Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.67
Peer-review started: November 12, 2019
First decision: December 20, 2019
Revised: January 8, 2020
Accepted: April 10, 2020
Article in press: April 10, 2020
Published online: April 28, 2020
Processing time: 167 Days and 12.5 Hours
Occult hepatitis B virus (HBV) infection, by definition, is a state in which infection with this virus does not manifest with the conventional diagnostic laboratory criteria reserved for the obvious form of HBV infection. As a result, occult HBV infection is commonly a surprise finding discovered accidently during the evaluation of other apparent liver diseases, such as hepatitis C virus (HCV) infection or non-alcoholic fatty liver disease and, more importantly, their evolution into life-threatening hepatocellular carcinoma. As infection with HCV and occult HBV is rarely considered when assessing these more obvious conditions, and in an attempt to offer a better understanding of this phenomenon, this study attempted to shed some light onto the uniqueness of occult HBV infection by addressing the natural history of HBV and HCV infections, as well as non-alcoholic fatty liver disease. This was carried out by taking into account the exclusive integration process undertaken by the HBV genome into infected host hepatocytes, with consideration given to conditions which afford reactivation of the occult infection and stress on the molecular mechanisms that underlie occult HBV infection. Finally, the clinical outcome of occult HBV infection and its relation to hepatocellular carcinoma is analyzed.
Core tip: Occult hepatitis B infection is a common clinical situation among chronic liver diseases including hepatitis C virus infection and non-alcoholic fatty liver disease. It is masked, not routinely diagnosed by common laboratory tools, it has a different clinical impact and may increase the incidence of hepatocellular carcinoma in patients with these chronic liver diseases. This systematic review analyzes the data on this clinical situation and highlights different studies which have investigated this clinical entity.
- Citation: Elalfy H, Besheer T, Elhammady D, El Mesery A, Shaltout SW, Abd El-Maksoud M, Amin AI, Bekhit AN, Abd El Aziz M, El-Bendary M. Pathological characterization of occult hepatitis B virus infection in hepatitis C virus-associated or non-alcoholic steatohepatitis-related hepatocellular carcinoma. World J Meta-Anal 2020; 8(2): 67-77
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/67.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.67
Despite an increased understanding of the immunopathogenesis and virology of hepatitis B, the directional course of chronic hepatitis B (CHB) viral infection remains uncertain. Nevertheless, it has been established that the natural history of this disease is primarily dependent on the age at time of exposure to the infecting virus[1].
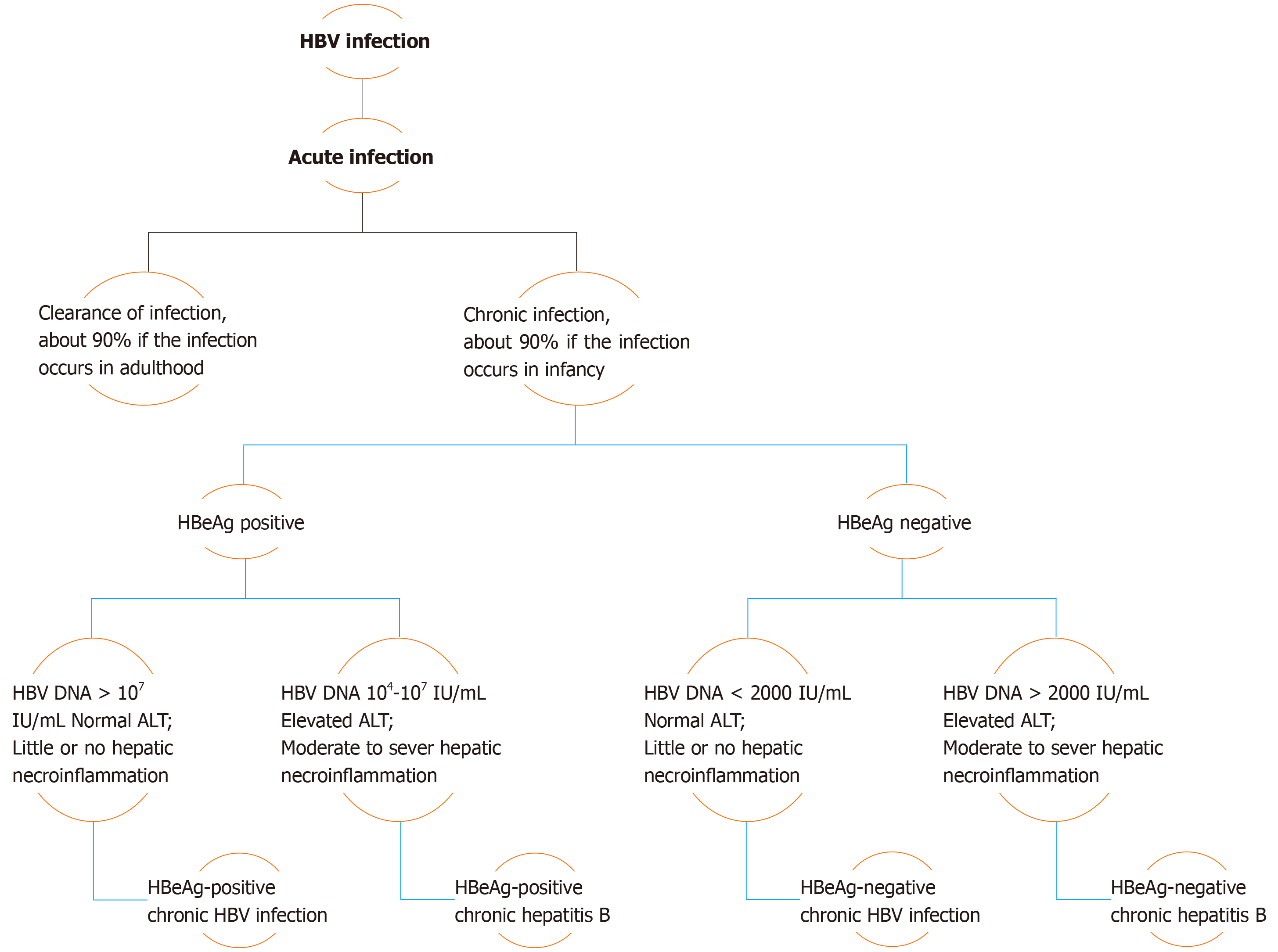
Chronicity is the hallmark of hepatitis B virus (HBV) infection acquired in infancy, with over 90% of cases developing CHB, while this same percentage of patients undergo resolution of the disease if the infection occurs in adulthood. Therefore, with consideration of the levels of hepatitis B e-antigen (HBeAg) and HBV DNA, in addition to alanine aminotransferase (ALT) values and extent of liver inflammation, the clinical practice guidelines for the management of HBV infection established by the EASL in 2017 have classified the natural history of chronic HBV infection into five phases[2] (Figure 1).
