Published online Apr 28, 2020. doi: 10.13105/wjma.v8.i2.54
Peer-review started: December 29, 2019
First decision: February 29, 2020
Revised: March 26, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: April 28, 2020
Processing time: 120 Days and 17.8 Hours
The number of patients with inflammatory bowel disease (IBD), a group of diseases mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC), has increased in recent decades. As a consequence, the number of people undergoing any drug treatment against these diseases has expanded. However, IBD conventional therapies present several limitations, which lead researchers to look for better alternatives to improve the quality of life of patients. Moreover, microbiome imbalance seems to play a crucial role in the pathogenesis of IBD, since important alterations in bacterial, viral, protist and fungal populations are observed in the gut microbiota of affected individuals. Given the importance of such life forms in that context, the use of probiotics becomes a plausible alternative for treating affected patients. Trials have been developed aiming the evaluation of probiotics potential to induce and to maintain remission in CD and UC. Regarding the tested microorganisms, various non-pathogenic bacteria and fungi have been assessed. However, consistent results have been obtained only with some of them, including Escherichia coli Nissle 1917, VSL#3, Saccharomyces boulardii, Lactobacillus, and Bifidobacterium. Therefore, this minireview aims to explore the role of microbiota in the genesis of such a disorder and to compile the most concrete data on probiotic-related efficiency in IBD treatment.
Core tip: The clinical management of ulcerative colitis and Crohn’s disease represent a major challenge in the gastroenterology field since conventional therapies present several limitations. Interestingly, changes in gut microbiota are linked to the development of these diseases. In this sense, the use of probiotics becomes a plausible alternative for treating affected individuals. Although several microorganisms have been tested for this purpose, satisfactory results have been obtained only with a portion of them. Therefore, this minireview aims to explore the role of microbiota in the pathogenesis of inflammatory bowel disease and to compile the most concrete data on probiotics efficiency in its treatment.
- Citation: Silva NOE, de Brito BB, da Silva FAF, Santos MLC, de Melo FF. Probiotics in inflammatory bowel disease: Does it work? World J Meta-Anal 2020; 8(2): 54-66
- URL: https://www.wjgnet.com/2308-3840/full/v8/i2/54.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i2.54
Inflammatory bowel disease (IBD) is a group of chronic diseases that significantly affects patients quality of life and is mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC)[1]. Although IBD pathophysiology is widely studied and intestinal microbiota seems to play a crucial role in this process, there are still several unclear points about that[2]. However, it is well known that the existence of positive first-degree relatives for these diseases, as well as environmental exposures including psychological stress, antimicrobial use, and dietary factors, are risk factors for IBD development[3-5].
More than 3.6 million people are estimated to be affected by IBD across the globe, though data scarcity from some regions hinders this calculation[6,7]. In addition, recent studies show that its prevalence has risen in recent decades, with an increase of 75% and 60% in the number of UC and CD patients, respectively, in North America and Europe over the last 20 years[7]. This data becomes even more important if we consider the significant negative impacts caused by these diseases in the quality of life of affected individuals, which include social, professional, sexual, self-esteem and functional prejudices[8,9].
Furthermore, current IBD therapy represents an important economic burden to health systems as it is considered one of the most expensive treatments in the gastroenterology field[10]. Besides that, conventional therapeutic options for IBD also present several limitations regarding the adverse effects associated with their use. Such negative points have motivated researchers to look for better alternatives aiming the clinical control of these diseases, and, in this sense, probiotics emerge as a new option, although there is still limited evidence supporting their use[1,11].
According to the World Health Organization, probiotics are “live organisms which when administered in adequate amounts confer a health benefit on the host”[12]. In this framework, the beneficial effects provided by these agents to IBD patients could arise from various mechanisms that potentially promote attenuation of bowel inflammatory activity, such as antimicrobial properties, immune modulation, and improvement of intestinal barrier integrity[13,14].
Various probiotics have been tested in IBD. However, satisfactory effects were observed only with a portion of them, including Escherichia coli Nissle 1917, VSL#3, Saccharomyces boulardii, Lactobacillus, and Bifidobacterium[15-18]. In this context, our study aims to review the main theories about the role of microbiota in IBD pathophysiology and to gather the most consistent results on probiotic-related effectiveness in the treatment of that condition.
The current evidence show that the intestinal microbiota is influenced by various factors and can vary between individuals and even be contrasting in different gastrointestinal areas[19]. Although the complete elucidation of the gut microbiota composition is challenging, it is well established that Bacteroidetes and Firmicutes are its main constituents[20]. It is believed that there is a relationship of commensalism between most microorganisms of the gastrointestinal tract (GIT) and host. Whereas the first ones benefit from the nutrients found in GIT environment, the second one takes advantage from important functions performed by the microbes[21].
Among these functions, we highlight the metabolization of nutrients - such as carbohydrates, lipids, and K and B vitamins[22-26], the protection against pathobionts - producing acids, thickening the protective wall and inducing production of immunoglobulins[27], and the immunomodulation of the innate and adaptive systems[28]. Besides that, the relationship between gut microbiota and human health has been widely discussed, not only in the gastroenterology field, but also when the elucidation of pathological manifestations outside GIT, such as allergic processes and neurodegenerative manifestations, is aimed[29,30].
