concept of EUS-guided gastroenteroanastomosis was first introduced by Fritscher-Ravens et al[7] in 2002, who used a through-the-scope suturing device under EUS guidance to create a gastroenteric anastomosis in pigs[8]. However, due to the complexity of the technique, further development stagnated for nearly a decade. A breakthrough occurred in 2012 when Binmoeller and Shah successfully performed EUS-GE in animal models using fully covered bi-flanged LAMS (AXIOS, Xlumena). The procedure achieved a 100% technical success rate without adverse events and resulted in mature fistulas upon follow-up[9]. Despite this progress, the technique remained technically demanding due to multiple procedural steps. In 2013, Itoi et al[10] introduced a novel approach using a double-balloon enteric tube and a specially designed bilateral stent, further simplifying the procedure in animal models. The LAMS and double-balloon techniques showed promise, with LAMS appearing more favorable due to its design tailored to create secure, fully covered, and leak-proof anastomoses. Early human applications explored alternative methods, including magnetic compression anastomosis[11] and NOTES-based procedures using electrocautery-enhanced LAMS[12]. Although technically successful, these approaches were limited by complexity and did not enter routine practice. Khashab et al[13] published the first clinical series of EUS-GE using LAMS in 2015. They reported a 90% technical and 100% clinical success rate in 10 patients, with no serious adverse events and sustained symptom resolution at 150 days of follow-up. This study marked the transition of EUS-GE from experimental to clinical practice.
INDICATIONS AND CONTRAINDICATIONS
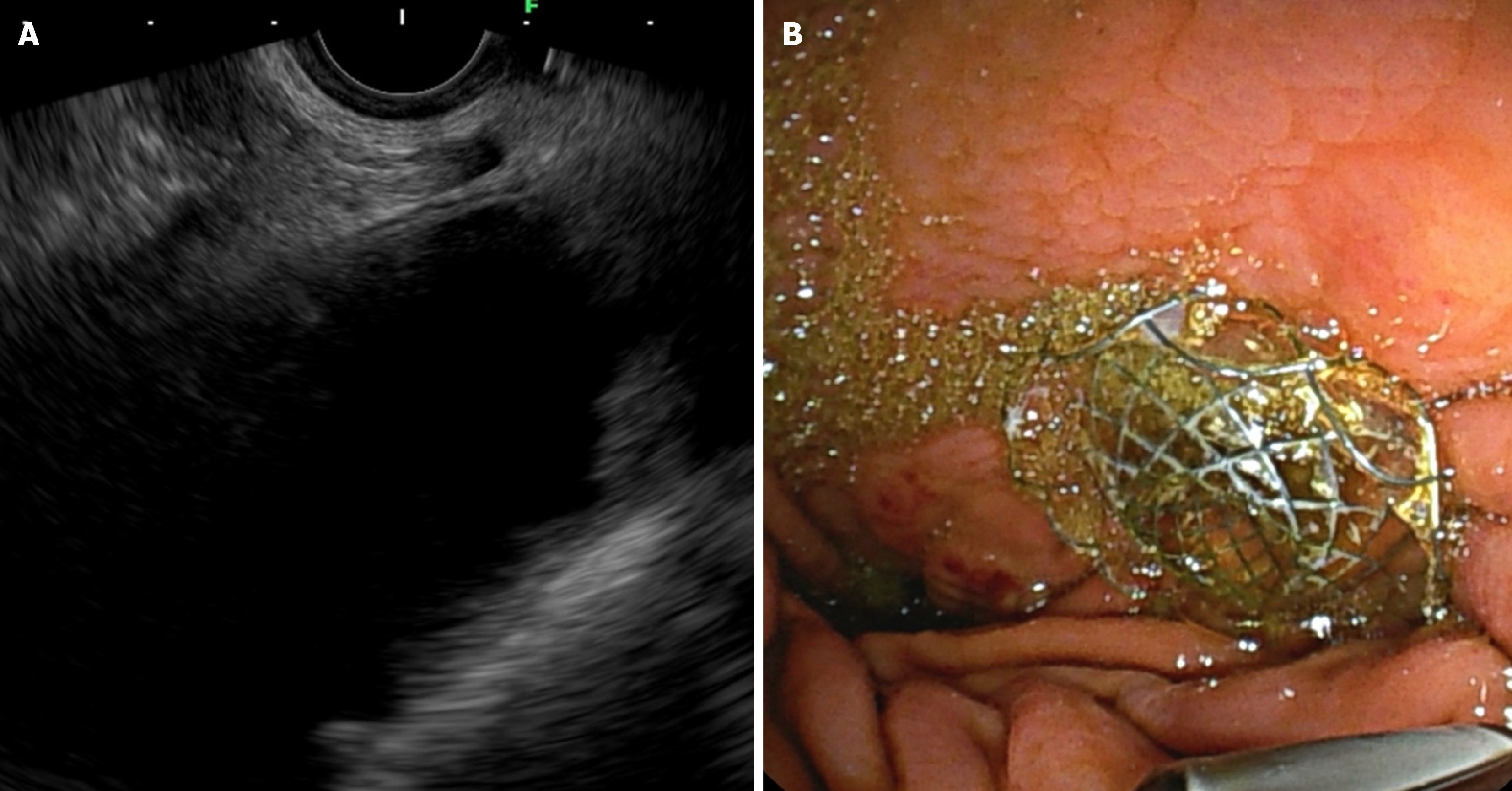
The EUS-GE is primarily used to treat malignant GOO. Currently, its indications have expanded to include benign conditions such as peptic ulcer disease, duodenal strictures resulting from chronic pancreatitis, and compressions due to peripancreatic fluid collections. EUS-GE has been successfully performed in rare cases involving Morbus Crohn, duodenal tuberculosis, and altered surgical anatomy. Reports indicate successful treatment of afferent loop syndrome using EUS-GE. Afferent loop syndrome is a complication of gastrointestinal surgery that can arise from either malignant or benign underlying diseases. Endoscopic stenting is not always a viable option, and repeated surgeries can be excessively invasive, particularly for vulnerable oncologic patients (Figure 1). In these scenarios, EUS-GE provides a suitable minimally invasive alternative. In cases of concurrent malignant duodenal and biliary obstruction, creating a gastroduodenostomy using LAMS may help facilitate access to the papilla of Vater, enabling endoscopic biliary decompression. An important technical prerequisite is to localize a suitable target small bowel loop as close to the gastric wall as possible. A distance greater than 2 cm is discouraged, as it may result in incomplete lumen apposition and anastomotic leaks. Tumor infiltration of the gastric wall, particularly at the puncture site, could make the procedure impossible or hazardous. Similarly, tumor extension to the fourth part of the duodenum or the ligament of Treitz may jeopardize the procedure. Strictures at the distal ends of the small or large intestine should be thoroughly assessed. Ascites is not a contraindication for the procedure, but it should be drained before the intervention to reduce the risk of complications[14,15].