Phase I includes patients with HBeAg-positive chronic HBV infection. Previously coined as “immune tolerant”, these patients are characterized as having detectable serum HBeAg associated with high HBV DNA levels, while ALT levels remain in the normal range (ULN, approximately 40 IU/L)[3]. Histologically, little or no necroinflammatory changes or fibrosis are detected in these cases. However, the presence of integration affiliated with the increased HBV DNA levels and clonal hepatocyte expansion suggest that the hepatocarcinogenic process may be initiated early in the course of this infection[3,4]. Phase 2 comprises HBeAg-positive CHB patients, formerly termed “immune reactive HBeAg positive”. In addition to detectable serum HBeAg and elevated HBV DNA levels, these patients are distinguished by increased ALT levels associated with moderate to severe hepatic necroinflammation with increased evolution to the development of fibrosis. Patients in phase 3, who have antecedently been called “inactive carriers”, are now known to have HBeAg-negative chronic HBV infection. Described by a lack of detectable serum HBeAg with absent or low (< 2000 IU/mL) levels of HBV DNA and normal ALT values, these patients have minimal liver necroinflammation and fibrosis.
Phase 4 of this categorization constitutes cases of HBeAg-negative CHB infection. These patients demonstrate absence of serum HBeAg in association with moderate to high levels of HBV DNA (> 2000 IU/mL). However, ALT levels in these subjects are elevated, the manner being either persistent or fluctuating, and hepatic necroinflammatory activity and fibrosis are evident. The final phase of the EASL classification, phase 5, is the hepatitis B surface antigen (HBsAg)-negative phase, which includes patients with negative serum HBsAg, regardless of the appearance of antibodies to HBsAg (anti-HBs), and the presence of detectable antibodies to hepatitis B core antigen (HBcAg) (anti-HBc). Known as “occult HBV infection”, patients in this phase most commonly exhibit undetectable HBV DNA in serum and ALT levels within the normal range. However, histological examination often demonstrates the presence of hepatic HBV DNA in the form of covalently closed circular DNA (cccDNA). As a consequence, reactivation of HBV infection may occur in patients undergoing immunosuppressive therapy[3].
Progression of hepatitis B infection to the stage of cirrhosis and the development of hepatocellular carcinoma (HCC) is of variable incidence and largely affected by immune response of the infected patient to the virus. Untreated CHB patients have a 5-year cumulative incidence of developing cirrhosis of approximately 8%-20%. Those who do progress to cirrhosis have a 5-year cumulative risk of advancing to hepatic decompensation of 20% and an annual risk of HCC of about 2%-5%[5].