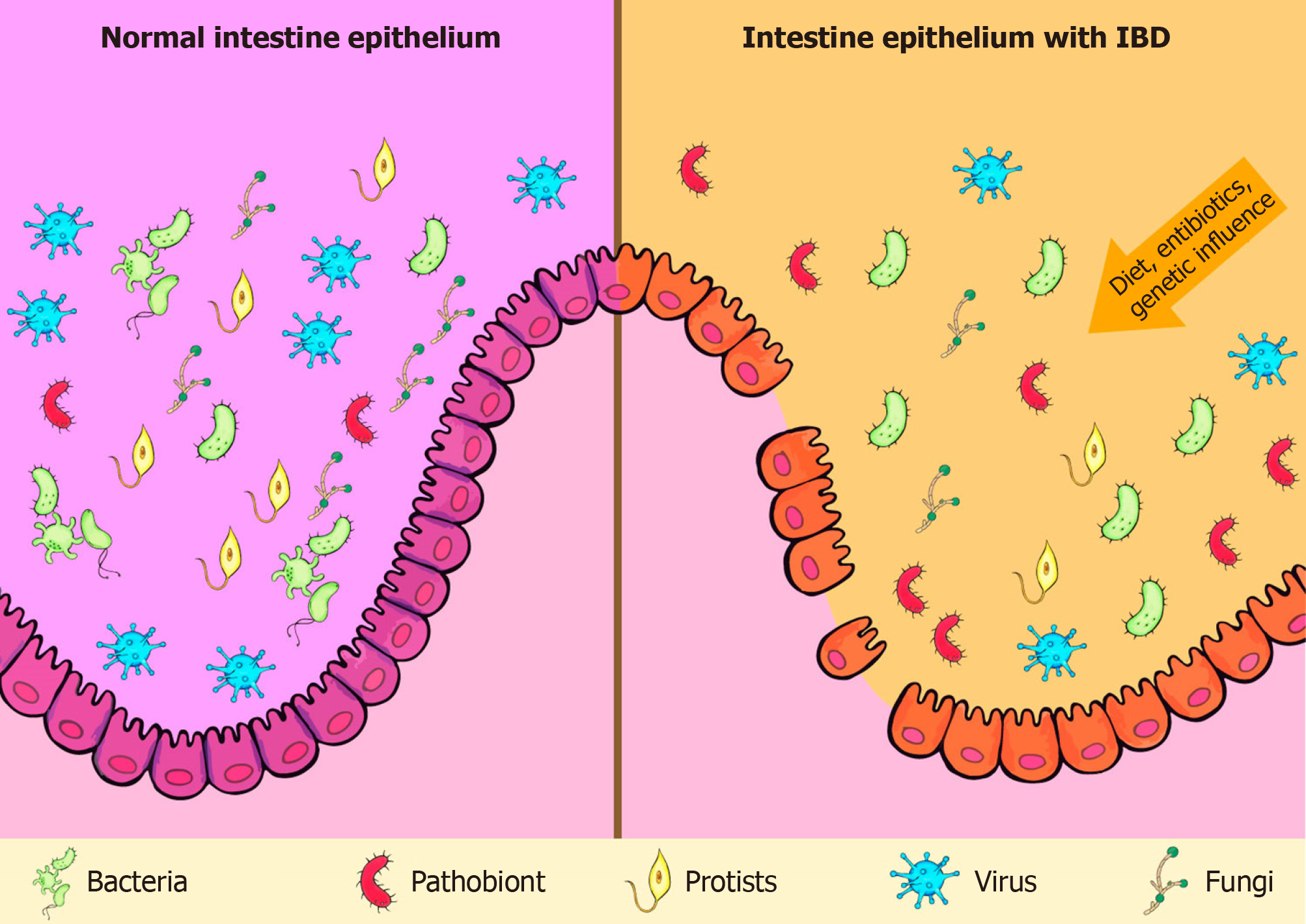
The role of gut microbiota in the pathogenesis of IBD has been extensively discussed. Although intestinal microbiota is mainly represented by bacteria, researches have also highlighted the importance of viruses, fungi, and protists in that process (Figure 1)[31-33]. Moreover, there is no consensus on whether the changes observed in the microbiota of IBD patients are causes or consequences of the disease.
Besides Bacteroides and Firmicutes, Actinobacteria and Proteobacteria phyla make up the group of the most common bacteria in human gut[31,34-38]. However, nowadays, it is being questioned whether this is a pattern among all individuals or whether factors such as genetic susceptibility and inheritance factors can change this profile and facilitate the occurrence of IBD[39].
The decrease of Bacteroides and Firmicutes phyla, as well as the increase of Proteobacteria and Actinobacteria, stand out as the main alterations in the microbiota from feces and intestinal mucosa of affected individuals[40,41]. Furthermore, the abnormal presence of pathogenic microorganisms might also contribute to the above-mentioned imbalance and to IBD emergence, since Mycobacterium avium paratuberculosis, Salmonella, Campylobacter, and Fusobacterium nucleatum have been positively associated with IBD[42-45]. Adherent-invasive E. coli is also supposed to be implicated in IBD pathogenesis, and recent evidences show that its presence not only propitiates the occurrence of IBD but also seems to predispose relapses in affected patients[46]. In addition, studies have correlated IBD relapses to Clostridium difficile infection[47]. Ultimately, Enterobacteriaceae and Streptococcus might also play a role in dysbiosis and further pathogenesis of IBD[48,49], with positive experimental trials for this relation[50].
Few studies evaluated the protective role of gut microbiota against IBD. Presti et al[51] suggest a protective role of Akkermansia muciniphila, since IBD patients had a lower presence of this species when compared to control and irritable bowel syndrome groups. Moreover, decreased abundance of Faecalibacterium prausnitzii in IBD has also been reported[51,52]. It is important to be highlighted that there is also a difference in the composition of the microbiome of IBD patients when comparing active and quiescent phases of the disease[53]. In view of the foregoing, it is conclusive in this topic that not only one, nor a few, but many bacteria can be related to IBD manifestations.
