Published online Jun 28, 2020. doi: 10.13105/wjma.v8.i3.210
Peer-review started: February 28, 2020
First decision: May 29, 2020
Revised: June 10, 2020
Accepted: June 17, 2020
Article in press: June 17, 2020
Published online: June 28, 2020
Processing time: 130 Days and 5 Hours
In recent years, a wide range of gastrointestinal endoscopy techniques have been developed, such as endoscopic submucosal dissection (ESD) and endoscopic retrograde cholangiopancreatography (ERCP). Although ESD and ERCP have an important role in gastrointestinal and biliary diseases, each technique has its limitations. Hybrid techniques that combine endoscopic and surgical procedures have emerged that have the advantages of different procedures and negate their limitations at the same time. Laparoscopic endoscopic cooperative surgery and modified laparoscopic endoscopic cooperative surgery combine ESD and laparoscopic techniques to resect submucosal tumors with minimum resection area. Air leak test by intraoperative endoscopy can effectively identify a mechanically insufficient anastomosis and decrease the complication rate. The rendezvous technique that combines percutaneous transhepatic biliary drainage and endoscopy can be performed as a rescue approach for the treatment of biliary obstruction, stenosis and bile duct injuries. For patients with simultaneous presence of stones in the gallbladder and the common bile duct, the laparo-endoscopic rendezvous technique can perform ERCP and laparoscopic cholecystectomy at the same time and reduces the risk of pancreatic injury caused by ERCP. Biliobiliary and bilioenteric anastomosis using magnetic compression anastomosis is another choice for biliary obstruction. The most common used approach to deliver the magnets is by percutaneous-peroral tract. Laparoscopic-assisted ERCP is a safe and highly effective therapy for patients who develop biliary diseases after Roux-en-Y gastric bypass surgery.
Core tip: A wide range of hybrid techniques that combine two or more of endoscopy, laparoscopy and percutaneous transhepatic biliary drainage have been developed. The hybrid techniques include laparoscopic and endoscopic cooperative surgery, air leak test by intraoperative endoscopy, magnetic compression anastomosis, the rendezvous technique and laparoscopic assisted endoscopic retrograde cholangiopancreatography. This review aims to introduce these hybrid techniques and their applications for the treatment of gastrointestinal and biliary diseases.
- Citation: Feng YL, Li J, Ye LS, Zeng XH, Hu B. Combined endoscopy/laparoscopy/percutaneous transhepatic biliary drainage, hybrid techniques in gastrointestinal and biliary diseases. World J Meta-Anal 2020; 8(3): 210-219
- URL: https://www.wjgnet.com/2308-3840/full/v8/i3/210.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i3.210
In recent years, a wide range of gastrointestinal endoscopy techniques have been developed, such as endoscopic submucosal dissection (ESD) and endoscopic retrograde cholangiopancreatography (ERCP). ESD is now widely carried out for early neoplastic lesions of the gastrointestinal tract and has advantages of minimal invasion, low cost, patient tolerance and better quality of life of patients[1]. However, ESD is confined to incision of mucosal and submucosal layers. Laparoscopy is able to perform the full thickness resection, but sometimes laparoscopy cannot determine the precise incision line from the peritoneal cavity. ERCP has matured into an essential technique for managing biliary and pancreatic disorders, but it can be technically difficult in some situations (e.g., completely biliary obstruction and altered anatomy) where percutaneous transhepatic biliary drainage (PTBD) may get access to the biliary tree.
In brief, none of these techniques can overcome all the difficulties encountered in the clinical practice. Therefore, many hybrid techniques that combine two or more of endoscopy, laparoscopy and PTBD have been developed that have the advantages of different procedures and negate their limitations at the same time. This review aims to introduce these hybrid techniques and their applications for the treatment of gastrointestinal and biliary diseases.
Resecting the gastrointestinal tumors
Gastrointestinal submucosal tumors (SMTs) are frequently seen in patients undergoing upper gastrointestinal endoscopy[2], and gastrointestinal stromal tumor is the most common type of SMT[3]. Usually, SMTs are treated by surgical approaches. Laparoscopic wedge resection has been confirmed a feasible option for SMT < 5 cm[4]. However, localization of small and intraluminal growing SMTs is difficult from the peritoneal cavity. As a result, excessive resection is needed to ensure the negative surgical margins, which can cause the deformity of the remaining stomach and gastric malfunction.