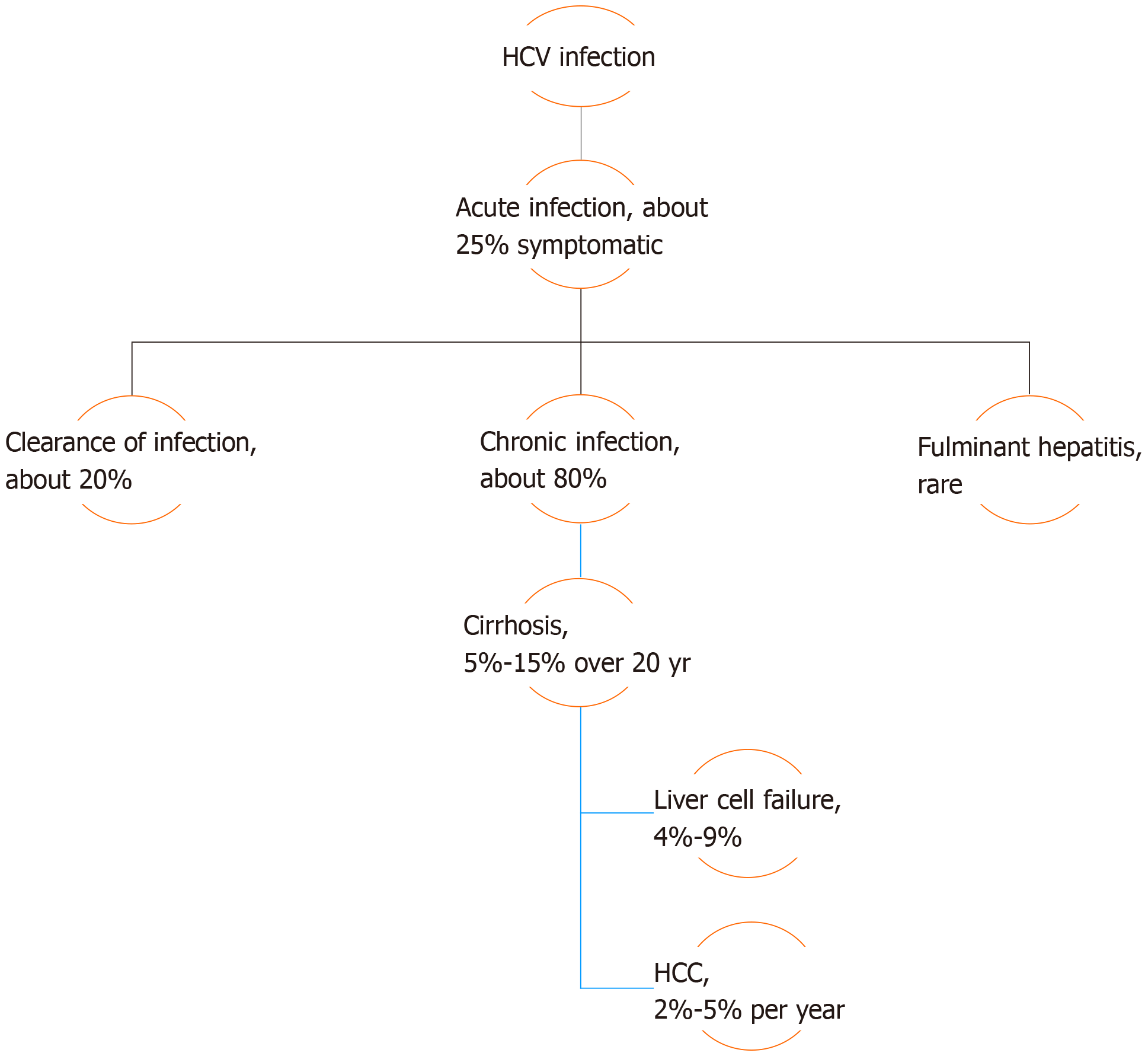
As occult HBV infection can only be diagnosed during assessment and evaluation of other liver diseases, such as HCV infection or non-alcoholic fatty liver disease (NAFLD), the natural history of these diseases should be taken into consideration. HCV infection may either manifest mildly, as in acute cases, or present with more serious symptoms in either acute or chronic cases. Chronicity develops in about 80% of patients infected with HCV, and is predicted by several factors such as male sex, age over 25 years at initial infection, mild asymptomatic acute infection, intake of immunosuppressive therapy, and co-infection with human immunodeficiency virus (HIV). The intensity of chronic hepatitis is variable between chronically infected patients but, in general, 5%-15% of patients with chronic HCV infection advance to develop liver cirrhosis over a period of 20 years. Of these cirrhotic patients, 4%-9% will progress to liver failure, with a 2%-5% annual risk of developing HCC[6] (Figure 2).
Progression of liver disease identified as simple steatosis to more advanced steatohepatitis with transformation to fibrosis and development of cirrhosis forms the basis for what is collectively defined as NAFLD. While progressive liver disease is a highly unlikely consequence of simple steatosis, the development of non-alcoholic steatohepatitis (NASH) and associated fibrosis have both been shown to be associated with poor prognosis in these patients. This was demonstrated in a study of patients with paired biopsies and showed that 49% of subjects demonstrating features of NASH at baseline proceeded to develop advanced fibrosis in contrast to only 17% of those presenting with simple steatosis[7]. However, no difference in mortality was detected among patients with NAFLD in a large population study utilizing blood tests and ultrasonography for assessment of this condition[8]. However, compared with the general population, the overall mortality rate was found to be higher in NAFLD patients assessed primarily by radiological evaluation with ultrasound[9]. Nevertheless, upon scrutinizing the results of an overlapping cohort with 26.4 years as the mean period of follow-up, only patients diagnosed with late-stage fibrosis had an increased risk of all-cause mortality when compared to the reference population[10]. The risk of developing HCC is increased in patients afflicted with cirrhosis in the setting of NAFLD, with about 1%-2% of primary hepatic cancer appearing in patients also suffering from NAFLD[11].
HBV is a member of the Hepadnaviridae family characterized by its incorporation of a partially double-stranded relaxed circular DNA of about 3.2-kb that comprises four open reading frames (ORF), each of which encodes for different virion proteins[12]. The S ORF encodes the large, middle, and small viral surface envelope glycoproteins collectively known as HBsAg, these being categorized into pre-S1, pre-S2 and S regions based on both structure and function. The C ORF encodes one of two proteins depending on the site of translation initiation, either the HBcAg or viral nucleocapsid, initiated from the core region of the viral genome, or the HBeAg initiated from the pre-core region. Viral polymerase, distinguished by reverse transcriptase activity, is encoded by the P ORF, while the small regulatory HBV X protein (HBxAg) is encoded by the X ORF[13,14].