The gut virobiota, unlike the bacterial microbiota, is not well described, neither in healthy individuals nor in IBD patients. It is known that the human gut virome is composed of eukaryotic viruses (e.g., herpesviruses, adenoviruses) and prokaryotic viruses (e.g., Microviridae and Caudovirales). However, many of them are not yet described, since there is a lack of studies on this area[54,55]. A study from 2016 aimed to identify the components of healthy human gut virome, which were divided into three different groups: the core, the common and the unique. The first group contains viruses found in more than half of the analyzed individuals, the second one is composed of species shared by many of the individuals, and the last one includes those found in a limited number of individuals. Drawing attention to the first group, it was noticed that the 23 bacteriophages that composed it were significantly reduced in IBD patients, bringing up the discussion that these common bacteriophages could have an important role in the pathogenesis of UC and CD when reduced[56].
Furthermore, differences have been observed between the gut virobiota of CD and UC patients. An increase of virobiota abundance in UC patients–mainly of Caudovirales bacteriophages-was reported by Zuo et al[57], with a concomitant identification of decreased viral diversity. Among CD patients, Pérez-Brocal et al[58] also observed a dysbiosis in virobiota, with abundance of phages that infect Clostridiales, Alteromonadales, and Clostridium. It was also detected a high abundance of the Retroviridae family in individuals with IBD.
Although mycome represents only 0.1% of the human gut microbiome, a study from 2017 demonstrated that it presents a significant variability between healthy and IBD positive individuals. In the latter, a higher presence of Candida albicans and a lower presence of Saccharomyces, when compared to the control group, was described. Furthermore, the fungal diversity was reduced in the IBD[59]. Tests on animal subjects also corroborates this theory, as mice treated with antifungal drugs presented a higher incidence of acute and chronic colitis when compared to control groups[60].
Regarding the protist microbiota, it is described that the presence of such microorganisms can represent a protective factor against IBD. Comparing the protozoans found in the feces of healthy and IBD positive patients, the latter presented a reduced number of Blastocystis, suggesting that it might play a role in the balance of human healthy gut environment[61].
Concerning individual characteristics that may facilitate the occurrence of IBD, incidence peaks in certain ages (around 25 years old and close to 60 years old) evoke a discussion about what changes in these periods predispose the occurrence of the first episodes of CD and UC. Interestingly, these stages of life are moments in which the microbiome undergoes significant alterations. The first peak is marked by the host adaptation to new microorganisms in intestinal microbiota, while, in the second one, a global decrease in these life forms is observed in the human gut[62].
In the last decades, the scientific community has increasingly investigated the role of host-microbial interactions in the human body immune regulation. Taking into consideration the gastrointestinal scenario, gut microbiome has been described as an integrating system that regulates the intestinal metabolism by means of environmental, genetic and immunological interactions. Therefore, it is expected that disturbances in such an important regulator can lead to complex diseases[63]. Indeed, the normal development of the immune system in the intestine have shown to be directly associated with adequate bacterial colonization during the early life and, in line with that, the result of a study indicated that germ-free mice present deficiencies in their immune functions[64,65]. It is also known that T and B immune cells from the intestinal mucosa play a crucial role in maintaining immune homeostasis, suppressing responses to non-pathogenic antigens and reinforcing the integrity of the intestinal mucosal barrier functions[66]. Among specific mechanisms through which bacteria influence immune response, it has already been observed that segmented filamentous bacteria induce the production of interleukin (IL)-17 and IL-22, which present a pro-inflammatory function[67]. Moreover, a series of 17 bacterial species have shown their potential to stimulate the expression of regulatory T cells and IL-10, which are associated with anti-inflammatory activity[68].
Regarding IBD, recent studies have described that it results from chronic intestinal inflammation which is due to a dysregulation in the expression of pro-inflammatory and anti-inflammatory molecules from the innate and adaptive responses of the intestinal immune system[69]. As an example, a study that evaluated 66 children with early onset IBD found a loss of function in the genes that encode and regulate IL-10 and the IL-10 receptor in those patients, what leads to deficient anti-inflammatory function in the gut environment, favoring the appearance of intestinal diseases[70]. Furthermore, other studies indicate that there is probably an important increase in the expression of pro-inflammatory cytokines in IBD, such as IL-1, IL-6, IL-18, TNF, IL-12, and IL-23, by antigen presenting cells, neutrophils, monocytes, and macrophages[71]. Given the importance of those molecules in IBD, therapeutic alternatives targeting them have been tested. Among which, anti TNF-α agents stand out since they present satisfactory effectiveness in the treatment of ulcerative colitis, being included in the current guidelines for IBD treatment[72]. In summary, the literature has not yet completely understood the role of immunopathogenesis in IBD. In addition, most available data are from association studies and from researches that evaluate molecules expression in patients that already manifested IBD, what impairs the understanding of the immunological phenomenons that occur during the onset of the disease.
Besides the significant negative impacts caused by the disease on the quality of life of patients, important economic impact is generated by IBD treatment, as it is considered one of the most expensive therapeutics in gastroenterology field[73]. Some guidelines have been published over the years to standardize and to guide IBD treatment[74,75]. The current consensus about this issue aim to improve the symptoms and quality of life of individuals, as well as to reduce the risk of complications and surgical interventions. Moreover, the immediate therapeutic target is the induction of clinical remission of the disease and, subsequently, its maintenance[76,77].