Figure 1 Endoscopic ultrasound-guided gastroenterostomy in afferent loop syndrome.
А: Echoendoscopic view demonstrating the afferent loop; В: Fluoroscopic view confirming the positioning and deployment of the stent.
TECHNIQUES
Many techniques can be employed to create endoscopic or echoendoscopic gastroenteroanastomosis. These methods require only general anesthesia, antibiotic prophylaxis, and either a linear echoendoscope or a combination of an echoendoscope, a regular gastroscope, and a double-channel scope[16-18]. In all cases, bi-flanged LAMS is utilized. A crucial step in every technique is the proper identification and stabilization of the target jejunal loop. Some authors categorize the techniques into device-assisted (EUS-guided double balloon-occluded gastrojejunostomy bypass- EPASS), direct techniques, and the wireless EUS-GE simplified technique (WEST)[19,20]. Variations and several combinations of techniques are also possible. According to the traditional downstream method, upper endoscopy is performed first, followed by guidewire cannulation of the stricture. The scope is then withdrawn, and a dilation balloon is inserted over the guidewire in the jejunum. Endosonographically, the inflated balloon in the jejunal limb is identified and punctured with a 19-gauge needle. A second guidewire is inserted through the needle downstream into the jejunum, and over this guidewire, the LAMS is deployed, creating a stable gastroenterostomy. This technique can be modified into the so-called “rendezvous” method: The guidewire, inserted through the needle after puncturing the balloon, is trapped in the dilation balloon or with the so-called “snare-over-balloon device”[13]. Using a stone extraction basket is also an option. The guidewire is then withdrawn through the stricture and out of the mouth. The LAMS is positioned over this guidewire while pulling it for stability[19]. Based on this technique, the guidewire is pulled out of the mouth to perform the so-called “retrograde EUS-GE enterogastrostomy”. A therapeutic gastroscope is then inserted into the jejunum over the guidewire, and the LAMS is placed under direct vision, initially opening the gastric flange. This technique is challenging and carries an increased risk of perforation when traversing the GOO[19]. The EPASS involves inserting a novel balloon occlusion catheter through the stricture into the jejunum. Two balloons are inflated (20 cm apart) to secure a segment of the jejunum between them, which is filled with contrast material and methylene blue. This portion of the jejunum is punctured under EUS guidance, and LAMS is deployed[19].