Upon entry of an infectious virion into the host hepatocyte, possibly by way of the pre-S protein[15], the process of replication begins with the uncoating and release of the relaxed circular DNA (RC-DNA) followed by its transport to the nucleus of the cell where it is re-organized into the stable form of the viral genome known as the cccDNA. This cccDNA is used as a template for transcription of a group of RNAs utilizing RNA polymerase II enzyme, including pre-genomic RNA (pgRNA), pre-core RNA and sub-genomic HBV RNAs. PgRNA functions as a template for completion of HBV DNA synthesis and serves as the messenger RNA for polymerase and core protein[16], while sub-genomic HBV RNA acts as mRNA for translation of the different sized surface proteins of HBsAg as well as HBxAg protein. At the cytoplasm, produced nucleocapsids are gathered into a glycoprotein envelope in the endoplasmic reticulum forming mature virions to be secreted extracellularly[17].
Development of occult HBV infection, as well as HCC, may possibly be explained by the phenomenon of hepatitis B viral integration into the host genome[18], a process that comprises the redistribution of HBV DNA sequences into host chromosomal DNA. This integration mechanism is often defective[19], and is associated with decreased virion production accompanied by undetectable HBV DNA and HBsAg in the serum of infected patients[7]. Integration is commonly seen in chronically infected HBV patients, particularly those with HBsAg-positive HCC[20], although it has also occasionally been seen in HBsAg-negative HCC cases. However, HBV viral integration has not been detected in patients co-infected with HCV[21-23].
Defects that have been associated with HBV DNA integration include loss of HBV core gene with consequent loss of core protein, leading to viral assembly at substandard levels with excessive aggregation of viral DNA in hepatocytes. This may possibly offer clarification for findings of detectable HBV DNA in the liver but not in serum of infected patients[18]. Excessive translation of the large protein of HBsAg causes impaired release of other forms of the surface protein, leading to their aggregation in the form of granules in the hepatocyte cytoplasm[24]. In addition, HBsAg expression is also affected by HBV DNA disruption and rearrangement when the downstream region of the S ORF is replaced with the pre-S1 promoter, leading to decreased S gene transcription associated with impaired secretion of S protein[18].
Patients with occult HBV infection (OBI) show HBV DNA existing in two forms, either as episomal free cccDNA or integrated into DNA of the host hepatocyte. The state of OBI may be induced by a number of situations including the subsequent resolution of an acute HBV infection, the “a” determinant arising from mutation in the HBsAg gene, co-infection with HCV or HIV, or epigenetic changes in the cell. OBI is defined by the absence of detectable HBsAg in serum regardless of the status of the antibodies against HBcAg (anti-HBc)[25].
Therefore, cases of immune suppression are associated with cessation of the decreased viral replication and suppressed gene expression that commonly accompany OBI, resulting in reactivation of viral replication consequent to the deterioration of control by the immune system. This provides crucial, albeit indirect, evidence of the important function of immunological control in induction of the state of occult HBV infection. However, recent studies have reported that reactivation of OBI also occurs with histone deacetylase inhibitor use, thus providing confirmation of the role of epigenetic mechanisms in maintaining HBV activity in check with modification of the structure and dynamics of viral cccDNA mini-microsome, these also being projected as probable causes of reactivation of HBV[26].
Conditions associated with a high risk of reactivation of OBI include hematological malignancies, in addition to transplantation with hematopoietic stem cells and immunosuppressive therapies consisting of anti-CD20 monoclonal antibody (Rituximab), drugs included in the CHOP regimen as well as fludarabine. However, only a small number of patients undergoing these therapeutic interventions experience severe clinical manifestations following alteration of the HBV serological profile[27].
On the other hand, reactivation of OBI appears to occur infrequently in sufferers of rheumatologic diseases subjected to high doses of corticosteroids or biological agents for prolonged periods of time. In addition, liver cancer patients going through transarterial chemoembolization (TACE) and those with inflammatory bowel diseases treated with biologics rarely experience OBI reactivation, although patients undergoing chemotherapy for solid tumors have increasing shown HBV reactivation. As a result of these inconsistencies, consensus regarding this topic remains inconclusive, with the entire scope of risk remaining obscure[27]. However, the possibility of reactivation of HBV in patients with OBI undergoing direct acting antiviral therapy for the treatment of HCV has been raised in recent reports, although there appears to be negligible risk in these instances and no clinical or virological consequences[27].
A double looped structure comprising amino acids 124 to 147 is known as the “a” determinant of HBsAg. On account of its abundant cysteine residues, it is known to take part in disulfide bond formation and maintenance of this region[28]. Mutations in the “a” determinant propose a serious health burden in that they are undetectable by certain commercial HBsAg assays as well as having the capability to infect both vaccinated and unvaccinated persons[29]. The first mutation in the “a” determinant of HBsAg to be reported was the sG145R mutation, which was described in a patient who was both actively and passively immunized[29], after which other mutations were later disclosed both inside and outside the “a” determinant[30,31]. In addition, this mutation most likely causes reinfection with HBV in patients following liver transplantation in spite of prophylaxis with hepatitis B immunoglobulin (HBIG), probably due to HBIG induced suppression of the secretion of HBsAg into serum without affecting replication of the HBV DNA[32].