Mesalazine, corticosteroids, immunosuppressive drugs, and monoclonal antibodies targeting TNF-α are some of the IBD therapeutic options, which are arranged along with their main adverse effects in Table 1. Some drug classes are used in both CD and UC management, and the therapeutics of this last condition significantly varies according disease activity and extent[78-80]. Furthermore, new research is being conducted on the incorporation of new corticosteroids, biosimilars, TGF-beta, immunomodulators, anti-TNF agents, and even intestinal microbiota manipulation in the treatment of affected individuals[81].
| Classes | Adverse effects | Ref. |
| Aminosalicylates | Mesalazine-nephrotoxicity and pancreatitis; sulfasalazine-blood dyscrasias | [10,11] |
| Antibiotics | Photosensitivity, tendonitis, tendon rupture, cartilage growth inhibition in fetuses and children oral candidiasis, gastrointestinal disorders, peripheral neuropathy | [14-16] |
| Corticosteroids | Acne, moon face and edema, sleep and mood disorders. Posterior subcapsular cataract, osteoporosis, myopathy, and susceptibility to infection. Acute adrenal insufficiency, arthralgia, increased intracranial pressure and pseudo-rheumatism syndrome | [7,20-22] |
| anti-TNF | Septicemia | [28] |
| Thiopurine | Hepatotoxicity, gastric intolerance and pancreatitis | [32] |
| Methotrexate | Nausea, vomiting and diarrhea | [34] |
It is well established that corticosteroid therapy with prednisone, methylpre-dnisolone or budesonide is indicated in the induction of CD remission[82,83]. However, such therapies present important limitations due to their adverse effects, that include cosmetic effects such as acne and moon face, as well as other multisystemic repercussions associated with a prolonged therapy from which stand out posterior subcapsular cataract, osteoporosis, and a higher susceptibility to infections[77,84,85]. Moreover, the abstinence to these drugs is associated with acute adrenal insufficiency, arthralgia, increased intracranial pressure and pseudo-rheumatism syndrome[86]. Budesonide may have fewer side effects when compared to other corticosteroids, but its use is not recommended in severe CD or exacerbations[87].
Clinical trials with antibiotic therapy generally use ciprofloxacin, metronidazole, rifaximin, clarithromycin, and antituberculosis regimens combined or not with steroids or immunosuppressants[88]. Those therapies are often suitable for infectious complications, especially in perianal disease[89]. Adverse effects of the main antibiotics used include photosensitivity, tendinitis, tendon rupture, cartilage growth inhibition in fetuses and children, oral candidiasis, gastrointestinal disorders and may cause peripheral neuropathy[90-92].
Although used in UC, aminosalicylates were initially considered effective in the treatment of mild CD. However, current meta-analyzes have not observed action in preventing relapse with sulfasalazine and mesalazine[93]. Blood dyscrasias are more frequent in use of the first one, whereas nephrotoxicity and pancreatitis are more common adverse effects when the latter treatment is chosen[94,95].
In active CD, the use of anti-TNF therapeutic strategy is effective. In this sense, adalimumab, infliximab and certolizumab are used in both induction and maintenance protocols of CD and UC[77,96]. Infection represents the worst adverse effect on anti-TNF use and, if it occurs, its use shall be suspended due to the risk of septicemia development[97]. Therefore, any presentation of systemic symptoms suggestive of infection in patients under that therapy demand the exclusion of opportunistic infections[98].
Thiopurines are represented by azathioprine or mercaptopurine and they may be used as an adjunctive treatment[99]. The efficacy of this drug class in inflammatory bowel disease is already evidenced by important studies and it is used for both induction and remission of CD[100]. Its main adverse reactions are hepatotoxicity, gastric intolerance and pancreatitis[101]. Another agent with an interesting immunosuppressive action is methotrexate, which can be also used in the scenarios the thiopurines are indicated[102]. Gastrointestinal changes represented by nausea, vomiting and diarrhea are its main adverse effects[103].
Facing the inconveniences associated with the above-mentioned side effects, IBD patients have gradually searched for alternative therapies[104]. Some potential therapies use plants, including Cannabis sativa, and their active ingredients. However, there is no robust evidence that prove their effectiveness in modifying the course of the disease[105]. Moreover, there is a higher prevalence of psychological disorders among IBD patients, such as stress, anxiety and depression[106,107]. These comorbidities calls attention for non-pharmacological therapies aiming the increase of patients’ quality of life, including cognitive and behavioral therapy, hypnotherapy, psychodynamic therapy, meditation, yoga, acupuncture, and exercise, but all of them present a limited level of evidence[104]. In that context, probiotics still have many conflicting works, however, they emerge as a new perspective for the treatment of these diseases[108].