Another option is to use the direct technique, which is appropriate in cases of complete obstruction when the stricture cannot be traversed with a guidewire or any device. A linear EUS scope is positioned in the stomach, and a suitable small bowel loop is identified and punctured with a 19G needle, then filled with saline mixed with contrast and methylene blue through the needle. Afterward, LAMS is placed as usual. This technique carries risks, and several complications may arise. For instance, complications include tenting and failing to traverse the small bowel wall with the needle, displacing the loop, puncturing the submucosa, creating a submucosal bleb, and misdeployment of the stent, especially when using a guidewire, among others.
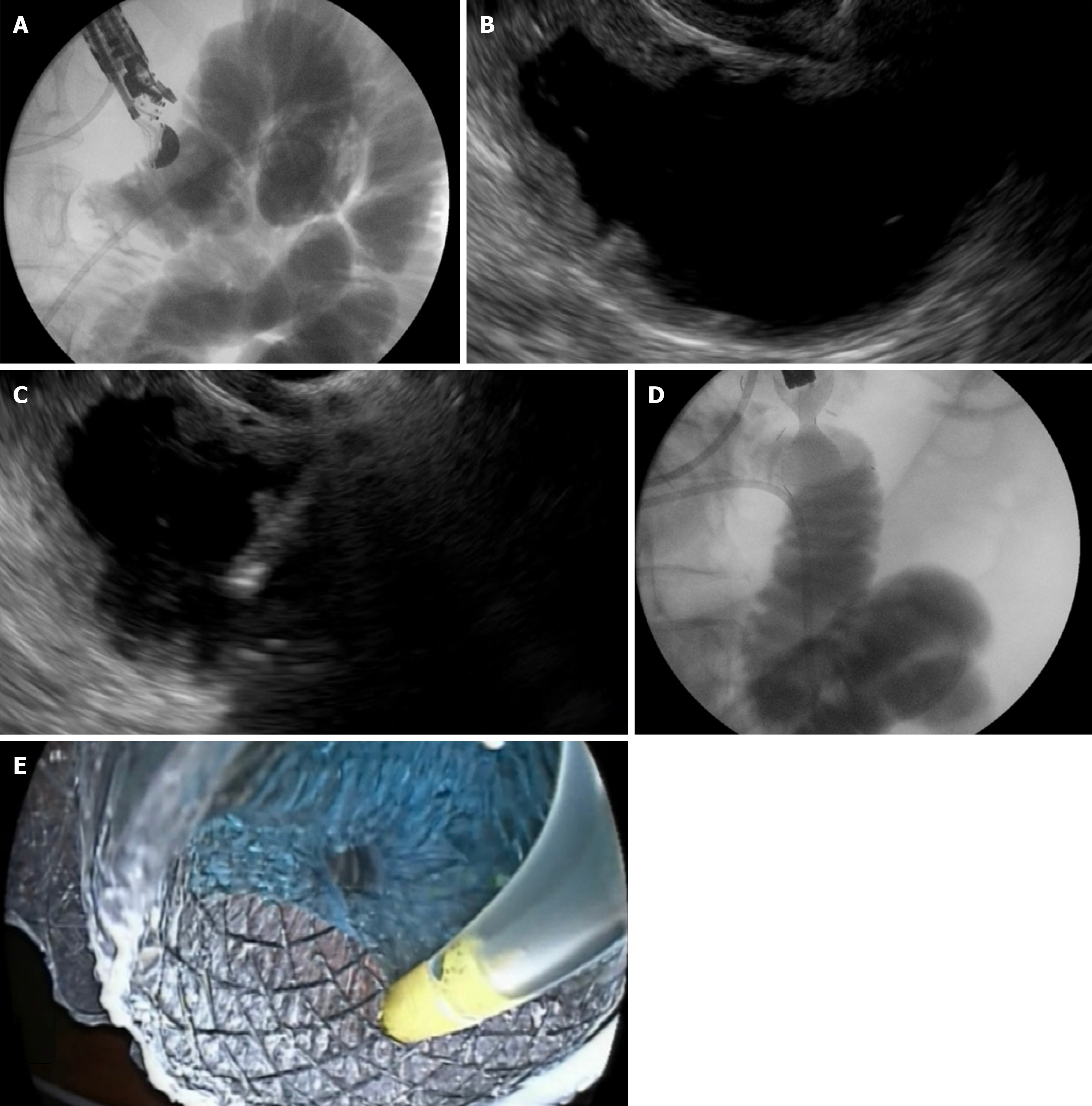
In the WEST technique (Wireless EUS-GE Simplified Technique) (Figure 2), a nasoenteric catheter is placed over a guidewire distal to the GOO in the small intestine. The bowel is filled with saline mixed with contrast and blue dye. Typically, a nasobiliary catheter is used, with 500-600 mL sufficient for a well-distended bowel. The LAMS is positioned under EUS guidance using the free-hand technique. The return of the blue solution through the stent indicates its correct placement[20].