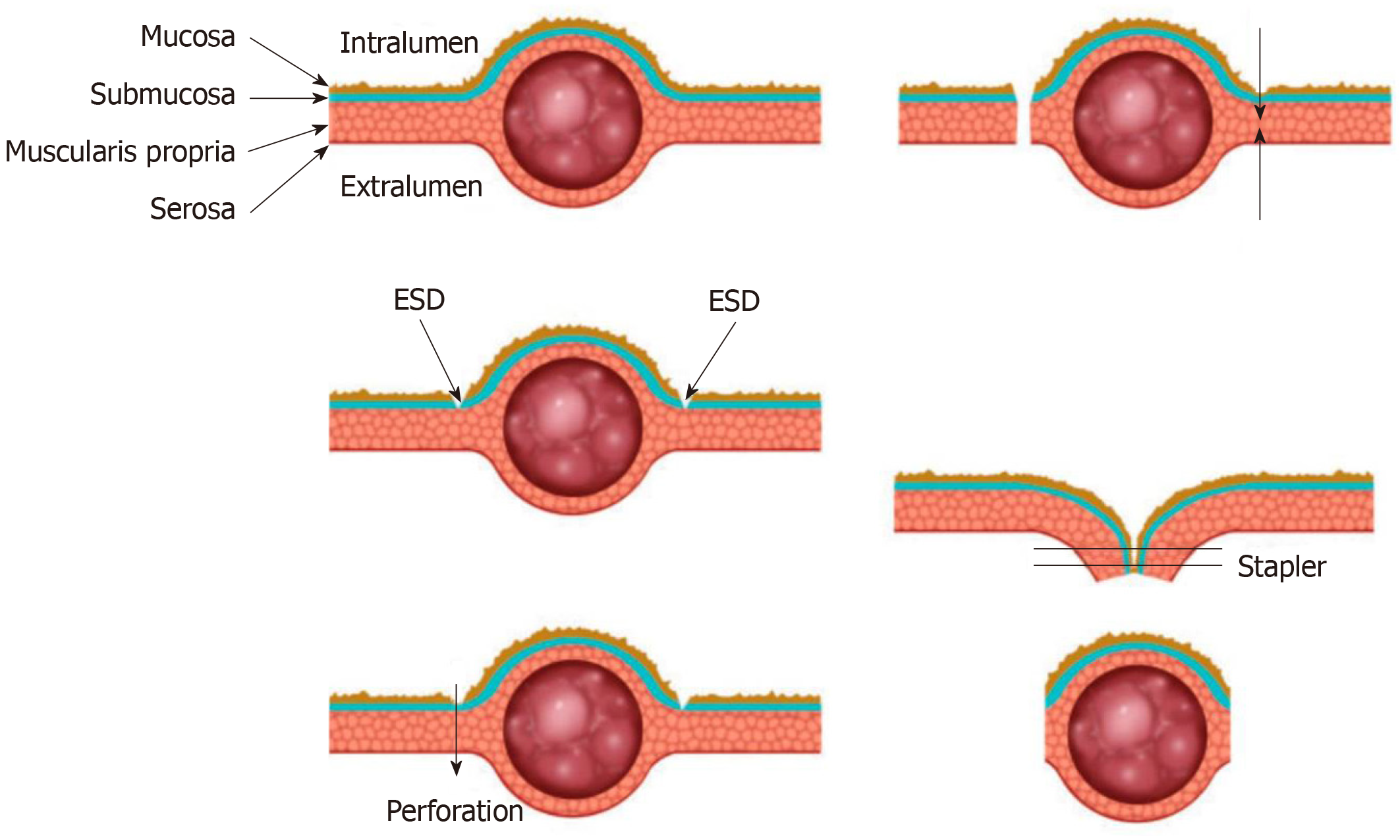
In order to decrease the resection area as much as possible, Hiki et al[5] firstly reported the conventional laparoscopic and endoscopic cooperative surgery (LECS) where the resection is performed jointly by the endoscopy and laparoscopy. Endoscopic submucosal dissection is used in this surgery. Firstly, the periphery of the tumor is marked by coagulation. Then three-fourths of the marked areas are cut down to the submucosal layer after submucosal injection. Next, a perforation of the gastric wall is created artificially, and the tip of the ultrasonically activated device is inserted into the perforation hole. Then three-fourths of the seromusclar layer is dissected along the incision line. After the tumor is inverted into the abdominal cavity, the serosa of the unresected tumor is grasped and retracted, and finally the incision line is closed by a laparoscopic stapler (Figure 1).
After the emergence of LECS, several modified LECS were developed excessively, including inverted LECS[6], laparoscopic assisted endoscopic full thickness resection[7,8], combination of laparoscopic and endoscopic approaches for neoplasia with non-exposure technique[9], non-exposed wall invasion surgery (NEWS)[10] and closed-NEWS[11]. Based on whether the gastric wall is open during the surgery, these techniques can be divided into exposed technique and non-exposed technique. Table 1 compares characteristics of these two techniques. Though there are differences among these techniques, in general they all consist of two main parts that are the ESD technique and the laparoscopic surgery. The endoscopist determines the precise margin of the tumor, and then the resection is performed jointly by the endoscopy and laparoscopy.