Since microbiota plays a crucial role in IBD pathophysiology, efforts have been directed towards the evaluation of the effectiveness of microbial-based therapies for its management, among which the use of probiotics rises as a promising alternative[109]. It is important to be highlighted that fecal microbiota transplantation is also a possibility that have been tried in this scenario, but a recent meta-analysis that included 18 studies did not demonstrate a consistent effectiveness of that method[110]. Regarding probiotics, several studies have been developed in order to evaluate their potential in inducing and maintaining remission in both CD and UC[111-113]. Moreover, encouraging results have been obtained with the use of non-pathogenic bacteria and fungi in the treatment of these patients (Table 2)[15,114,115].
| Probiotic | Clinical activity in IBD | Ref. |
| Escherichia coli Nissle 1917 | Induction and maintenance of UC remission | [16,57,58,62] |
| VSL#3 | Induction and maintenance of UC remission; prevention of relapses in chronic pouchitis | [65,66,68,69] |
| Saccharomyces boulardii | Clinical remission of UC | [68-70] |
| Bifidobacterium longum | Objective improvements in UC parameters | [71] |
| Lactobacillus acidophilus La-5 + Bifidobacterium BB-12 | Probable improvement of intestinal parameters in IBD | [72] |
In 1997, the Escherichia coli Nissle 1917 (EcN) was tested in a double-blind trial in order to evaluate its efficacy in maintaining UC remission[16]. That study included 120 patients and observed an equivalence between this probiotic and mesalazine in preventing disease relapses, whose rates were 11.3% in mesalazine group and 16.0% in probiotics group, with a relapse-free time of 103 ± 4 d vs 106 ± 5 d, respectively. Since then, other studies on EcN efficacy were performed[116-118], and two meta-analyses reaffirmed the results found in the above-mentioned study[111,112]. The first of them included six trials, embracing 719 patients, and found that EcN induced remission in 61.6% of patients, while in mesalazine that rate was 69.5%[111]. The most recent one, in its turn, comprehended 10 studies, totaling 1049 patients, and observed a related ratio (RR) of 0.94 (95%CI: 0.8-1.03, P = 0.21) in remission rate and of 1.04 (95%CI: 0.82-1.31, P = 0.77) in relapse rate when EcN and Mesalazine groups were compared[114]. Moreover, a current practice position from European Crohn’s and Colitis Organization (ECCO) consider that EcN may be effective in inducing and maintaining remission in UC[109].
Probiotics formulations containing multiple species with different combinations of microorganisms are also commonly applied[115]. The VSL#3 is a widely studied and commercialized combined preparation that contains eight strains of lactic acid-producing bacteria (L. plantarum, L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, B. breve, B. longum, B. infantis, and Streptococcus salivarius subsp. thermophilus)[17]. This formulation was firstly tested in 2000 for maintenance of clinical remission in patients with UC and chronic pouchitis in a double-blind placebo-controlled trial. That study included 40 patients during disease remission and its results pointed to the efficacy of this agent in preventing clinical relapses when compared to placebo[119]. Their results showed that only 15% of the patients who received the probiotic therapy presented relapses within 9 months, while all of the individuals from placebo group (100%) experienced such intercurrences (P < 0.01). After that, encouraging results were observed in the use of VSL#3 aiming the remission of acute mild-to-moderate UC[120-122]. Increased regulatory cytokines levels and reduced pro-inflammatory cytokines and toll-like receptors (TLRs) expression are supposed to be induced by this probiotic[123]. According to a new study that used a murine model, the inhibition of NF-κB and TNF-α expression by means of TLR4-NF-κB signal pathway might play an important role in such promising VSL#3 effects on UC[124]. Recently, a meta-analyses concluded that VSL#3 is effective in preventing pouchitis episodes and may have beneficial effects in inducting UC remission (RR = 1.67, 95%CI: 1.06-2.63, P = 0.03) and in avoiding UC relapses (RR = 0.29, 95%CI: 0.10-0.83, P = 0.02) when compared to placebo.
Besides the presence of Lactobacillus and Bifidobacterium in VSL#3 composition, these genera are also evaluated singly or in other combinations, being them the most clinically tested genera in IBD[114]. With regards to Bifidobacterium, a recent double-blind study including 195 patients found that B. breve strain Yakult fermented milk had no effect in maintaining remission in UC patients[18]. On the other hand, a randomized, placebo-controlled, double-blinded trial that included 56 patients demonstrated that B. longum 536 strain promoted a reduction in UC Disease Activity Index (UCDAI) after 8 wk of treatment (3.8 ± 0.4 at baseline vs 2.6 ± 0.4 at week 8, P < 0.01), while no significant improvement in UCDAI was observed among patients that received placebo (4.5 ± 0.5 at baseline vs 3.2 ± 0.6 at week 8, P = 0.88)[125]. Another trial with 305 IBD patients showed that Lactobacillus acidophilus La-5 associated with Bifidobacterium BB-12 probably improves intestinal parameters of affected individuals by means of increasing the prevalence of probiotic bacteria in intestine and colon[126].
The fungus Saccharomyces boulardii, a yeast that induces anti-inflammatory activity, has also been studied in IBD[15]. Some clinical trials observed satisfactory effects when using S. boulardii for the prevention of relapses in CD patients and in clinical remission of UC. A randomized non blinded study with 32 CD patients showed that the clinical relapses rates during six months in S. boulardii plus mesalazine group (6.25%) were lower than in those patients that used mesalazine alone (37.5%)[127], while the other one found improved bowel permeability among patients in whom this probiotic was added to baseline therapy[128]. Regarding UC, a pilot study found an improvement in Rachmilewitz clinical activity index among treated individuals[129]. However, these researches included small populations and were performed using distinct S. boulardii doses.
It is important to be highlighted that the number of randomized controlled trials that evaluate the efficacy of probiotics in IBD remains low. Besides that, the meta-analyses about this therapy modality present potential biases due to the reduced number of included studies[11,111,112]. Furthermore, the lack of standardization of the therapeutic protocols leads to probiotics administration in different doses and frequency in distinct studies. Moreover, a study showed that probiotics composed by identical microorganisms, when underwent to different manufacturing methods, present distinct metabolic characteristics[130]. Complementarily, recent research showed that the effectiveness of a multispecies probiotic formulation depends on microbial metabolic properties, which affect its anti-inflammatory activity[131].
Considering the apparent pivotal role of microbiota in IBD genesis and the negative points observed in its conventional treatment, the application of microbial-based therapies seems to be a plausible alternative for affected patients with UC disease. To date, the use of probiotics seems to have no consistent benefit in treating DC. Although more evidence is needed in the evaluation of probiotics efficacy, promising results have been obtained in UC, mainly regarding E. coli Nissle 1917 and VSL#3. Lastly, standardizing therapeutic protocols and probiotics manufacturing methods could improve future studies, minimizing their potential biases.
| 1. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 2. | Geboes K, Van Eyken P. Inflammatory bowel disease unclassified and indeterminate colitis: the role of the pathologist. J Clin Pathol. 2009;62:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 3. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3414] [Article Influence: 179.7] [Reference Citation Analysis (12)] |
| 4. | Eckburg PB, Relman DA. The role of microbes in Crohn's disease. Clin Infect Dis. 2007;44:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 415] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2174] [Article Influence: 98.8] [Reference Citation Analysis (2)] |
| 7. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3601] [Article Influence: 257.2] [Reference Citation Analysis (6)] |
| 8. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1601] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 9. | Umanskiy K, Fichera A. Health related quality of life in inflammatory bowel disease: the impact of surgical therapy. World J Gastroenterol. 2010;16:5024-5034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 764] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 11. | Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, Korzenik J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis. 2015;9:86-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Food and Agriculture Organization and World Health Organization Expert Consultation. Health and nutritional properties of powder milk and live lactic acid bacteria. 2001 Oct 4 [cited 22 December 2019]. In: Probiotics in food - Health and nutritional properties and guidelines for evaluation [Internet]. Cordoba 2001: FAO Food and Nutrition Paper. Available from: ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf. |
| 13. | Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2970] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 15. | Dalmasso G, Cottrez F, Imbert V, Lagadec P, Peyron JF, Rampal P, Czerucka D, Groux H, Foussat A, Brun V. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131:1812-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 483] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Mora D, Filardi R, Arioli S, Boeren S, Aalvink S, de Vos WM. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: the case of VSL#3. Microb Biotechnol. 2019;12:1371-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 18. | Matsuoka K, Uemura Y, Kanai T, Kunisaki R, Suzuki Y, Yokoyama K, Yoshimura N, Hibi T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig Dis Sci. 2018;63:1910-1919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5684] [Article Influence: 270.7] [Reference Citation Analysis (3)] |
| 20. | Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | Kabat AM, Srinivasan N, Maloy KJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. 2014;35:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Zhao C, Dong H, Zhang Y, Li Y. Discovery of Potential Genes Contributing to the Biosynthesis of Short-Chain Fatty Acids and Lactate in Gut Microbiota From Systematic Investigation in. E. coli. NPJ Biofilms Microbiomes. 2019;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1397] [Article Influence: 77.6] [Reference Citation Analysis (2)] |
| 24. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1513] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 25. | Baddini Feitoza A, Fernandes Pereira A, Ferreira da Costa N, Gonçalves Ribeiro B. Conjugated linoleic acid (CLA): effect modulation of body composition and lipid profile. Nutr Hosp. 2009;24:422-428. [PubMed] |
| 26. | Devillard E, McIntosh FM, Paillard D, Thomas NA, Shingfield KJ, Wallace RJ. Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology. 2009;155:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 27. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1970] [Article Influence: 179.1] [Reference Citation Analysis (79)] |
| 28. | Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 936] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 29. | Rachid R, Chatila TA. The role of the gut microbiota in food allergy. Curr Opin Pediatr. 2016;28:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Jiang C, Li G, Huang P, Liu Z, Zhao B. The Gut Microbiota and Alzheimer's Disease. J Alzheimers Dis. 2017;58:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 613] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 31. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8109] [Article Influence: 506.8] [Reference Citation Analysis (4)] |
| 32. | Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 511] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 33. | Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8:e66019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 581] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 34. | DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 745] [Article Influence: 74.5] [Reference Citation Analysis (23)] |
| 35. | Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 615] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 36. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3507] [Article Influence: 184.6] [Reference Citation Analysis (1)] |
| 37. | Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2236] [Cited by in RCA: 2652] [Article Influence: 189.4] [Reference Citation Analysis (0)] |
| 38. | Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2376] [Cited by in RCA: 2214] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 39. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 706] [Article Influence: 100.9] [Reference Citation Analysis (2)] |
| 40. | Ryan FJ, Ahern AM, Fitzgerald RS, Laserna-Mendieta EJ, Power EM, Clooney AG, O'Donoghue KW, McMurdie PJ, Iwai S, Crits-Christoph A, Sheehan D, Moran C, Flemer B, Zomer AL, Fanning A, O'Callaghan J, Walton J, Temko A, Stack W, Jackson L, Joyce SA, Melgar S, DeSantis TZ, Bell JT, Shanahan F, Claesson MJ. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat Commun. 2020;11:1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (1)] |
| 41. | Bamola VD, Ghosh A, Kapardar RK, Lal B, Cheema S, Sarma P, Chaudhry R. Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb Ecol Health Dis. 2017;28:1322447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Schultz BM, Paduro CA, Salazar GA, Salazar-Echegarai FJ, Sebastián VP, Riedel CA, Kalergis AM, Alvarez-Lobos M, Bueno SM. A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases. Front Immunol. 2017;8:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Kirk KF, Méric G, Nielsen HL, Pascoe B, Sheppard SK, Thorlacius-Ussing O, Nielsen H. Molecular epidemiology and comparative genomics of Campylobacter concisus strains from saliva, faeces and gut mucosal biopsies in inflammatory bowel disease. Sci Rep. 2018;8:1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, Lynch T, Allen-Vercoe E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 46. | Mirsepasi-Lauridsen HC, Vallance BA, Krogfelt KA, Petersen AM. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 47. | Clayton EM, Rea MC, Shanahan F, Quigley EM, Kiely B, Hill C, Ross RP. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Herrera P, Kwon YM, Ricke SC. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe. 2009;15:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC, Glickman JN, Gevers D, Huttenhower C, Garrett WS. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014;8:1403-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 50. | Lo Presti A, Zorzi F, Del Chierico F, Altomare A, Cocca S, Avola A, De Biasio F, Russo A, Cella E, Reddel S, Calabrese E, Biancone L, Monteleone G, Cicala M, Angeletti S, Ciccozzi M, Putignani L, Guarino MPL. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front Microbiol. 2019;10:1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 51. | Becker C, Neurath MF, Wirtz S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015;56:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 52. | Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, Wang G, Xia B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 53. | Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 54. | Virgin HW. The virome in mammalian physiology and disease. Cell. 2014;157:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 435] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 55. | Karst SM. Viral Safeguard: The Enteric Virome Protects against Gut Inflammation. Immunity. 2016;44:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Manrique P, Bolduc B, Walk ST, van der Oost J, de Vos WM, Young MJ. Healthy human gut phageome. Proc Natl Acad Sci USA. 2016;113:10400-10405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 57. | Zuo T, Lu XJ, Zhang Y, Cheung CP, Lam S, Zhang F, Tang W, Ching JYL, Zhao R, Chan PKS, Sung JJY, Yu J, Chan FKL, Cao Q, Sheng JQ, Ng SC. Gut mucosal virome alterations in ulcerative colitis. Gut. 2019;68:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 58. | Pérez-Brocal V, García-López R, Nos P, Beltrán B, Moret I, Moya A. Metagenomic Analysis of Crohn's Disease Patients Identifies Changes in the Virome and Microbiome Related to Disease Status and Therapy, and Detects Potential Interactions and Biomarkers. Inflamm Bowel Dis. 2015;21:2515-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 958] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 60. | Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe. 2016;19:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 61. | Audebert C, Even G, Cian A; Blastocystis Investigation Group, Loywick A, Merlin S, Viscogliosi E, Chabé M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep. 2016;6:25255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 62. | Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O'Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4586-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1277] [Article Influence: 85.1] [Reference Citation Analysis (3)] |
| 63. | Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1418] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 64. | Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 65. | Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 884] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 66. | Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1347] [Article Influence: 134.7] [Reference Citation Analysis (1)] |
| 67. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3630] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 68. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2211] [Article Influence: 170.1] [Reference Citation Analysis (3)] |
| 69. | Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 70. | Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister ED, Baumann U, Puchalka J, Bohne J, Egritas O, Dalgic B, Kolho KL, Sauerbrey A, Buderus S, Güngör T, Enninger A, Koda YK, Guariso G, Weiss B, Corbacioglu S, Socha P, Uslu N, Metin A, Wahbeh GT, Husain K, Ramadan D, Al-Herz W, Grimbacher B, Sauer M, Sykora KW, Koletzko S, Klein C. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 71. | Ng SC, Benjamin JL, McCarthy NE, Hedin CR, Koutsoumpas A, Plamondon S, Price CL, Hart AL, Kamm MA, Forbes A, Knight SC, Lindsay JO, Whelan K, Stagg AJ. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn's disease. Inflamm Bowel Dis. 2011;17:2027-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Pugliese D, Felice C, Papa A, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Anti TNF-α therapy for ulcerative colitis: current status and prospects for the future. Expert Rev Clin Immunol. 2017;13:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 73. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1043] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 74. | Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT; AGA Institute Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 75. | Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology. 2019;156:748-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 76. | World Gastroenterology Organisation Global Guidelines. Inflammatory Bowel Disease. 2015 August [cited 21 December 2019]. In: [18 pages]. Available from: https://www.worldgastroenterology.org/guidelines/global-guidelines/inflammatory-bowel-disease-ibd/inflammatory-bowel-disease-ibd-english. |
| 77. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P. ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1509] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 78. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 79. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1653] [Article Influence: 118.1] [Reference Citation Analysis (6)] |
| 80. | Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, Colombel JF, D'Haens G, Ghosh S, Marteau P, Kruis W, Mortensen NJ, Penninckx F, Gassull M; European Crohn's and Colitis Organisation (ECCO). European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohns Colitis. 2008;2:24-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 81. | Weisshof R, El Jurdi K, Zmeter N, Rubin DT. Emerging Therapies for Inflammatory Bowel Disease. Adv Ther. 2018;35:1746-1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Macfarlane GT, Cummings JH. Probiotics, infection and immunity. Curr Opin Infect Dis. 2002;15:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Summers RW, Switz DM, Sessions JT, Becktel JM, Best WR, Kern F, Singleton JW. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979;77:847-869. [PubMed] |
| 84. | Schoon EJ, Bollani S, Mills PR, Israeli E, Felsenberg D, Ljunghall S, Persson T, Haptén-White L, Graffner H, Bianchi Porro G, Vatn M, Stockbrügger RW; Matrix Study Group. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn's disease. Clin Gastroenterol Hepatol. 2005;3:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, Pritchard ML, Sandborn WJ. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 640] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 86. | Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut. 2000;46 Suppl 1:i1-i8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249-266. [PubMed] |
| 88. | Nitzan O, Elias M, Peretz A, Saliba W. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol. 2016;22:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 161] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 89. | Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 90. | Park SK, Kim KJ, Lee SO, Yang DH, Jung KW, Duk Ye B, Byeon JS, Myung SJ, Yang SK, Kim JH, Sik Yu C. Ciprofloxacin usage and bacterial resistance patterns in Crohn's disease patients with abscesses. J Clin Gastroenterol. 2014;48:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Bertino J, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798-817; discussion 797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 92. | Sarna JR, Furtado S, Brownell AK. Neurologic complications of metronidazole. Can J Neurol Sci. 2013;40:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Ford AC, Kane SV, Khan KJ, Achkar JP, Talley NJ, Marshall JK, Moayyedi P. Efficacy of 5-aminosalicylates in Crohn's disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 94. | Van Staa TP, Travis S, Leufkens HG, Logan RF. 5-aminosalicylic acids and the risk of renal disease: a large British epidemiologic study. Gastroenterology. 2004;126:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 96. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. 2015;148:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 97. | Colombel JF, Loftus EV, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, Zinsmeister AR, Sandborn WJ. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 623] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 98. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 760] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 99. | Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 100. | Axelrad JE, Roy A, Lawlor G, Korelitz B, Lichtiger S. Thiopurines and inflammatory bowel disease: Current evidence and a historical perspective. World J Gastroenterol. 2016;22:10103-10117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Warner B, Johnston E, Arenas-Hernandez M, Marinaki A, Irving P, Sanderson J. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol. 2018;9:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Chan ES, Cronstein BN. Mechanisms of action of methotrexate. Bull Hosp Jt Dis (2013). 2013;71 Suppl 1:S5-S8. [PubMed] |
| 103. | Gomollón F, Rubio S, Charro M, García-López S, Muñoz F, Gisbert JP, Domènech E; En Representación de GETECCU. [Reccomendations of the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) on the use of methotrexate in inflammatory bowel disease]. Gastroenterol Hepatol. 2015;38:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Torres J, Ellul P, Langhorst J, Mikocka-Walus A, Barreiro-de Acosta M, Basnayake C, Ding NJS, Gilardi D, Katsanos K, Moser G, Opheim R, Palmela C, Pellino G, Van der Marel S, Vavricka SR. European Crohn's and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:673-685e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn's disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455-458. [PubMed] |
| 106. | Cámara RJ, Ziegler R, Begré S, Schoepfer AM, von Känel R; Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS) group. The role of psychological stress in inflammatory bowel disease: quality assessment of methods of 18 prospective studies and suggestions for future research. Digestion. 2009;80:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, Rawsthorne P, Miller N, Rogala L, McPhail CM, Bernstein CN. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 108. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1639] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 109. | Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96:170-178. [PubMed] |
| 110. | Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 111. | Losurdo G, Iannone A, Contaldo A, Ierardi E, Di Leo A, Principi M. Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2015;24:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 112. | Jia K, Tong X, Wang R, Song X. The clinical effects of probiotics for inflammatory bowel disease: A meta-analysis. Medicine (Baltimore). 2018;97:e13792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 114. | Fontana L, Bermudez-Brito M, Plaza-Diaz J, Muñoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr. 2013;109 Suppl 2:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (3)] |
| 115. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 712] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 116. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 707] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 117. | Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 846] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 118. | Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med. 2010;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 961] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 120. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 121. | Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino' S, D'Amico T, Sebkova L, Sacca' N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 361] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 122. | Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 123. | Ng SC, Plamondon S, Kamm MA, Hart AL, Al-Hassi HO, Guenther T, Stagg AJ, Knight SC. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm Bowel Dis. 2010;16:1286-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Wang H, Li S, Li H, DU F, Guan J, Wu Y. Mechanism of Probiotic VSL#3 Inhibiting NF-κB and TNF-α on Colitis through TLR4-NF-κB Signal Pathway. Iran J Public Health. 2019;48:1292-1300. [PubMed] |
| 125. | Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, Kusaka T, Uose S, Hisatsune H, Tojo M, Noda T, Arasawa S, Izuta M, Kubo A, Ogawa C, Matsunaka T, Shibatouge M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016;28:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 126. | Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Effects of Probiotics on Gut Microbiota in Patients with Inflammatory Bowel Disease: A Double-blind, Placebo-controlled Clinical Trial. Korean J Gastroenterol. 2015;65:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci. 2000;45:1462-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 413] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 128. | Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A, Carolina Carneiro Aguirre A, Paiva Martins F, Marcos Andrade Goulart E, Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol. 2008;43:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 129. | Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 130. | Biagioli M, Capobianco D, Carino A, Marchianò S, Fiorucci C, Ricci P, Distrutti E, Fiorucci S. Divergent Effectiveness of Multispecies Probiotic Preparations on Intestinal Microbiota Structure Depends on Metabolic Properties. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 131. | Biagioli M, Laghi L, Carino A, Cipriani S, Distrutti E, Marchianò S, Parolin C, Scarpelli P, Vitali B, Fiorucci S. Metabolic Variability of a Multispecies Probiotic Preparation Impacts on the Anti-inflammatory Activity. Front Pharmacol. 2017;8:505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Abdolghaffari AH, Marteau P, Mazzarella G S-Editor: Wang J L-Editor: A E-Editor: Qi LL