Figure 2 Step-by-step visualization of endoscopic ultrasound-guided gastroenterostomy procedure.
A: Fluoroscopic view showing a 7Fr nasobiliary catheter placed into the jejunum over a guidewire; B: Echoendoscopic view displaying the duodenum and jejunum distended with contrast material and methylene blue before direct puncture; C: Real-time puncture of the stomach and jejunal wall using the freehand technique under endoscopic ultrasound guidance; D: Fluoroscopic confirmation of successful stent deployment; E: Endoscopic confirmation ensuring correct positioning and lumen patency of the deployed stent.
Some authors described the NOTES approach for performing EUS-GE. The gastric wall is punctured under endosonographic guidance using a 19G needle, and a guidewire is inserted into the peritoneal cavity, close to the ligament of Treitz. The EUS scope is carefully removed, keeping the guidewire in place. A double-channel gastroscope is inserted into the stomach over the guidewire. An incision in the gastric wall is then made using a needle knife, followed by balloon dilation of the tract. The endoscope enters the peritoneal cavity and identifies a suitable small bowel limb. A forceps is passed through one of the operating channels to hold and retract the limb, and a needle knife is passed through the second channel to perform an incision in the bowel wall. LAMS is then inserted over a guidewire in the jejunum, and the distal flange is deployed. The scope is then pulled back into the stomach, retracting the limb and opening the proximal flange[12,15].
The technique is not standardized, and each method has advantages and disadvantages. The recently published guideline for therapeutic endoscopic ultrasound by the European Society of Gastrointestinal Endoscopy does not recommend a specific technique[21]. The literature does not offer sufficient data to compare various EUS-GE methods.
In a retrospective comparative study published in 2023, Monino et al[22] compare the WEST technique and the direct technique regarding technical and clinical success, as well as adverse events. Seventy-one patients were included in the study. Technical success was higher in the WEST group [95.1% vs 73.3%; relative risk estimates from odds ratio (OR) 3.2, 95%CI: 0.94-10.9; P = 0.01]. The rate of adverse events was lower in the WEST group (14.6% vs 46.7%; OR: 2.3, 95%CI: 1.2-4.5; P = 0.007). There was no difference in clinical success.
Despite limited literature, the WEST technique shows the best results and is likely the preferred method.
OUTCOMES OF EUS-GE
Current literature shows that EUS-GE is highly effective, with technical and clinical success rates exceeding 90%, rivaling surgical bypass and duodenal stenting. The first published data from 2015 summarizes 10 cases (3 malignant, 7 benign) with a 90% technical success rate, 100% clinical success, no adverse events (AEs), an average procedure time of 96 minutes, and a hospital stay of 2.2 days[13]. These results indicate EUS-GE as a promising option for benign and malignant GOO. In a 2016 multicenter registry[23], 26 patients underwent EUS-GE for malignant (n = 17) and benign (n = 9) GOO. Technical success was 92%, clinical success 85%, and AEs occurred in 11.5%. Techniques varied across centers, including NOTES and non-electrocautery LAMS, requiring multi-step dilation[23]. A 2015 prospective study by Itoi et al[24] evaluated EPASS in 20 patients. The initial technical success rate was 81.8%, improving to 100% after switching from an over-the-wire method to a "freestyle" technique. Long-term LAMS patency surpassed duodenal stenting and, in some cases, surgical bypass. A 2021 retrospective case series reported on 33 patients (28 malignant, five benign), achieving 100% technical success, 91% clinical success, and a 30% AE rate, including stent migration, bleeding, and infection[25]. Twelve percent experienced fatal AEs, and 15 percent required reintervention (P value not reported). The average hospital stay was three days[25]. A 2023 multicenter study (25 patients) using enhanced electrocautery LAMS (Hot Spaxus) showed 100% technical success, 68% clinical success at 7 days, 100% at 30 days, an 11.4-hour average time to oral diet, and a 4-day hospital stay, with no AEs or stent dysfunction over 7.6 months[26]. A single-center study evaluating nasojejunal