Similar mutations characterized by being undetectable by some commercial HBsAg assays as well as infecting vaccinated subjects, are mutations with the added health complication of inducing lamivudine resistance in patients undergoing this treatment[29]. These include the Q563S mutation in HBV polymerase, surface gene mutation sS207R, the V539I mutation in the “C” domain of the polymerase, sS143L substitution in the “a” determinant of HBsAg, and M204I and L180M/M204I mutations of the polymerase gene[33,34].
Furthermore, deficient HBsAg expression associated with low replication is also seen with the G-to-A mutation of the surface gene at position 458. Due to the close proximity of this position to the 5’ splice site of the S gene mRNA, this mutation impedes S gene mRNA splicing through a co-/posttranscriptional mechanism with resultant defective export of S gene mRNA or dysregulated RNA folding[35]. Genotype D strains of HBV are the only ones that have evolved to accommodate the accumulation of substitutions thus advocating positive selection, as well as having an acceptor site at nucleotide 202. Splicing at this site as well as at the nucleotide 2986 donor site produced intracellular viral particles lacking the surface protein, this resulting in the accumulation of mutations subsequent to alleviation of coding restrictions[36].
Pre-S region deletion mutations are associated with eradication of HLA-restricted B-cell and T-cell epitopes, and are another form of mutation also accompanied by inadequate expression of the surface proteins of HBV[37,38]. Deletions and mutations in the pre-S gene result in modified expression of HBsAg as well as reduced HBeAg and HBV DNA levels in hepatocyte cell lines[39]. In addition, pre-S1 and pre-S2 region mutations were found to be associated with low secretion of HBsAg in cell culture systems[40].
Contradictory data exists with regards to the clinical outcome of occult HBV co-infection with HCV. A significant association was noted between occult HBV infection and cirrhosis in patients infected with HCV in a study by Cacciola et al[41], in spite of the enhanced response to interferon therapy reported in these co-infected patients. A higher likelihood of developing HCC in patients co-infected with HCV and occult HBV has also been reported[42], although no correlation was found between the presence of infection with occult HBV and the severity of liver disease related to HCV[43]. Co-infection with HBV and HCV is associated with reduced levels of HBV replication accompanied by diminished expression of hepatic HBsAg[44,45], but patients infected with HBV alone demonstrated significantly higher rates of spontaneous HBsAg clearance[46].
Inhibition of HBV replication and HBV protein production by HCV occurs through a variety of underlying mechanisms, including co-localization of HBV and HCV genomes within the nucleus in which the inhibitory effect of HCV on HBV replication was demonstrated using a double fluorescent in situ hybridization technique[47], although this result could not be replicated in Huh-7 cells[48]. In addition, replication of HBV and expression of HBsAg can be inhibited by HCV core protein[49]. HBV core and polymerase proteins interact with the package signal (Σ) situated at the 5’ end of HBV pgRNA to start the process of encapsidation[50]. Direct interaction of HCV core protein with HBx protein causes obstruction of the core and polymerase binding to this package signal within the hepatocyte, thus preventing encapsidation of HBV with consequent inhibition of HBV gene expression[29]. Furthermore, HCV NS2 protein downregulates secretion of HBsAg and HBeAg accompanied by suppression of different cellular and viral promoters[51].
Another viral infection in which occult HBV infection is commonly seen is HIV, in which HBV DNA is also detected intermittently. As a result, recurrent sampling has been proposed for HBV DNA detection in HIV-positive patients, although this recommendation remains controversial[52].
Apolipoprotein B mRNA-Editing Enzyme Catalytic Polypeptide (APOBEC) has been shown to play a role in cytidine deamination and is known to inhibit and edit replication of HIV[53]. Similarly, expression of APOBEC3G has been associated with a 50-fold reduction in HBV DNA[54]. Inhibition of HBV replication by APOBECs occurs in either a deamination-dependent or a deamination-independent manner.
APOBECs have been shown to edit up to 35% of the HBV genome present in the liver[55], including regions encoding for the surface proteins, the polymerase, and the HBx protein[56]. While little data exists demonstrating the non-cytolytic clearance of HBV by APOBEC proteins, this has steadily been improving over the past few years with the appearance of more studies[29].
On the other hand, deamination-independant inhibition can be mediated by enhanced nuclease susceptibility of the pre-genomic RNA associated with HBV core protein[57]. In addition, targeting of single-stranded or hybrid HBV DNA is another mechanism of inhibiting early stage HBV DNA[58]. Secretion of both HBsAg and HBeAg can also be inhibited by APOBEC3B, although the mechanism remains unknown[59].
Occult hepatitis B virus infection may be caused by impedance of the immune response to HBV. Modulation of both replication of HBV and synthesis of HBV proteins are the outcome of a number of mechanisms related to the host immune response, including apoptosis, T-cell responses of both cytolytic and non-cytolytic nature, and polymorphisms of vitamin D receptor (VDR)[29]. Compared to patients with CHB, those with OBI demonstrated lower expression levels of the apoptotic factor Fas, which plays a role in the apoptosis of infected hepatocytes and removal of old hepatocytes[60]. Another potential mechanism for occult HBV is the non-cytolytic immune responses seen to be associated with indistinquishable HBsAg levels[61]. Similarly, VDR gene polymorphisms were associated with occult HBV infections and were characterized by variable levels of HBV DNA with HBeAg loss[62,63].