| Exposed LECS | Non-exposed LECS | ||
| Techniques | Conventional LECS, Inverted LECS, LAEFR | CLEAN-NET | NEWS, Closed-NEWS |
| Opening of gastric wall | Yes | No | No |
| Advantages | Minimum resection area; more evidence of efficacy and feasibility | Non-exposed | Precise resection of both serosal and mucosal layers |
| Limitations | Possibility of tumor seeding and gastric juice contamination into the abdominal cavity | Potential risk of margin positive or excessive resection | Not applicable to tumor > 3 cm due to peroral retrieval |
As a less invasive approach, LECS has advantages of minimum resecting area and reserving function of organs at the greatest extent. In addition, LECS can be applied to tumors located in the esophagogastric junction or pyloric ring that cannot be removed by laparoscopic wedge resection[12,13]. The exposed LECS has a risk of tumor seeding and contamination of gastric juice in the peritoneal cavity due to the artificial perforation of the gastric wall[14]. The non-exposed LECS avoids the gastric open during the surgery and thus expands the indication of LECS for gastric epithelial neoplasms[15]. A series of studies on LECS and modified LECS have been conducted, showing that these techniques are feasible and safe for gastric SMTs[2,16-19].
Besides gastric tumors, LECS has been used to resect tumors in other parts of the gastrointestinal tract. There are a few reports of LECS for early superficial duodenal tumors (SDT), showing that this technique may be safe and feasible and could be an option for surgical SDT resection[37-25]. Standard treatment for SDT has not been established. Though ESD has been considered safe and effective for early gastric tumors, ESD for early duodenal cancer is associated with a high risk of perforation during and after surgery as a result of the narrow lumen and thin walls of the duodenum[26,27]. In LECS, the laparoscopic suture and monitoring may help to prevent the occurrence of perforation. Therefore, compared to ESD, LECS might be a safer approach for the treatment of SDT. For colon polyps and colorectal tumors that cannot be removed by conventional endoscopic techniques, LECS may also be an alternative choice[28].
Endoscopic localization is essential in both endoscopic procedures and surgeries. In laparoscopy, endoscopic tattooing that uses suspensions of carbon particles is a commonly used approach to localize the tumor during laparoscopy. However, intraoperative endoscopic localization may be difficult to arrange between endoscopic and surgical teams. Hu et al[29] reported performing tumor resection using an ultrasonic scalpel through a gastric fistula formed by percutaneous endoscopic gastrostomy. Some novel methods may provide other choices for preoperative localization of tumors. Ohdaira et al[30] applied a magnet-string-clip system to gastric mucosa in 15 patients with early gastric cancer, and the tumor site was detected in all cases during laparoscopic gastrectomy. Hyun et al[31] introduced an endoscopic fluorescent band ligation method. The fluorescent rubber bands were endoscopically placed on the mucosa of porcine stomachs and colons, and the bands were clearly identified using the near-infrared fluorescence laparoscopy system during subsequent surgery.
Anastomotic leak (AL) is one of the most frequent and devastating complications after many gastrointestinal surgeries[32,33]. Among measures that have been used to prevent AL, intraoperative air leak test (ALT) is the most widely used to identify a mechanically insufficient anastomosis[32]. The bowel proximal to the anastomosis is clamped, and then air is insufflated into the bowel lumen using a syringe or endoscope with the anastomosis under irrigation of saline. Leakage is detected by the bubbles arising from the anastomosis.
Compared to syringe, the intraoperative endoscopy can simultaneously provide air insufflation with adequate and steady pressure for ALT[33]. More importantly, it enables real-time assessment of anastomotic integrity, bleeding, vascular insufficiency and allows for repeatability if a leak is repaired[34]. The intraoperative ALT is easy, quick and associated with little or no risk[35]. One prospective randomized controlled trial showed that intraoperative endoscopy had significant lower rate of AL and lower need for reoperation than simple visual inspection in laparoscopic Roux-en-Y gastric bypass (RYGB)[36]. For colorectal surgeries, intraoperative endoscope has also been confirmed safe and effective[37-39].
ERCP has become the first choice of treatment for many biliary diseases, including bile duct injuries, obstruction and stenosis. Endoscopic treatment of the biliary stricture relies on initial passage of a guidewire across the stricture, followed by subsequent stricture dilation and stent placement[40]. However, this maneuver is not possible when the biliary duct is completely obstructed or transected. The rendezvous technique could be a choice for the recanalization of bile duct in this situation.
The rendezvous technique that combines endoscopic and percutaneous transhepatic approach was initially described for duodenoscopic sphincterotomy in the 1980s[41,42]. A guide wire is placed via the PTBD route, advanced into the duodenum, then grasped by grasping forceps or snares of the duodenoscope and pulled out of the duodenoscope. Then a catheter is advanced into the bile duct over the guidewire for drainage. Stents or balloons can also be placed to dilate the stricture of the bile duct. The procedure can also be completed in a reverse way where a guide wire placed endoscopically is grasped and pulled out through the PTBD route[43,44].