tube-assisted EUS-GE in 30 patients reported 96.67% technical success, 100% clinical success in the cases with technical success, and 6.6% AEs[27]. Stent misdeployment and late stent migration were successfully managed endoscopically[27]. A 2021 study examined EUS-GE with large-diameter (20 mm) LAMS in 31 patients, reporting 100% technical success and 93.55% clinical success[28]. Large-diameter LAMS proved highly effective in allowing a regular diet[28]. A 2022 multicenter study compared 15 mm and 20mm LAMS in 267 patients, showing no differences in technical (95.5%) or clinical success (> 80%). However, more patients with 20mm LAMS tolerated a solid diet (91.2% vs 81.2%, P = 0.04). AE rates were 12.4%, with 2% of AEs being fatal[29]. A 2018 study on benign GOO included 26 patients across five centers[30]. Technical success was 96.1%, clinical success was 84%, and AEs occurred in three cases (stent misdeployment and gastric leak requiring surgery). Reintervention was necessary in 4.8%[30]. A systematic review/meta-analysis (12 studies, 285 patients) found pooled technical success at 92%, clinical success at 90%, AEs at 12%, and reintervention at 9%[31]. A 2020 meta-analysis (26 studies, 1493 patients) reported 94% technical success, 89.9% clinical success, and a 13.1% AE rate[32]. A meta-analysis (12 studies, 290 patients) reported 93.5% technical success, 90.1% clinical success, and 11.7% AEs (mostly mild-moderate)[33]. Stent misdeployment was the most common AE, treated endoscopically in nearly all cases. Fatal AEs were 2.9%, occurring in fragile patients with advanced malignancies[33]. Despite most studies being retrospective, EUS-GE is an effective treatment for GOO. The freestyle technique minimizes misdeployment risk, and large-diameter LAMS improve dietary tolerance. AE rates range from 13% to 30%, with fatal AEs at 2%-12%, emphasizing the need for safety improvements.
EUS-GE VERSUS DUODENAL STENTING
Duodenal stenting (DS) is well-established, with high technical success and low AEs, allowing rapid oral intake. However, tumor ingrowth, tissue overgrowth, and frequent reintervention are limitations. Uncovered DS is unsuitable for benign cases, and uncovered stents are a suboptimal choice for oncologic patients with longer life expectancy. EUS-GE offers an alternative by creating an anastomosis away from the tumor and using fully covered LAMS in benign cases[34]. A 2020 meta-analysis (Chandan et al[35], five studies, 659 patients) comparing EUS-GE and DS found both had technical and clinical success rates > 90%, but EUS-GE had a lower reintervention rate (4% vs 24.6%). A 2023 meta-analysis (13 studies, 1762 patients) showed EUS-GE had higher clinical success (93.62% vs 85.57%), lower AE rates (8.97% vs 19.63%), and fewer reinterventions (3.77% vs 25.13%)[36]. A 2016 multicenter study (82 patients with malignant GOO) found no significant differences in technical (86.7% vs 94.2%, P = 0.2) or clinical success (83.3% vs 67.3%, P = 0.12)[37]. However, symptom recurrence and reintervention were significantly lower in the EUS-GE group (4% vs 28.6%, P = 0.015). A retrospective study (100 patients: 22 EUS-GE, 78 DS) found lower reintervention rates in the EUS-GE group (8.3% vs 32%, P = 0.021), with higher initial clinical success (95.8% vs 76.3%, P = 0.042)[38]. A 2024 propensity score-matched study (88 patients) reported treatment failure rates of 13/44 (DS) vs 4/44 (EUS-GE). Median failure time was 22 weeks for DS vs 76 weeks for EUS-GE (P = 0.002)[39]. No differences were observed in technical (> 90%) or clinical success (72.7% vs 79.5%). An international multicenter study (176 patients) found no difference in technical success, but EUS-GE had higher clinical success (91% vs 75%, P = 0.008). Stent dysfunction was significantly lower in EUS-GE (1% vs 26%), and AEs were lower, though not statistically significant (10% vs 21%, P = 0.09)[40]. The first randomized controlled trial (2024, 97 patients) compared EUS-GE (n = 48) and DS (n = 49) in malignant GOO[41]. Reintervention within six months was lower in EUS-GE (4% vs 29%, P = 0.002), with a risk ratio of 0.15 (95%CI: 0.04-0.61). Stent patency was longer, and one-month GOOS scores were better (2.41 vs 1.91, P = 0.012). There were no differences in 30-day mortality, technical success, quality of life, or AE rates (23% vs 24%).