Occult HBV infection may be the result of modulated HBV replication and virion production due to control of transcriptional activity by methylation[29]. Cytosine methylation of CpG dinucleotides within CpG islands inside the gene promoters has been linked to silencing of genes[64]. Furthermore, methylated HBV cccDNA has been detected in liver tissues of infected humans, this being associated with a 90% diminution of HBsAg secretion in hepatocyte cell lines[38]. In addition, HBeAg-negative individuals demonstrated methylated cccDNA in a higher ratio to total cccDNA when compared to HBeAg-positive subjects[65].
In addition, enhanced replication of HBV in cell culture is associated with hyperacetylation of cccDNA-bound histones, a finding similarly demonstrated with histone deacetylase inhibitors resulting in acetylated histones bound to cccDNA leading to elevated HBV replication with high levels of HBV transcripts. Conversely, hypoacetylation of cccDNA-bound histones is linked to diminished viral loads associated with recruitment of histone deacetylase[66]. Remodelling of the HBV minichromosome with regulation of HBV replication is the culmination of histone phosphorylation and methylation[67].
Integration of HBV DNA sequences into the host genome is an established mechanism by which chronic HBV patients develop HCC[68]. During the integration process, interruption and rearrangement of genes into chromosomal DNA may result in either loss of serum HBsAg, reduced virus production, or the appearance of undetectable serum HBV DNA. Therefore, particularly in patients suffering from chronic HBV infection for several years, the fundamental mechanism underlying occult HBV is integration of HBV DNA[29].
Loss of HBV core gene during HBV DNA integration results in HBV core protein loss which is associated with minimal levels of viral assembly with aggregation of unencapsidated HBV DNA in the hepatocyte, this offering an explanation as to why HBV-related HCC is associated with undetectable serum HBV DNA although it may be easily detected in liver tissure[18].
Immune complexes containing HBsAg have been demonstrated in the serum of patients with acute HBV infection, those with chronic HBV infection, as well as HBsAg carriers who are asymptomatic[69]. HBsAg may be become entrapped with anti-HBc, thus hindering detection of HBsAg by conventional serological assays. In addition, these HBsAg-containing immune complexes have been found in HBsAg-negative occult HBV infected patients with HCC[70]. In fact, about 40% of blood donors identified as HBsAg-negative but having positive anti-HBc were found to have detectable HBV DNA, most of these being shown as having HBV-containing immune complexes. These circulating immune complexes demonstrated an absence of nucleic acid changes that may cause changes in the major epitopes of HBsAg, thus providing further confirmation of the role of these immune complexes in OBI[71].
Although the clinical characteristics of HCC developing in the setting of OBI has not been extensively studied, several attempts have been made to explore this point. A recent study by El-Maksoud et al[72], performed on 50 Egyptian patients with HCC who had undergone either resection or transplantation, found that over 30% of chronic hepatitis C patients with HCC had OBI. Although no significant association could be ascertained between OBI and clinical tumor characteristics such as size and number, BCLC staging or vascular invasion, findings showed that these patients were younger with histology demonstrating a more advanced grade of 3 or 4. In addition, most of these patients had serological positivity for HBcAg, a possible indicator for prediction of occult infections[72]. These findings have been supported by a number of studies[73-75]. Therefore, the complexity yet uniqueness of the state of OBI grant it an exceptional status that is worthy of consideration when investigating any liver disease.
As OBI does not demonstrate the diagnostic laboratory criteria of conventional HBV infection, it is often missed in patients with HCC developing in the settings of HCV infection or NAFLD. Addressing the natural history of these conditions, and the molecular mechanisms by which hepatitis B virus becomes occult then reactivated in circumstances of immunosuppression emphasizes the unique characteristics of this infection. Studies conducted on occult HBV in patients with HCC on top of HBV have collectively concluded that although no significant association could be determined between OBI and tumor characteristics, the presence of OBI in up to 30% of patients, these being mostly younger patients who also tested positive for HBcAg, does in fact warrant its consideration during the investigative workup of other liver diseases.
| 1. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (3)] |
| 2. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4011] [Article Influence: 445.7] [Reference Citation Analysis (1)] |
| 3. | European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 4. | Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology. 2016;151:986-998.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 5. | Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36:1239-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 522] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 7. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 408] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 9. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2148] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 10. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1778] [Article Influence: 161.6] [Reference Citation Analysis (2)] |
| 11. | Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 786] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 12. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1714] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 13. | Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 747] [Cited by in RCA: 667] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 14. | Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Neurath AR, Kent SB, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350-3357. [PubMed] |
| 17. | Locarnini S, McMillan J, Bartholomeusz A. The hepatitis B virus and common mutants. Semin Liver Dis. 2003;23:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Raimondo G, Burk RD, Lieberman HM, Muschel J, Hadziyannis SJ, Will H, Kew MC, Dusheiko GM, Shafritz DA. Interrupted replication of hepatitis B virus in liver tissue of HBsAg carriers with hepatocellular carcinoma. Virology. 1988;166:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Brechot C, Kremsdorf D, Soussan P, Pineau P, Dejean A, Paterlini-Brechot P, Tiollais P. Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC): molecular mechanisms and novel paradigms. Pathol Biol (Paris). 2010;58:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kawai S, Yokosuka O, Imazeki F, Maru Y, Saisho H. State of HBV DNA in HBsAg-negative, anti-HCV-positive hepatocellular carcinoma: existence of HBV DNA possibly as nonintegrated form with analysis by Alu-HBV DNA PCR and conventional HBV PCR. J Med Virol. 2001;64:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Matsuzaki Y, Chiba T, Hadama T, Asaoka H, Doy M, Shoda J, Tanaka N, Kinoshita M. HBV genome integration and genetic instability in HBsAg-negative and anti-HCV-positive hepatocellular carcinoma in Japan. Cancer Lett. 1997;119:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Momosaki S, Nakashima Y, Kojiro M, Tabor E. HBsAg-negative hepatitis B virus infections in hepatitis C virus-associated hepatocellular carcinoma. J Viral Hepat. 2005;12:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Tamori A, Nishiguchi S, Kubo S, Narimatsu T, Habu D, Takeda T, Hirohashi K, Shiomi S. HBV DNA integration and HBV-transcript expression in non-B, non-C hepatocellular carcinoma in Japan. J Med Virol. 2003;71:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Chisari FV, Filippi P, McLachlan A, Milich DR, Riggs M, Lee S, Palmiter RD, Pinkert CA, Brinster RL. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol. 1986;60:880-887. [PubMed] |
| 25. | Makvandi M. Update on occult hepatitis B virus infection. World J Gastroenterol. 2016;22:8720-8734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Saffioti F, Raimondo G. What do we know about hepatitis B virus infection?. AMPM (Atti della Accademia Peloritana dei Pericolaanti-Classe di Scienze Medico-Biologiche). 2017;SD2 1-13. [DOI] [Full Text] |
| 28. | Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 776] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 29. | Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Carman WF, Korula J, Wallace L, MacPhee R, Mimms L, Decker R. Fulminant reactivation of hepatitis B due to envelope protein mutant that escaped detection by monoclonal HBsAg ELISA. Lancet. 1995;345:1406-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Chiou HL, Lee TS, Kuo J, Mau YC, Ho MS. Altered antigenicity of 'a' determinant variants of hepatitis B virus. J Gen Virol. 1997;78:2639-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Schilling R, Ijaz S, Davidoff M, Lee JY, Locarnini S, Williams R, Naoumov NV. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003;77:8882-8892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Torresi J, Earnest-Silveira L, Deliyannis G, Edgtton K, Zhuang H, Locarnini SA, Fyfe J, Sozzi T, Jackson DC. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Wakil SM, Kazim SN, Khan LA, Raisuddin S, Parvez MK, Guptan RC, Thakur V, Hasnain SE, Sarin SK. Prevalence and profile of mutations associated with lamivudine therapy in Indian patients with chronic hepatitis B in the surface and polymerase genes of hepatitis B virus. J Med Virol. 2002;68:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Hass M, Hannoun C, Kalinina T, Sommer G, Manegold C, Günther S. Functional analysis of hepatitis B virus reactivating in hepatitis B surface antigen-negative individuals. Hepatology. 2005;42:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | van Hemert FJ, Zaaijer HL, Berkhout B, Lukashov VV. Occult hepatitis B infection: an evolutionary scenario. Virol J. 2008;5:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Chaudhuri V, Tayal R, Nayak B, Acharya SK, Panda SK. Occult hepatitis B virus infection in chronic liver disease: full-length genome and analysis of mutant surface promoter. Gastroenterology. 2004;127:1356-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Vivekanandan P, Kannangai R, Ray SC, Thomas DL, Torbenson M. Comprehensive genetic and epigenetic analysis of occult hepatitis B from liver tissue samples. Clin Infect Dis. 2008;46:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Fang Y, Teng X, Xu WZ, Li D, Zhao HW, Fu LJ, Zhang FM, Gu HX. Molecular characterization and functional analysis of occult hepatitis B virus infection in Chinese patients infected with genotype C. J Med Virol. 2009;81:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Melegari M, Bruno S, Wands JR. Properties of hepatitis B virus pre-S1 deletion mutants. Virology. 1994;199:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 479] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 42. | Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40:4068-4071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Chu CM, Yeh CT, Liaw YF. Low-level viremia and intracellular expression of hepatitis B surface antigen (HBsAg) in HBsAg carriers with concurrent hepatitis C virus infection. J Clin Microbiol. 1998;36:2084-2086. [PubMed] |