Because the guide wire may be damaged during withdraw and the procedure is cumbersome, a few modified techniques have been developed to avoid these problems, such as parallel cannulation technique[45-47]. With the advance in endoscopic ultrasonography (EUS) technology, the EUS guided rendezvous technique has been developed, where the bile duct is punctured under the EUS guidance, and a guide wire is advanced antegrade through the papilla to perform a transpapillary procedure[48].
The rendezvous technique increases the success rate of biliary duct cannulation and facilitates the treatment of biliary tract diseases. It is reported with a high technical success rate of 80%-100%[49-53] and a significantly lower complication rate when compared to percutaneous transhepatic cholangiography[53]. The rendezvous technique can also be used to establish the continuity of the bile duct when surgical bile duct injury occurs, with a high primary success rate and a long term success rate of 55%[44].
Besides recanalization of bile ducts, the rendezvous technique is also reported to remove stones in the bile ducts[43,54,55]. Lithotomy by percutaneous transhepatic approach was performed firstly, but there were stones remaining in the intrahepatic duct or common bile duct (CBD). After the guide wire was grasped, the endoscopy was inserted further with the guide wire into the hepaticojejunostomy anastomotic region or CBD and lithotomy was performed for the remaining stones.
Besides the rendezvous technique, biliobiliary and bilioenteric anastomosis using magnetic compression anastomosis (MCA) is another choice for the treatment of severe biliary strictures or complete obstructions. The working principle of MCA is that the magnetic compression force leads to gradual tissue necrosis within magnets while with tissue healing at the edge of the magnet simultaneously[56].
Two magnets are needed for the procedure, parent and daughter magnet. These two magnets can be delivered by a variety of methods, but the most common route is by the percutaneous-peroral approach[57]. One magnet is delivered through the PTBD route into the anastomosis site, and the other magnet is delivered endoscopically. When inserting a magnet into the CBD, full sphincterotomy or balloon dilation is usually required, and a metal stent may be inserted to facilitate further magnet delivery[58,59]. After recanalization and magnets removal, biliary stents can also be placed to prevent restenosis[59].
Bilioenteric anastomosis is a common operation to bypass extrahepatic biliary obstructions[60]. The conventional hand-sewn is time-consuming and associated with a high risk of complications[60]. In contrast, the MCA is considered to be associated with little complication because fistula formation after MCA requires a relatively long time. Also, there is no dilation of fibrotic tissue in the progress of fistula formation, so the risk of restenosis upon recoiling of fibrotic tissue is low[57].
RYGB surgery is one of the most common bariatric procedures to treat obesity[61]. However, the patients have a high risk of biliary disease with up to 40% developing symptomatic cholelithiasis[61,62]. In addition, ERCP is challenging due to the surgically altered anatomy. Laparoscopic-assisted ERCP (LA-ERCP) is an option for these patients.
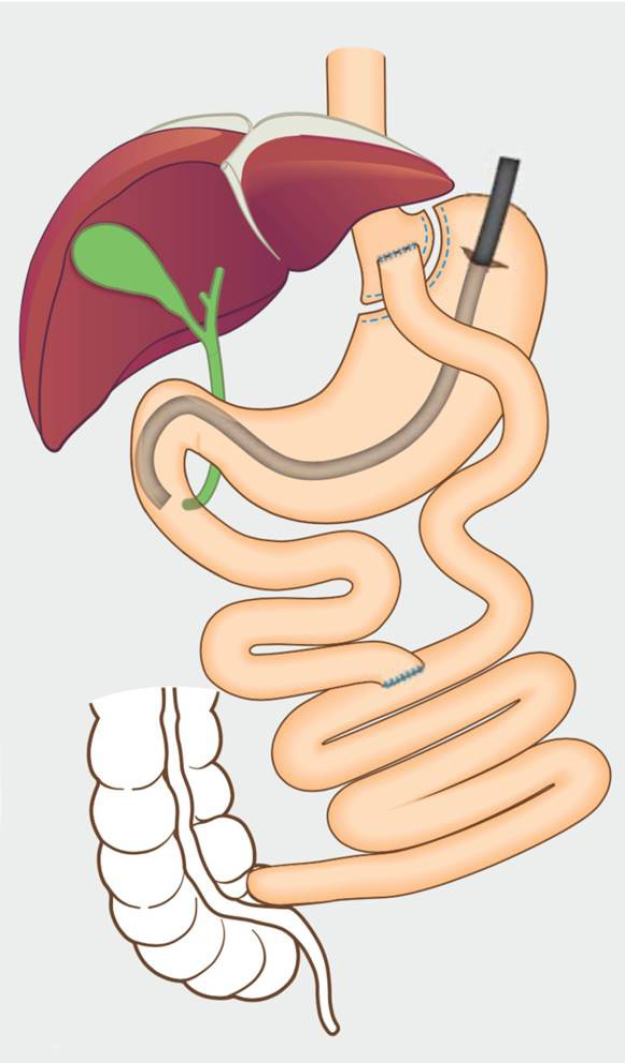
A gastrostomy is performed by the laparoscopy, and a port is placed into the remnant stomach. Then ERCP is performed by a conventional side-view duodenoscope via this port (Figure 2). After completion of the procedure, the port is removed, and the defect is closed by a suture or stapler. The transgastric route is commonly used to perform the LA-ERCP, and transjejunal route has also been reported[63]. Because the jejunal loop can easily reach the abdominal wall, the transjejunal LA-ERCP can be performed in all Roux-en-Y cases, even when the gastric remnant is not attainable. However, the transjejunal route needs a colonoscope to reduce the risk of intestinal injuries as a result of limited visual field of side-viewing of the duodenoscope.
LA-ERCP is a safe and highly effective therapy for patients who develop biliary diseases after RYGB surgery[64]. One advantage of LA-ERCP is the high successful rate, which was reported to be approximately 90%-100%[65]. Another one is that the successful rate remains high in long-limb reconstruction cases because a limb length of > 150 cm is associated with a high failure rate in other ERCP techniques[66]. In addition, LA-ERCPs would be favored if the patient also requires cholecystectomy. Therefore, LA-ERCP is preferred in patients with long limbs who require concomitant cholecystectomy[65].
The simultaneous presence of stones in the gallbladder and the CBD is a common clinical circumstance[67]. ERCP and laparoscopic cholecystectomy (LC) are considered as standard approaches to treat CBD stones and gallstones, respectively[68,69]. To perform ERCP and LC at the same time, the rendezvous intraoperative ERCP with transcystic guide-wire-assisted cannulation technique was developed as a one-stage intervention[70,71]. An antegrade guidewire is inserted and advanced through Vater’s papilla into the duodenum by a surgeon. Subsequently, the guidewire is grasped by a snare and pulled out through the working channel of the duodenoscope and then cannulation of the CBD is performed.
The major advantage of the rendezvous procedure is a lower risk of pancreatic injury caused by the ERCP. The transcystic guide wire facilitates the endoscopic procedure and thus ensures elective CBD cannulation and avoids the inadvertent cannulation of the pancreatic duct. In addition, the antegrade approach avoids the problem of discordant patient positioning encountered when ERCP and LC are performed at the same time but separately. A recent meta-analysis compared different combinations of laparoscopic and intraoperative techniques (LC plus preoperative, intraoperative and postoperative ERCP and LC plus laparoscopic CBD exploration) and showed that the rendezvous approach was associated with the highest rates of safety and success[67]. The major limitation is that an experienced endoscopist may not be available for the procedure, and it may be difficult to arrange and carry out the rendezvous procedures in the operating room[68,72]. Moreover, using intraoperative cholangiography to detect CBD stones is essential before performing the rendezvous procedure[68]. Therefore, in centers where preoperative ERCP is routinely used to detect CBD stones, this technique is not applicable.
A wide range of hybrid techniques have been developed for the treatment of gastrointestinal and biliary diseases. These techniques expand the indications of therapeutic endoscopy, make it easier and safer to perform difficult procedures and decrease the agony of patients. Some of the techniques are only reported in few cases and further detailed evaluation of feasibility and efficacy is needed. For those that have been confirmed safe and effective, how to choose between hybrid techniques and conventional methods could be difficult. Further prospective investigations should be conducted to determine the best treatment options.
| 1. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 2. | Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A, Iizuka T, Udagawa H, Kaise M. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (9)] |
| 4. | Goh BK, Goh YC, Eng AK, Chan WH, Chow PK, Chung YF, Ong HS, Wong WK. Outcome after laparoscopic versus open wedge resection for suspected gastric gastrointestinal stromal tumors: A matched-pair case-control study. Eur J Surg Oncol. 2015;41:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 355] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 6. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Abe N, Takeuchi H, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M. Recent developments in gastric endoscopic submucosal dissection: towards the era of endoscopic resection of layers deeper than the submucosa. Dig Endosc. 2013;25 Suppl 1:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Mitsui T, Goto O, Shimizu N, Hatao F, Wada I, Niimi K, Asada-Hirayama I, Fujishiro M, Koike K, Seto Y. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H, Fujiwara T. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. 2017;20:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 13. | Hwang SH, Park DJ, Kim YH, Lee KH, Lee HS, Kim HH, Lee HJ, Yang HK, Lee KU. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc. 2009;23:1980-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 15. | Matsuda T, Nunobe S, Ohashi M, Hiki N. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl Gastroenterol Hepatol. 2017;2:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Aoyama J, Goto O, Kawakubo H, Mayanagi S, Fukuda K, Irino T, Nakamura R, Wada N, Takeuchi H, Yahagi N, Kitagawa Y. Clinical outcomes of non-exposed endoscopic wall-inversion surgery for gastric submucosal tumors: long-term follow-up and functional results. Gastric Cancer. 2020;23:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Balde AI, Chen T, Hu Y, Redondo N JD, Liu H, Gong W, Yu J, Zhen L, Li G. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc. 2017;31:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 18. | Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kanehira E, Kanehira AK, Tanida T, Takahashi K, Obana Y, Sasaki K. CLEAN-NET: a modified laparoendoscopic wedge resection of the stomach to minimize the sacrifice of innocent gastric wall. Surg Endosc. 2020;34:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Miyazaki Y, Takiguchi S, Kurokawa Y, Takahashi T, Fukuda Y, Yamasaki M, Makino T, Tanaka K, Motoori M, Kimura Y, Nakajima K, Mori M, Doki Y. Endoscopy-assisted laparoscopic submucosal dissection for a duodenal epithelial tumor. Asian J Endosc Surg. 2019;12:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Tsushimi T, Mori H, Harada T, Nagase T, Iked Y, Ohnishi H. Laparoscopic and endoscopic cooperative surgery for duodenal neuroendocrine tumor (NET) G1: Report of a case. Int J Surg Case Rep. 2014;5:1021-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Nagasawa Y, Okauchi H, Kojima M, Setoyama H, Hasegawa M, Mizuta H, Tsujikawa T, Tani M, Kurumi Y. Laparoscopic-endoscopic cooperative surgery for a duodenal neuroendocrine tumor: A case report. Asian J Endosc Surg. 2017;10:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Irino T, Nunobe S, Hiki N, Yamamoto Y, Hirasawa T, Ohashi M, Fujisaki J, Sano T, Yamaguchi T. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Yorimitsu N, Oyama T, Takahashi A, Takehana T, Shiozawa S. Laparoscopy and endoscopy cooperative surgery is a safe and effective novel treatment for duodenal neuroendocrine tumor G1. Endoscopy. 2020;52:E68-E70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Yanagimoto Y, Omori T, Jeong-Ho M, Shinno N, Yamamoto K, Takeuchi Y, Higashino K, Uedo N, Sugimura K, Matsunaga T, Miyata H, Ushigome H, Takahashi Y, Nishimura J, Yasui M, Asukai K, Yamada D, Tomokuni A, Wada H, Takahashi H, Ohue M, Yano M, Sakon M. Feasibility and Safety of a Novel Laparoscopic and Endoscopic Cooperative Surgery Technique for Superficial Duodenal Tumor Resection: How I Do It. J Gastrointest Surg. 2019;23:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501-12508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Suzuki S, Fukunaga Y, Tamegai Y, Akiyoshi T, Konishi T, Nagayama S, Saito S, Ueno M. The short-term outcomes of laparoscopic-endoscopic cooperative surgery for colorectal tumors (LECS-CR) in cases involving endoscopically unresectable colorectal tumors. Surg Today. 2019;49:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Yuan XL, Wang SF, Ye LS, Wu CC, Zeng XH, Khan N, Hu B. Resection of a giant gastric mass using an ultrasonic scalpel with endoscopic assistance. Endoscopy. 2018;50:E227-E228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Ohdaira T, Nagai H. Intraoperative localization of early-stage upper gastrointestinal tumors using a magnetic marking clip-detecting system. Surg Endosc. 2007;21:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Hyun JH, Kim SK, Kim KG, Kim HR, Lee HM, Park S, Kim SC, Choi Y, Sohn DK. A novel endoscopic fluorescent band ligation method for tumor localization. Surg Endosc. 2016;30:4659-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wu Z, van de Haar RC, Sparreboom CL, Boersema GS, Li Z, Ji J, Jeekel J, Lange JF. Is the intraoperative air leak test effective in the prevention of colorectal anastomotic leakage? A systematic review and meta-analysis. Int J Colorectal Dis. 2016;31:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Yang SY, Han J, Han YD, Cho MS, Hur H, Lee KY, Kim NK, Min BS. Intraoperative colonoscopy for the assessment and prevention of anastomotic leakage in low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Park JH, Jeong SH, Lee YJ, Kim TH, Kim JM, Kim DH, Kwag SJ, Kim JY, Park T, Jeong CY, Ju YT, Jung EJ, Hong SC. Safety and efficacy of post-anastomotic intraoperative endoscopy to avoid early anastomotic complications during gastrectomy for gastric cancer. Surg Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis. 2014;16:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Valenzuela-Salazar C, Rojano-Rodríguez ME, Romero-Loera S, Trejo-Ávila ME, Bañuelos-Mancilla J, Delano-Alonso R, Moreno-Portillo M. Intraoperative endoscopy prevents technical defect related leaks in laparoscopic Roux-en-Y gastric bypass: A randomized control trial. Int J Surg. 2018;50:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. 1990;77:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Sasaki K, Ishihara S, Nozawa H, Kawai K, Hata K, Kiyomatsu T, Tanaka T, Nishikawa T, Otani K, Yasuda K, Murono K, Watanabe T. Successful Management of a Positive Air Leak Test during Laparoscopic Colorectal Surgery. Dig Surg. 2018;35:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Allaix ME, Lena A, Degiuli M, Arezzo A, Passera R, Mistrangelo M, Morino M. Intraoperative air leak test reduces the rate of postoperative anastomotic leak: analysis of 777 laparoscopic left-sided colon resections. Surg Endosc. 2019;33:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Bukhari MA, Haito-Chavez Y, Ngamruengphong S, Brewer Gutierrez O, Chen YI, Khashab MA. Rendezvous Biliary Recanalization of Complete Biliary Obstruction With Direct Peroral and Percutaneous Transhepatic Cholangioscopy. Gastroenterology. 2018;154:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Shorvon PJ, Cotton PB, Mason RR, Siegel JH, Hatfield AR. Percutaneous transhepatic assistance for duodenoscopic sphincterotomy. Gut. 1985;26:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Mason RR, Cotton PB. Combined duodenoscopic and transhepatic approach to stenosis of the papilla of Vater. Br J Radiol. 1981;54:678-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Kimura K, Kudo K, Kurihara T, Yoshiya S, Mano Y, Takeishi K, Itoh S, Harada N, Ikegami T, Yoshizumi T, Ikeda T. Rendezvous Technique Using Double Balloon Endoscope for Removal of Multiple Intrahepatic Bile Duct Stones in Hepaticojejunostomy After Living Donor Liver Transplant: A Case Report. Transplant Proc. 2019;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Schreuder AM, Booij KAC, de Reuver PR, van Delden OM, van Lienden KP, Besselink MG, Busch OR, Gouma DJ, Rauws EAJ, van Gulik TM. Percutaneous-endoscopic rendezvous procedure for the management of bile duct injuries after cholecystectomy: short- and long-term outcomes. Endoscopy. 2018;50:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Mönkemüller KE, Linder JD, Fry LC. Modified rendezvous technique for biliary cannulation. Endoscopy. 2002;34:936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Lee TH, Park SH, Lee SH, Lee CK, Lee SH, Chung IK, Kim HS, Kim SJ. Modified rendezvous intrahepatic bile duct cannulation technique to pass a PTBD catheter in ERCP. World J Gastroenterol. 2010;16:5388-5390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Dickey W. Parallel cannulation technique at ERCP rendezvous. Gastrointest Endosc. 2006;63:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Tsuchiya T, Itoi T, Sofuni A, Tonozuka R, Mukai S. Endoscopic ultrasonography-guided rendezvous technique. Dig Endosc. 2016;28 Suppl 1:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Tomizawa Y, Di Giorgio J, Santos E, McCluskey KM, Gelrud A. Combined interventional radiology followed by endoscopic therapy as a single procedure for patients with failed initial endoscopic biliary access. Dig Dis Sci. 2014;59:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Neal CP, Thomasset SC, Bools D, Sutton CD, Garcea G, Mann CD, Rees Y, Newland C, Robinson RJ, Dennison AR, Berry DP. Combined percutaneous-endoscopic stenting of malignant biliary obstruction: results from 106 consecutive procedures and identification of factors associated with adverse outcome. Surg Endosc. 2010;24:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Chang JH, Lee IS, Chun HJ, Choi JY, Yoon SK, Kim DG, You YK, Choi MG, Choi KY, Chung IS. Usefulness of the rendezvous technique for biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis. Gut Liver. 2010;4:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Yang MJ, Kim JH, Hwang JC, Yoo BM, Kim SS, Lim SG, Won JH. Usefulness of combined percutaneous-endoscopic rendezvous techniques after failed therapeutic endoscopic retrograde cholangiography in the era of endoscopic ultrasound guided rendezvous. Medicine (Baltimore). 2017;96:e8991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Bokemeyer A, Müller F, Niesert H, Brückner M, Bettenworth D, Nowacki T, Beyna T, Ullerich H, Lenze F. Percutaneous-transhepatic-endoscopic rendezvous procedures are effective and safe in patients with refractory bile duct obstruction. United European Gastroenterol J. 2019;7:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Kimura K, Kudo K, Yoshizumi T, Kurihara T, Yoshiya S, Mano Y, Takeishi K, Itoh S, Harada N, Ikegami T, Ikeda T. Electrohydraulic lithotripsy and rendezvous nasal endoscopic cholangiography for common bile duct stone: A case report. World J Clin Cases. 2019;7:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Itoi T, Ishii K, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Tsuji S, Umeda J, Moriyasu F. Single Balloon Enteroscopy-Assisted ERCP Using Rendezvous Technique for Sharp Angulation of Roux-en-Y Limb in a Patient with Bile Duct Stones. Diagn Ther Endosc. 2009;2009:154084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Fan C, Zhang H, Yan X, Ma J, Wang C, Lv Y. Advanced Roux-en-Y hepaticojejunostomy with magnetic compressive anastomats in obstructive jaundice dog models. Surg Endosc. 2018;32:779-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Jang SI, Choi J, Lee DK. Magnetic compression anastomosis for treatment of benign biliary stricture. Dig Endosc. 2015;27:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Parlak E, Koksal AS, Kucukay F, Eminler AT, Toka B, Uslan MI. A novel technique for the endoscopic treatment of complete biliary anastomosis obstructions after liver transplantation: through-the-scope magnetic compression anastomosis. Gastrointest Endosc. 2017;85:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Jang SI, Lee KH, Yoon HJ, Lee DK. Treatment of completely obstructed benign biliary strictures with magnetic compression anastomosis: follow-up results after recanalization. Gastrointest Endosc. 2017;85:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Fan C, Yan XP, Liu SQ, Wang CB, Li JH, Yu L, Wu Z, Lv Y. Roux-en-Y choledochojejunostomy using novel magnetic compressive anastomats in canine model of obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2012;11:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Mohammad B, Richard MN, Pandit A, Zuccala K, Brandwein S. Outcomes of laparoscopic-assisted ERCP in gastric bypass patients at a community hospital center. Surg Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Paranandi B, Joshi D, Mohammadi B, Jenkinson A, Adamo M, Read S, Johnson GJ, Chapman MH, Pereira SP, Webster GJ. Laparoscopy-assisted ERCP (LA-ERCP) following bariatric gastric bypass surgery: initial experience of a single UK centre. Frontline Gastroenterol. 2016;7:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Dalmonte G, Valente M, Bosi S, Gnocchi A, Marchesi F. Transjejunal Laparoscopic-Assisted ERCP: a Technique to Deal with Choledocholithiasis After Roux-En-Y Reconstruction. Obes Surg. 2019;29:2005-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Bowman E, Greenberg J, Garren M, Guda N, Rajca B, Benson M, Pfau P, Soni A, Walker A, Gopal D. Laparoscopic-assisted ERCP and EUS in patients with prior Roux-en-Y gastric bypass surgery: a dual-center case series experience. Surg Endosc. 2016;30:4647-4652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Krutsri C, Kida M, Yamauchi H, Iwai T, Imaizumi H, Koizumi W. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy. World J Gastroenterol. 2019;25:3313-3333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (3)] |
| 66. | Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Ricci C, Pagano N, Taffurelli G, Pacilio CA, Migliori M, Bazzoli F, Casadei R, Minni F. Comparison of Efficacy and Safety of 4 Combinations of Laparoscopic and Intraoperative Techniques for Management of Gallstone Disease With Biliary Duct Calculi: A Systematic Review and Network Meta-analysis. JAMA Surg. 2018;153:e181167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 68. | Noel R, Arnelo U, Swahn F. Intraoperative versus postoperative rendezvous endoscopic retrograde cholangiopancreatography to treat common bile duct stones during cholecystectomy. Dig Endosc. 2019;31:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Qian Y, Xie J, Jiang P, Yin Y, Sun Q. Laparoendoscopic rendezvous versus ERCP followed by laparoscopic cholecystectomy for the management of cholecysto-choledocholithiasis: a retrospectively cohort study. Surg Endosc. 2020;34:2483-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 70. | Swahn F, Nilsson M, Arnelo U, Löhr M, Persson G, Enochsson L. Rendezvous cannulation technique reduces post-ERCP pancreatitis: a prospective nationwide study of 12,718 ERCP procedures. Am J Gastroenterol. 2013;108:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 71. | Swahn F, Regnér S, Enochsson L, Lundell L, Permert J, Nilsson M, Thorlacius H, Arnelo U. Endoscopic retrograde cholangiopancreatography with rendezvous cannulation reduces pancreatic injury. World J Gastroenterol. 2013;19:6026-6034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Winder JS, Juza RM, Alli VV, Rogers AM, Haluck RS, Pauli EM. Concomitant laparoscopic cholecystectomy and antegrade wire, rendezvous cannulation of the biliary tree may reduce post-ERCP pancreatitis events. Surg Endosc. 2020;34:3216-3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | James HJ, James TW, Wheeler SB, Spencer JC, Baron TH. Cost-effectiveness of endoscopic ultrasound-directed transgastric ERCP compared with device-assisted and laparoscopic-assisted ERCP in patients with Roux-en-Y anatomy. Endoscopy. 2019;51:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Skok P S-Editor: Wang JL L-Editor: Filipodia E-Editor: Qi LL