STENT MISDEPLOYMENT
Stent misdeployment (SM) is a specific complication and likely the most feared aspect of EUS-GE. AEs associated with SM can be dangerous and challenging to manage. Most reported fatal complications are linked to SM, which is the main reason this procedure is neither very popular nor widely accepted nor implemented[42-48].
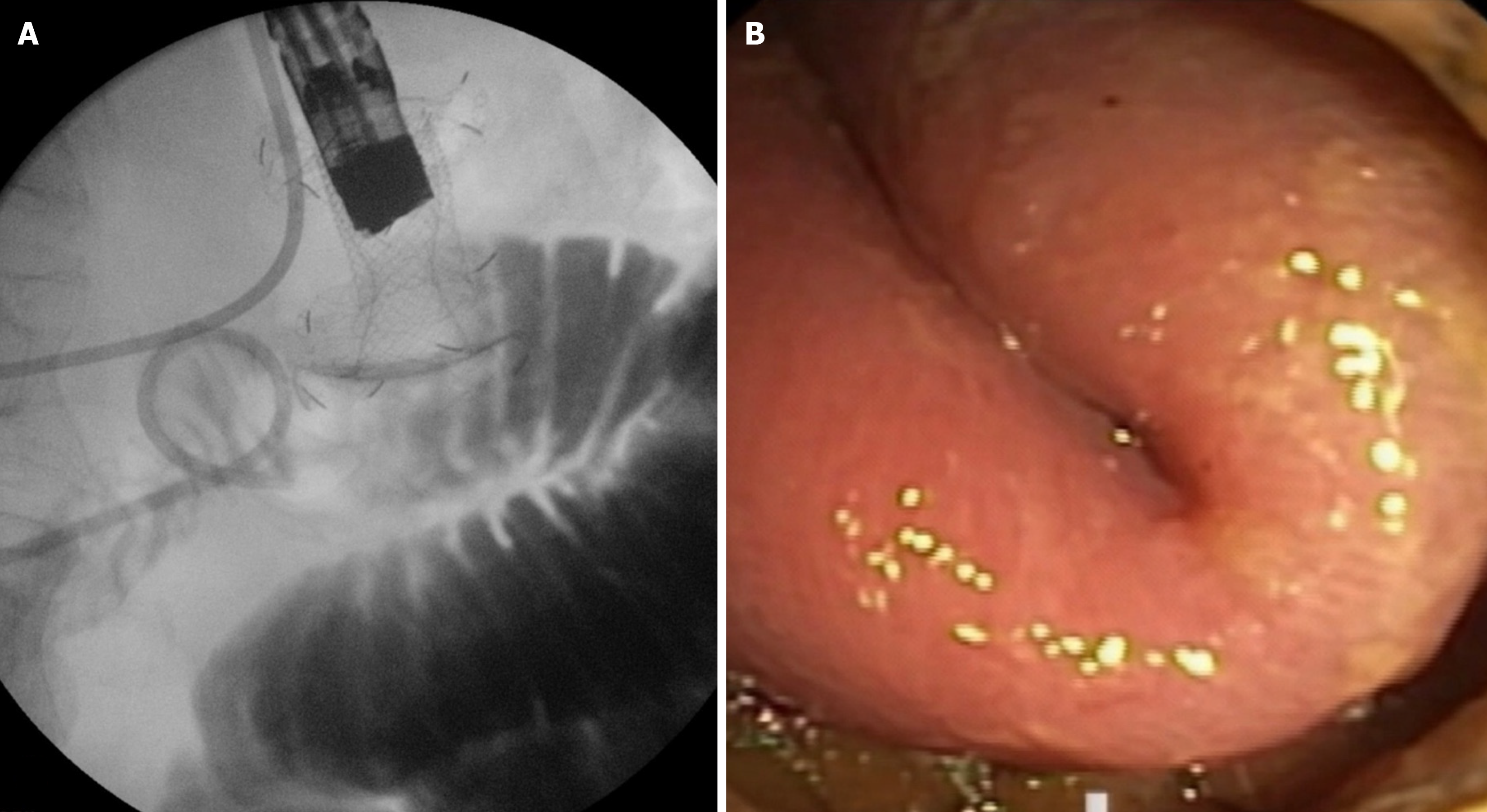
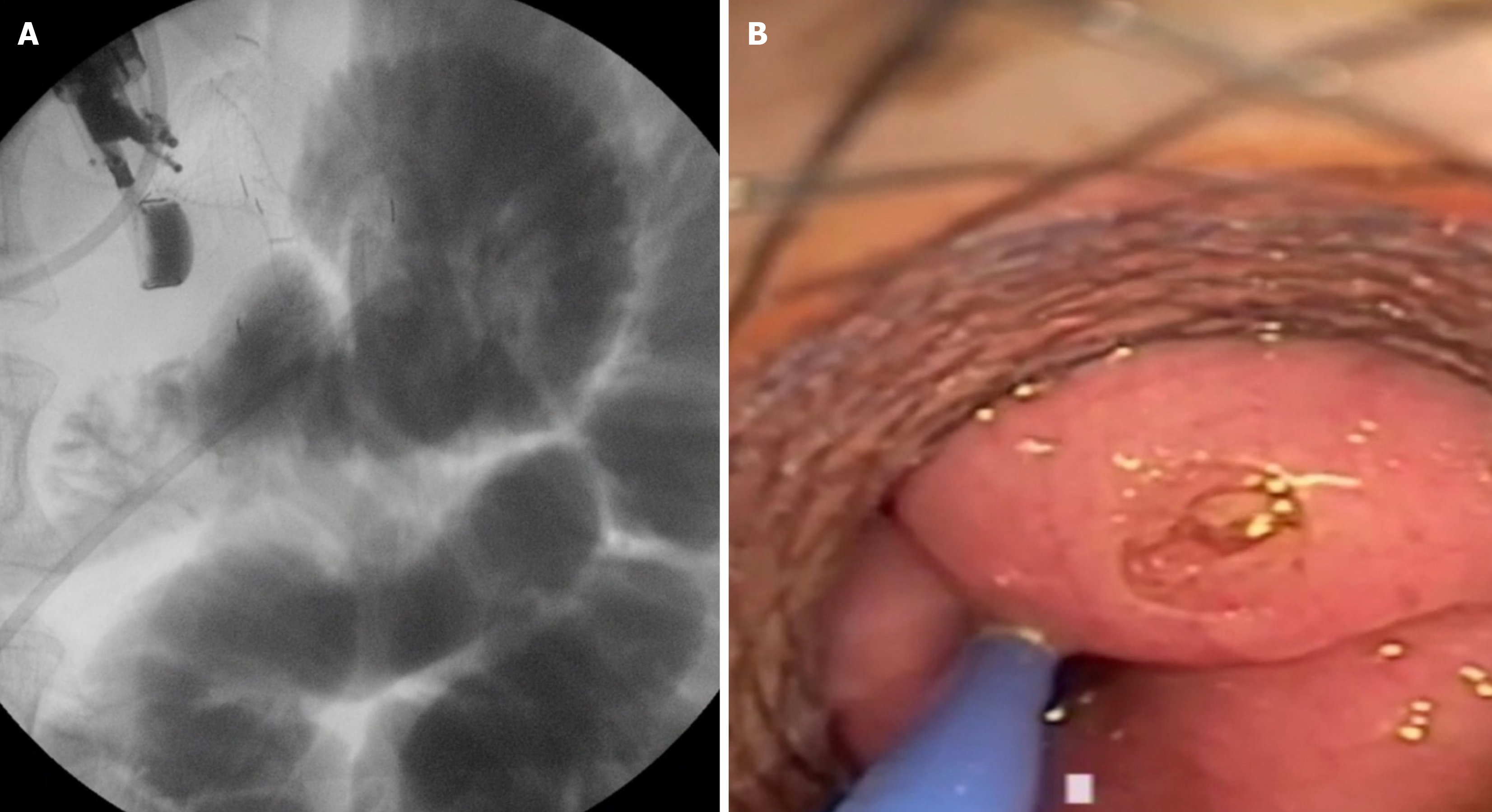
There is only one multicentric retrospective study that thoroughly examines SM and provides insights into its management[49]. It includes 16 tertiary centers and 467 procedures. The reported rate of SM is 9.85%. Interestingly, 73.2% occurred during the first 13 procedures and progressively decreased after the initial cases; only 17% occurred after 25 cases. Most SMs were managed endoscopically, and only 10.9% required emergency surgery. The authors propose a classification of SM, dividing them into four types. Type I occurs when the distal flange of the LAMS is deployed in the peritoneal cavity, while the proximal flange is in the stomach (Figure 3). The small bowel remains intact and shows no evidence of enterotomy. This is the most common type of SM, accounting for 63.1%. Most Type I SMs are classified as mild according to the ASGE lexicon. Treatment involves the endoscopic removal of the LAMS, followed by the closure of the gastric defect, either using an over-the-scope clip or a through-the-scope clip. The procedure can be repeated, or a duodenal stent may be placed. Emergency surgery is rarely necessary. Type II SM occurs when the distal flange of the LAMS is released into the peritoneum