| 45. | Crespo J, Lozano JL, de la Cruz F, Rodrigo L, Rodríguez M, San Miguel G, Artiñano E, Pons-Romero F. Prevalence and significance of hepatitis C viremia in chronic active hepatitis B. Am J Gastroenterol. 1994;89:1147-1151. [PubMed] |
| 46. | Sheen IS, Liaw YF, Lin DY, Chu CM. Role of hepatitis C and delta viruses in the termination of chronic hepatitis B surface antigen carrier state: a multivariate analysis in a longitudinal follow-up study. J Infect Dis. 1994;170:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Rodríguez-Iñigo E, Bartolomé J, Ortiz-Movilla N, Platero C, López-Alcorocho JM, Pardo M, Castillo I, Carreño V. Hepatitis C virus (HCV) and hepatitis B virus (HBV) can coinfect the same hepatocyte in the liver of patients with chronic HCV and occult HBV infection. J Virol. 2005;79:15578-15581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Eyre NS, Phillips RJ, Bowden S, Yip E, Dewar B, Locarnini SA, Beard MR. Hepatitis B virus and hepatitis C virus interaction in Huh-7 cells. J Hepatol. 2009;51:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Chen SY, Kao CF, Chen CM, Shih CM, Hsu MJ, Chao CH, Wang SH, You LR, Lee YH. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Hirsch RC, Lavine JE, Chang LJ, Varmus HE, Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as wel as for reverse transcription. Nature. 1990;344:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 264] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Dumoulin FL, von dem Bussche A, Li J, Khamzina L, Wands JR, Sauerbruch T, Spengler U. Hepatitis C virus NS2 protein inhibits gene expression from different cellular and viral promoters in hepatic and nonhepatic cell lines. Virology. 2003;305:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Gupta S, Singh S. Occult hepatitis B virus infection in ART-naive HIV-infected patients seen at a tertiary care centre in north India. BMC Infect Dis. 2010;10:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007;282:2587-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 54. | Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 55. | Vartanian JP, Henry M, Marchio A, Suspène R, Aynaud MM, Guétard D, Cervantes-Gonzalez M, Battiston C, Mazzaferro V, Pineau P, Dejean A, Wain-Hobson S. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6:e1000928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Noguchi C, Ishino H, Tsuge M, Fujimoto Y, Imamura M, Takahashi S, Chayama K. G to A hypermutation of hepatitis B virus. Hepatology. 2005;41:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Rösler C, Köck J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizsäcker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Nguyen DH, Gummuluru S, Hu J. Deamination-independent inhibition of hepatitis B virus reverse transcription by APOBEC3G. J Virol. 2007;81:4465-4472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | Zhang W, Zhang X, Tian C, Wang T, Sarkis PT, Fang Y, Zheng S, Yu XF, Xu R. Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell Microbiol. 2008;10:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Martin CM, Welge JA, Shire NJ, Shata MT, Sherman KE, Blackard JT. Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals. Cytokine. 2009;47:194-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Bremer CM, Saniewski M, Wend UC, Torres P, Lelie N, Gerlich WH, Glebe D. Transient occult hepatitis B virus infection in a blood donor with high viremia. Transfusion. 2009;49:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Huang YW, Liao YT, Chen W, Chen CL, Hu JT, Liu CJ, Lai MY, Chen PJ, Chen DS, Yang SS, Kao JH. Vitamin D receptor gene polymorphisms and distinct clinical phenotypes of hepatitis B carriers in Taiwan. Genes Immun. 2010;11:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Suneetha PV, Sarin SK, Goyal A, Kumar GT, Shukla DK, Hissar S. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol. 2006;44:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1865] [Cited by in RCA: 2031] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 65. | Guo Y, Li Y, Mu S, Zhang J, Yan Z. Evidence that methylation of hepatitis B virus covalently closed circular DNA in liver tissues of patients with chronic hepatitis B modulates HBV replication. J Med Virol. 2009;81:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 67. | Gong Q, Chen S, Guo J, Sun H, Zheng G, Liu Q, Ren H, He S. Chromosome remodeling related to hepatitis B virus replication in HepG2 cells. DNA Cell Biol. 2011;30:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Urashima T, Saigo K, Kobayashi S, Imaseki H, Matsubara H, Koide Y, Asano T, Kondo Y, Koike K, Isono K. Identification of hepatitis B virus integration in hepatitis C virus-infected hepatocellular carcinoma tissues. J Hepatol. 1997;26:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Anh-Tuan N, Novák E. Detection and quantitation of hepatitis-B surface antigen immune complexes (HBsAg-ICs) by an antigen-specific method. I. Detection and quantitation of in vitro prepared HBsAg-ICs. J Immunol Methods. 1980;33:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Brown SE, Howard CR, Steward MW, Ajdukiewicz AB, Whittle HC. Hepatitis B surface antigen containing immune complexes occur in seronegative hepatocellular carcinoma patients. Clin Exp Immunol. 1984;55:355-359. [PubMed] |
| 71. | Yotsuyanagi H, Yasuda K, Moriya K, Shintani Y, Fujie H, Tsutsumi T, Nojiri N, Juji T, Hoshino H, Shimoda K, Hino K, Kimura S, Iino S, Koike K. Frequent presence of HBV in the sera of HBsAg-negative, anti-HBc-positive blood donors. Transfusion. 2001;41:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | El-Maksoud MA, Habeeb MR, Ghazy HF, Nomir MM, Elalfy H, Abed S, Zaki MES. Clinicopathological study of occult hepatitis B virus infection in hepatitis C virus-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2019;31:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Coppola N, Onorato L, Iodice V, Starace M, Minichini C, Farella N, Liorre G, Filippini P, Sagnelli E, de Stefano G. Occult HBV infection in HCC and cirrhotic tissue of HBsAg-negative patients: a virological and clinical study. Oncotarget. 2016;7:62706-62714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Muto J, Sugiyama M, Shirabe K, Mukaide M, Kirikae-Muto I, Ikegami T, Yoshizumi T, Yamashita YI, Maehara Y, Mizokami M. Frequency and Characteristics of Occult Hepatitis B Infection Among Hepatocellular Carcinoma Patients in Japan. Ann Hepatol. 2018;17:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Hassan ZK, Hafez MM, Mansor TM, Zekri AR. Occult HBV infection among Egyptian hepatocellular carcinoma patients. Virol J. 2011;8:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamal SA, Kao JT S-Editor: Gong ZM L-Editor: Webster JR E-Editor: Qi LL