but with the presence of enterotomy (Figure 4). The reported rate is approximately 30.4%, making it the second most common type of SM according to the study by Ghandour et al[50]. Several endoscopic treatment strategies are feasible, including repeating the procedure and placing a new LAMS, using a NOTES approach, or placing a fully covered esophageal stent through the scope to bridge the gap between the enterotomy and the LAMS attached to the perforated stomach. In this case, surgery is also rarely required. Type III SM has been described in only one instance in Ghandour et al’s study (2.2%)[50]. This occurs when the distal flange of the LAMS is deployed and remains attached to the jejunal wall, while the proximal flange slips into the peritoneal cavity after being deployed in the stomach. Attempts at endoscopic salvage were made, but this case required emergency surgery. Type IV SM refers to the creation of a gastrocolic anastomosis. It is also rare, with only two cases reported: One recognized during the procedure and the other identified three weeks later. Both cases were successfully managed with endoscopic closure techniques. The proposed classification of SM enables interventional endosonographers to standardize the terminology and select the optimal treatment option according to the specific type of SM, as not all cases are identical. It is also crucial to highlight that the average frequency of SM is 10% (ranging from 2% to 27%), and surgical intervention is required in no more than 10% of instances. Ninety percent can be effectively managed conservatively or through endoscopic techniques[51].
Figure 3 Type I stent misdeployment.
A: Fluoroscopic view showing the distal flange of the lumen-apposing metal stent deployed in the peritoneal cavity while the proximal flange remains in the stomach; B: Endoscopic view confirming the incomplete anastomosis with the small bowel intact.
Figure 4 Type II stent misdeployment.
A: Fluoroscopic view demonstrating the distal flange of the lumen-apposing metallic stents deployed in the peritoneum with evidence of an enterotomy; B: Endoscopic view revealing the perforation at the small bowel site, requiring further intervention.
LEARNING CURVE
EUS-GE is a valuable option for treating GOO, which poses challenges for duodenal stenting and surgical gastroenterostomy. It has several advantages over these traditional interventions. The procedure is gaining popularity, although data on the learning curve for EUS-GE are limited. Defining the learning curve is crucial for developing training programs and establishing quality parameters for the procedure.
Data on the learning curve (LC) for EUS-GE were first reported by Jovani et al[52] in a retrospective study. The procedures performed by a single endoscopist were analyzed. The LC was defined as the number of cases needed to achieve proficiency and mastery. Cumulative sum (CUSUM) curve analysis was utilized. The authors included 73 patients, all treated by a single endoscopist. The mean procedure time served as the target value. Analyzing the CUSUM curve clarified that 25 cases were required to achieve proficiency, and 40 cases were needed to master the technique[52].
In another study by Tyberg et al[53], a prospective registry of consecutive patients who underwent EUS-GE performed by a single endoscopist was utilized. Twenty-three patients were included, and the median procedure time was 88 minutes. The cumulative sum chart indicated that the 7th procedure achieved 88 minutes of procedure time. In conclusion, seven procedures are necessary to achieve efficiency.
CONCLUSION
EUS-GE is an emerging modality for treating GOO that is more durable than duodenal stenting and offers several advantages over surgical GJ, particularly regarding morbidity, mortality, recovery time, costs, and the time required to resume oral intake. However, there remains no consensus on the periprocedural management of patients. The optimal method for performing EUS-GE is still unclear. Although early data on EUS-GE is promising, the procedure cannot yet be endorsed as the standard of care for GOO. Randomized studies are needed to compare EUS-GE with traditional techniques. Furthermore, there is limited data available on long-term stent patency, migration rates, and outcomes beyond several months. It remains uncertain how to manage patients with benign conditions long-term, including whether we need to remove or exchange the stent and how often. It is crucial to remember that patients with malignant diseases will live longer due to advancements in oncologic therapy, and we will soon face similar issues with them. Defining the learning curve is urgently needed to develop training programs and establish quality indicators for the procedure. Refining and simplifying the technique will enhance the procedure's safety and make its implementation feasible outside tertiary centers. Once accomplished, further expansion of indications will be the next step, including entering the fields of bariatric endoscopy and treating refractory gastroparesis as part of the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 154505; European Society of Gastrointestinal Endoscopy, No. 45909537.
Specialty type: Gastroenterology and hepatology
Country of origin: Bulgaria
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade D
Novelty: Grade B, Grade B, Grade D
Creativity or Innovation: Grade B, Grade B, Grade D
Scientific Significance: Grade B, Grade B, Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Gweon TG; Tsibouris P S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH