Published online Mar 18, 2024. doi: 10.13105/wjma.v12.i1.90229
Peer-review started: November 27, 2023
First decision: December 28, 2023
Revised: January 5, 2024
Accepted: January 31, 2024
Article in press: January 31, 2024
Published online: March 18, 2024
Processing time: 101 Days and 14.4 Hours
The rising prevalence of carbapenem-resistant Acinetobacter baumannii (CRAB) in neonatal intensive care units (NICUs) represents an escalating challenge in health
To explore the prevalence of CRAB colonization in NICUs, focusing on neonates, healthcare workers, and the en
We conducted according to PRISMA 2020 checklist guidelines, a comprehensive literature search across multiple databases including MEDLINE (Ovid), EMBASE (Ovid), Global Health (Ovid), Web of Science, and Global Index Me
We analyzed 737 records from five databases, ultimately including 13 studies from ten countries. For neonates, the pooled prevalence was 4.8% (95%CI: 1.1% to 10.5%) with the highest rates observed in South-East Asia (10.5%; 95%CI: 2.4% to 23.3%). Among HCWs, a single Indian study reported a 3.3% prevalence. Environmental samples showed a prevalence of 2.3% (95%CI: 0% to 9.3%), with the highest rates in South-East Asia (10%; 95%CI: 4.2% to 17.7%). Significant heterogeneity was found across studies, and no publication bias was detected.
This systematic review highlights a significant prevalence of CRAB colonization in neonates across various regions, particularly in South-East Asia, contrasting with lower rates in high-income countries. The study reveals a gap in research on HCWs colonization, with only a single study from India reporting moderate prevalence. Environmen
Core Tip: This study reveals a notable prevalence of carbapenem-resistant Acinetobacter baumannii colonization in neonatal intensive care units. The analysis revealed a pooled prevalence of 4.8% in neonates, with a considerable gap in research on healthcare workers colonization and a 2.3% prevalence in environmental samples. The substantial heterogeneity across studies and the observed regional variations underlines the need for more targeted research.
- Citation: Mbaga DS, Kenmoe S, Esemu SN, Bowo-Ngandji A, Keneh NK, Tatah Kihla Akoachere JF, Gonsu HK, Ndip Ndip R, Ebogo-Belobo JT, Kengne-Ndé C, Tendongfor N, Assam Assam JP, Ndip LM, Njiki Bikoï J, Riwom Essama SH. Epidemiology of carbapenem-resistant Acinetobacter baumannii colonization in neonatal intensive care units: A systematic review and meta-analysis. World J Meta-Anal 2024; 12(1): 90229
- URL: https://www.wjgnet.com/2308-3840/full/v12/i1/90229.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i1.90229
The escalation of carbapenem-resistant Acinetobacter baumannii (CRAB) in neonatal intensive care units (NICUs) is a mounting concern in the healthcare settings. A study has highlighted the significance of CRAB healthcare-acquired in
The asymptomatic nature of CRAB colonization and the possibility of its transmission to vulnerable neonates with developing immune systems through healthcare workers (HCWs) or the NICU environment exacerbate this risk[4,5]. This scenario is challenging because the absence of symptoms in colonized individuals makes early detection and isolation difficult, increasing the risk of transmission to vulnerable newborns. The findings from a study investigating nosocomial rectal CRAB colonization in a tertiary-care hospital identified several significant risk factors associated with CRAB colonization, notably the use of permanent devices (OR 10.15, 95%CI: 2.27 to 45.39), mechanical ventilation (OR 40.01, 95%CI: 4.05 to 395.1), urinary catheters (OR 4.9, 95%CI: 1.52 to 16.19), a poorer prognosis (OR 5.45, 95%CI: 1.87 to 15.89), increased length of stay (OR 1.03, 95%CI: 1.01 to 1.05), and carbapenem use (OR 5.39, 95%CI: 1.14 to 25.44)[6]. Effective management in NICUs demands a comprehensive strategy encompassing regular screening of neonates and HCWs, strict hand hygiene, thorough environmental cleaning and disinfection, and adherence to infection control protocols[7]. CRAB, identified by the WHO and the Infectious Diseases Society of America (IDSA) as a high-priority pathogen, poses a significant threat due to its resistance to a wide range of antibiotics[8,9]. CRAB’s resistance to a broad range of antibiotics, including cephalosporins, fluoroquinolones, and commonly used hospital antibiotics like piperacillin, ticarcillin, and ampicillin, limits treatment options[10]. Colistin and polymyxin B show the lowest resistance rates, suggesting potential therapeutic alternatives[10]. The prevalence of CRAB colonization in NICUs is subject to significant variation, reflecting disparities in healthcare practices, hospital environments, geographic locations, and patient demographics. Despite the critical impact of CRAB in NICUs, current epidemiological understanding, particularly regarding neonates, HCWs, and the NICU environment, remains limited. The objective of this review is to examine the prevalence of CRAB colonization in NICUs, focusing on neonates, HCWs, and the NICU environment.
The protocol was registered on the International Prospective Register of Systematic Reviews, PROSPERO, as CRD
The search strategy included looking through five databases, such as MEDLINE (Ovid), EMBASE (Ovid), Global Health (O
After removing duplicates from the detected papers across bibliographic databases, the titles and abstracts of the re
The selection was done by two independent reviewers (DSM and SK) based on the predefined inclusion and exclusion criteria. The reviewers individually examined all of the publication titles and abstracts to find potentially qualifying stu
SK and DSM independently examined the data retrieved from selected studies. Data was extracted online by google form and summarized in a Microsoft Excel file. From each study we collected first author names, publication year, reason of exclusion if study were excluded, study design, country of study, sampling method, setting, levels of care, number of si
The studies that met the inclusion criteria were rated for methodological quality by two investigators (SK and DSM) independently. Quality assessment of the included studies were done by using the Hoy et al[12] tools (Supplementary Table 2). Any disagreements were settled verbally, and consensus was obtained.
The analysis was carried out with R software version 4.0.3 utilising the statistical software packages meta (version 4.18-2) and metafor (version 3.0-2)[13,14]. The pooled percentage and 95% confidence interval (CI) were calculated using a ran
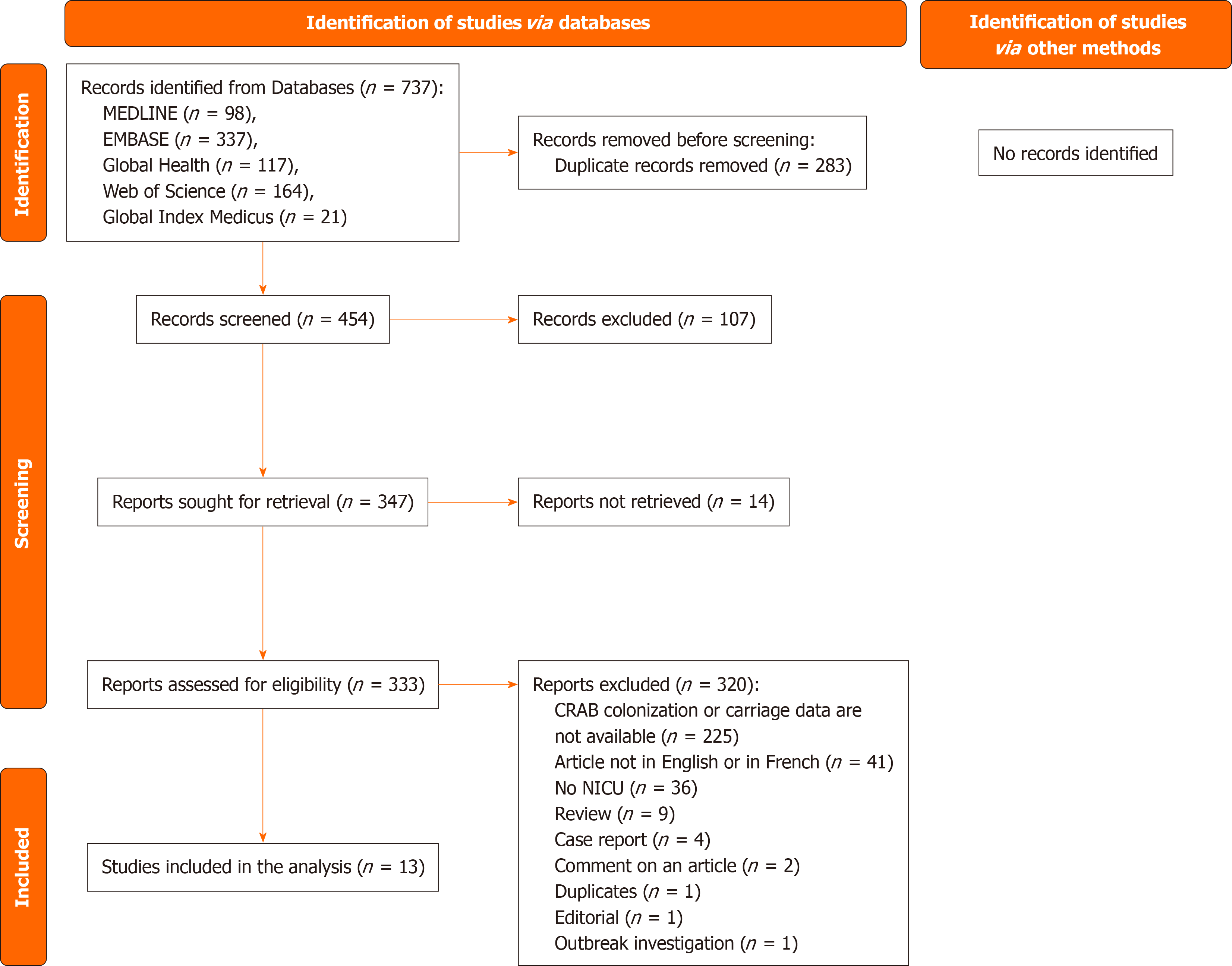
A total of 737 records were identified from five databases: MEDLINE (n = 98), EMBASE (n = 337), Global Health (n = 117), Web of Science (n = 164), and Global Index Medicus (n = 21). Of these, 454 records were screened, leading to the exclusion of 107 reports. Subsequently, 347 reports were sought for retrieval, but 14 could not be retrieved. Upon assessing the 333 retrieved reports for eligibility, 320 were excluded for various reasons, including the absence of CRAB colonization or carriage data (n = 225), language barriers (n = 41), lack of NICU data (n = 36), and other categorizations such as reviews, case reports, article comments, duplicates, editorials, and outbreak investigations. Ultimately, 13 studies were included in the analysis (Figure 1)[19-31].
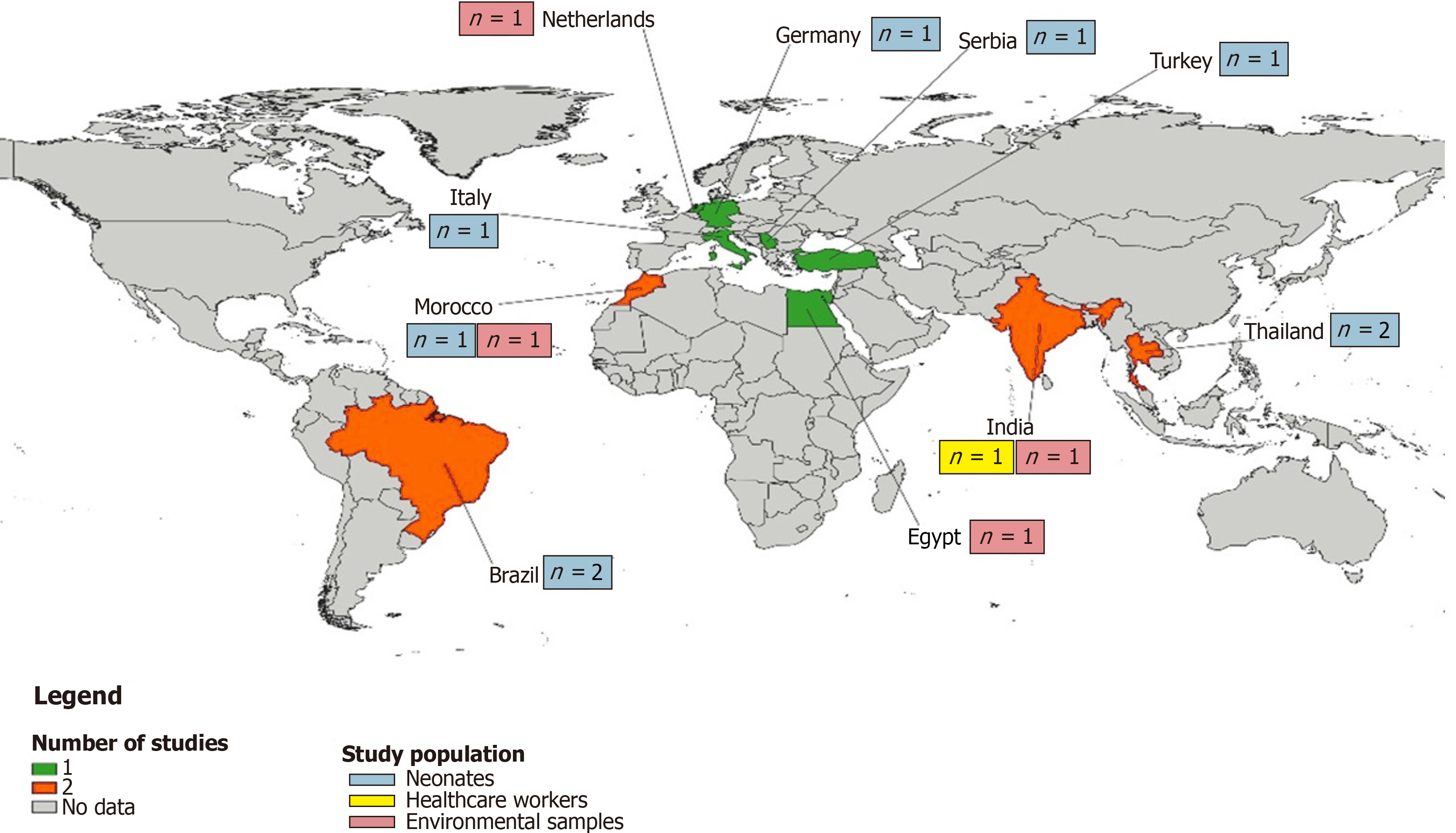
We gathered published data from ten countries, with Brazil, Morocco, and Thailand each contributing two studies, and Egypt, Germany, India, Italy, Netherlands, Serbia, and Türkiye each providing one (Supplementary Table 3). Geographically, the majority of the studies hailed from Europe (n = 5), followed by the Eastern Mediterranean and South-East Asia each with three studies, and America with two studies (Figure 2). When segmented by income, most of the studies were from upper-middle-income countries (n = 6), with lower-middle-income countries providing four and high-income countries three. The majority of these studies were recent, with various participant recruitment periods ranging from January 1989 to February 2020. Concerning the populations under study, neonates dominated the research (n = 9), com
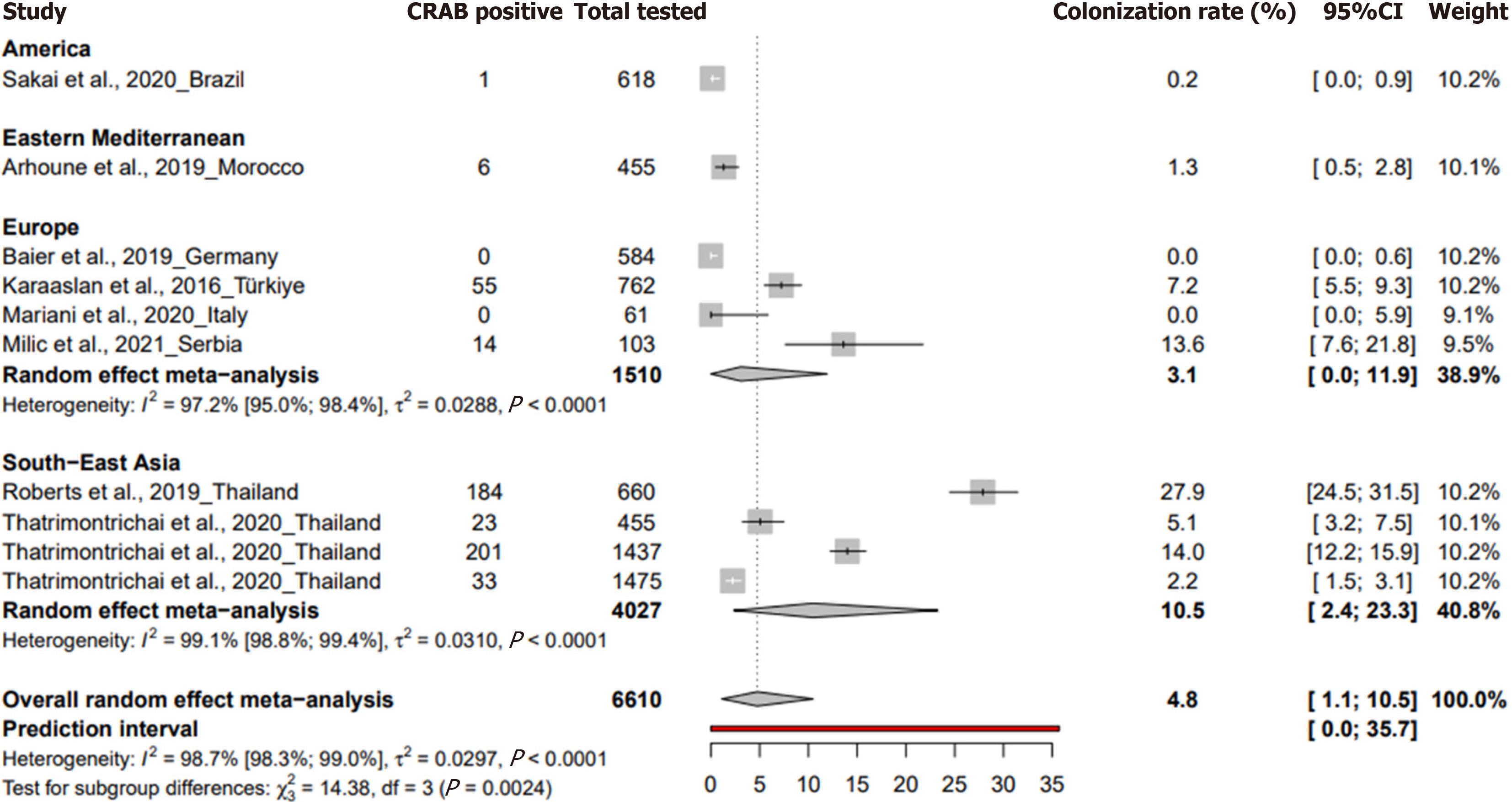
The prevalence of CRAB colonization in neonatal intensive care units for neonates was 4.8% (95%CI: 1.1 to 10.5%) based on 10 studies with 6610 participants, with a heterogeneity of I² = 98.7% (95%CI: 98.3 to 99), indicating significant heterogeneity (Figure 3). A study in Germany during the study period from November 2016 to March 2018 reported a preva
| Prevalence % (95%CI) | 95% prediction interval | N studies | N participants | 1H (95%CI) | 2I2 (95%CI) | P heterogeneity | P egger test | |
| Neonates | 4.8 [1.1-10.5] | [0-35.7] | 10 | 6610 | 8.7 [7.6-9.9] | 98.7 [98.3-99] | < 0.001 | 0.718 |
| HCWs | 3.3 [0-13.8] | NA | 1 | 30 | NA | NA | 1 | NA |
| Environmental samples | 2.3 [0-9.3] | [0-51.9] | 4 | 530 | 2.7 [1.7-4.2] | 86.4 [67-94.4] | < 0.001 | 0.989 |
For HCWs, the prevalence was 3.3% (95%CI: 0 to 13.8) from a singular study conducted in India and involving 30 participants.
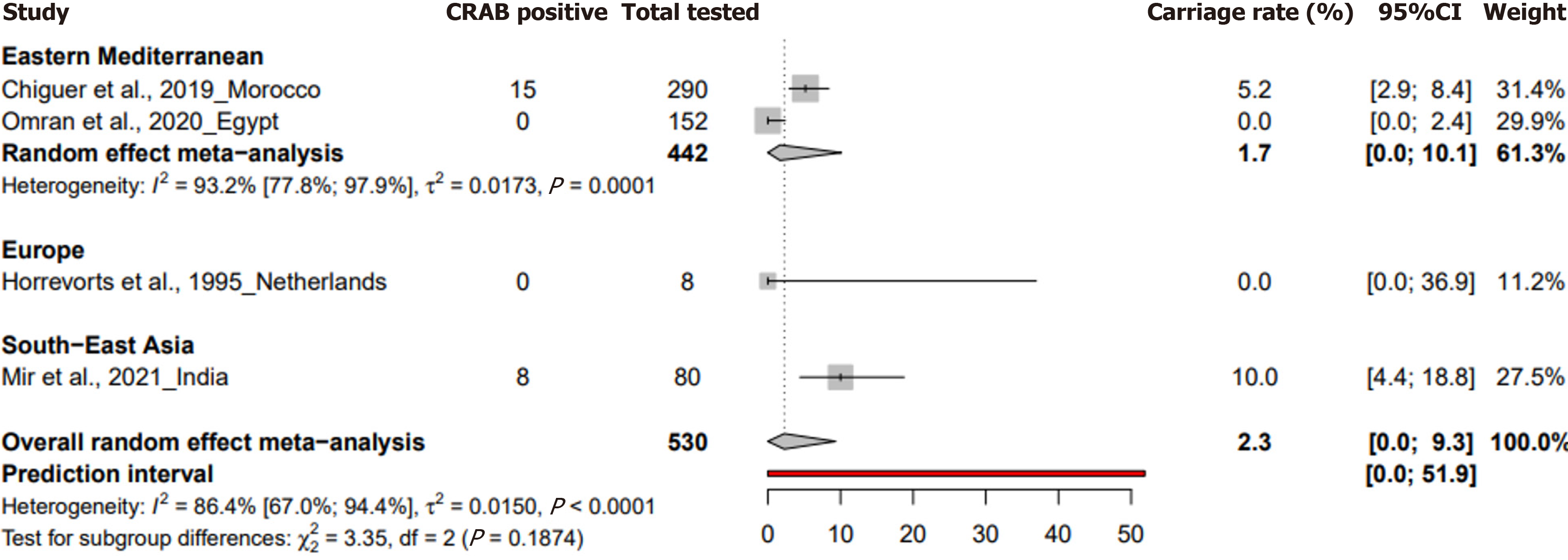
In the case of environmental samples, the prevalence was reported at 2.3% (95%CI: 0 to 9.3) from four studies with 530 samples, showcasing a heterogeneity of I² = 86.4% (95%CI: 67 to 94.4) (Figure 4). P values for heterogeneity were signi
In a subgroup analysis of a systematic review aimed at describing the colonization of CRAB in neonatal intensive care units, among neonates, Serbia reported the highest prevalence at 13.6% (95%CI: 7.6% to 21%) followed by Thailand with 10.5% (95%CI: 2.4% to 23.3%) and Türkiye with 7.2% (95%CI: 5.5% to 9.2%) (Supplementary Table 5). When grouped by WHO regions, South-East Asia had the highest prevalence at 10.5% (95%CI: 2.4% to 23.3%), while Europe reported a pre
The present systematic review is the first to examine CRAB colonization in neonates, HCWs, and environmental samples in NICUs in ten countries, encompassing 13 included studies. The review found a substantial variability in CRAB colo
The review identifies a pooled prevalence of 4.8% (95%CI: 1.1% to 10.5%) among neonates, with notable geographical variability, highlighting the influence of regional socioeconomic factors and healthcare practices. South-East Asia showed the highest prevalence at 10.5% (95%CI: 2.4% to 23.3%), contrasting with minimal rates in high-income countries. This disparity in CRAB colonization rates may be attributed to infection control standards, healthcare infrastructures, distinct local healthcare protocols, environmental conditions, and variations in antibiotic usage, which warrant further detailed investigation to understand their contributions to these regional differences[7,32,33]. However, a significant limitation is the absence of data on neonatal length of stay in NICUs in included studies, a critical factor in assessing colonization risk[34]. Another limitation of this study is the absence of data from low-income countries, which potentially limits the gene
The review also points to a significant knowledge gap regarding HCW colonization, with only one study from India indicating a 3.3% prevalence. Given the potential of HCWs as vectors for asymptomatic transmission of CRAB to highly susceptible neonates, this lack of data hampers the development of comprehensive infection control strategies in non-outbreak settings in NICUs[35]. Environmental samples revealed a pooled prevalence of 2.3% (95%CI: 0% to 9.3%), with a peak prevalence of 10% in South-East Asia (95%CI: 4.2% to 17.7%), suggesting that hospital environments, particularly in resource-limited settings, can act as reservoirs for CRAB, facilitating its spread within NICUs[5,36].
Antimicrobial resistance patterns in neonates are poorly represented in the literature, with only one Brazilian study included, reporting a 100% resistance rate to several antibiotics except for colistin. This finding aligns with Lima's 2019 study, which documented high resistance rates to various antibiotics in CRAB isolates from burn injury patients[10]. The emerging challenge in treating CRAB infections is evident, highlighting the urgent need for judicious antibiotic use and alternative therapeutic strategies[5,37].
In terms of preventive measures, the review includes a study from Thailand, demonstrating a significant reduction in CRAB prevalence in NICUs following specific interventions. This contrasts with Tomczyk's 2019 review, which provides a broader view of effective infection prevention and control measures across various healthcare settings[38]. The specific challenges and needs of neonatal populations in NICUs, however, remain under-researched, underscoring the necessity for more focused interventional studies on effective preventive strategies for this vulnerable group.
This systematic review finds a notable prevalence of CRAB colonization in neonates, with significant regional differences, being higher in South-East Asia and lower in high-income countries. The research on HCWs colonization is limited, with only one study from India indicating a moderate prevalence. Environmental samples also show a moderate CRAB con
The surge of carbapenem-resistant Acinetobacter baumannii (CRAB) in neonatal intensive care units (NICUs) has emerged as a significant healthcare concern, particularly due to its role in healthcare-acquired infections (HAIs). CRAB doubles the mortality risk compared to patients with carbapenem-susceptible Acinetobacter baumannii.
The asymptomatic nature of CRAB colonization, especially in NICU settings, and its potential transmission through healthcare workers (HCWs) or the environment, intensify the risks to vulnerable neonates with developing immune sys
This review aims to examine the prevalence of CRAB colonization in NICUs, focusing on neonates, HCWs, and the NICU environment.
Our systematic review was conducted following the PRISMA 2020 guidelines. We initiated our search across MEDLINE (Ovid), EMBASE (Ovid), Global Health (Ovid), Web of Science, and Global Index Medicus. We also conducted a manual search through the references of relevant papers. Our inclusion criteria focused on studies in English or French that investigated CRAB colonization in neonates, HCWs, and environmental samples using culture or molecular techniques. Studies that did not focus on NICUs, were duplicates, or lacked adequate data were excluded. A random-effects model was applied to calculate the pooled prevalence and 95% confidence intervals, with subgroup analysis stratified by re
Our systematic review collated data from 13 studies across ten countries. We found that neonates had a pooled CRAB colonization prevalence of 4.8%, though this varied widely by region, with South-East Asia reporting the highest rates. The prevalence in HCWs was only documented in a single study from India, suggesting a significant research gap in understanding the role of HCWs as potential vectors in CRAB transmission. Environmental samples exhibited CRAB pre
The study revealed significant geographical variability in CRAB colonization rates, with a pooled prevalence of 4.8% among neonates and notable higher rates in South-East Asia and lower in high-income countries. A critical gap in research was identified regarding HCW colonization, with only a single study from India reporting a prevalence of 3.3%. Environmental samples showed a 2.3% pooled prevalence, with the highest rates again in South-East Asia.
This study underscores the necessity of tailored research and intervention strategies in NICUs to address the unique challenges of neonatal populations and combat the threat of CRAB colonization effectively.
| 1. | Ayobami O, Willrich N, Harder T, Okeke IN, Eckmanns T, Markwart R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Emerg Microbes Infect. 2019;8:1747-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, Kawai K. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 3. | Thatrimontrichai A, Apisarnthanarak A, Chanvitan P, Janjindamai W, Dissaneevate S, Maneenil G. Risk factors and outcomes of carbapenem-resistant Acinetobacter baumannii bacteremia in neonatal intensive care unit: a case-case-control study. Pediatr Infect Dis J. 2013;32:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ng DHL, Marimuthu K, Lee JJ, Khong WX, Ng OT, Zhang W, Poh BF, Rao P, Raj MDR, Ang B, De PP. Environmental colonization and onward clonal transmission of carbapenem-resistant Acinetobacter baumannii (CRAB) in a medical intensive care unit: the case for environmental hygiene. Antimicrob Resist Infect Control. 2018;7:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Jiang Y, Ding Y, Wei Y, Jian C, Liu J, Zeng Z. Carbapenem-resistant Acinetobacter baumannii: A challenge in the intensive care unit. Front Microbiol. 2022;13:1045206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | Meschiari M, Kaleci S, Orlando G, Selmi S, Santoro A, Bacca E, Menozzi M, Franceschini E, Puzzolante C, Bedini A, Sarti M, Venturelli C, Vecchi E, Mussini C. Risk factors for nosocomial rectal colonization with carbapenem-resistant Acinetobacter baumannii in hospital: a matched case-control study. Antimicrob Resist Infect Control. 2021;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Wong SC, Chau PH, So SY, Lam GK, Chan VW, Yuen LL, Au Yeung CH, Chen JH, Ho PL, Yuen KY, Cheng VC. Control of Healthcare-Associated Carbapenem-Resistant Acinetobacter baumannii by Enhancement of Infection Control Measures. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N; WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2062] [Cited by in RCA: 4259] [Article Influence: 532.4] [Reference Citation Analysis (1)] |
| 9. | Nelson R. IDSA releases “hit list”. Lancet Infect Dis. 2006;6:265. [DOI] [Full Text] |
| 10. | Lima WG, Silva Alves GC, Sanches C, Antunes Fernandes SO, de Paiva MC. Carbapenem-resistant Acinetobacter baumannii in patients with burn injury: A systematic review and meta-analysis. Burns. 2019;45:1495-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 5619] [Article Influence: 1123.8] [Reference Citation Analysis (0)] |
| 12. | Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1948] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 13. | Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2715] [Cited by in RCA: 4366] [Article Influence: 272.9] [Reference Citation Analysis (1)] |
| 14. | Schwarzer G. meta: An R Package for Meta-Analysis. 2007; 7: 40-45. |
| 15. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 31161] [Article Influence: 779.0] [Reference Citation Analysis (1)] |
| 16. | Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3226] [Cited by in RCA: 3276] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 17. | Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 1017] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 42511] [Article Influence: 1465.9] [Reference Citation Analysis (4)] |
| 19. | Arhoune B, Oumokhtar B, Hmami F, El Fakir S, Moutaouakkil K, Chami F, Bouharrou A. Intestinal carriage of antibiotic resistant Acinetobacter baumannii among newborns hospitalized in Moroccan neonatal intensive care unit. PLoS One. 2019;14:e0209425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Baier C, Pirr S, Ziesing S, Ebadi E, Hansen G, Bohnhorst B, Bange FC. Prospective surveillance of bacterial colonization and primary sepsis: findings of a tertiary neonatal intensive and intermediate care unit. J Hosp Infect. 2019;102:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Chiguer M, Maleb A, Amrani R, Abda N, Alami Z. Assessment of surface cleaning and disinfection in neonatal intensive care unit. Heliyon. 2019;5:e02966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Horrevorts A, Bergman K, Kollée L, Breuker I, Tjernberg I, Dijkshoorn L. Clinical and epidemiological investigations of Acinetobacter genomospecies 3 in a neonatal intensive care unit. J Clin Microbiol. 1995;33:1567-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Karaaslan A, Soysal A, Altinkanat Gelmez G, Kepenekli Kadayifci E, Söyletir G, Bakir M. Molecular characterization and risk factors for carbapenem-resistant Gram-negative bacilli colonization in children: emergence of NDM-producing Acinetobacter baumannii in a newborn intensive care unit in Turkey. J Hosp Infect. 2016;92:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Maciel WG, da Silva KE, Croda J, Cayô R, Ramos AC, de Sales RO, de Almeida de Souza GH, Bampi JVB, Limiere LC, Casagrande JC, Gales AC, Simionatto S. Clonal spread of carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. J Hosp Infect. 2018;98:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Mariani M, Bandettini R, LA Masa D, Minghetti D, Baldelli I, Serveli S, Mesini A, Saffioti C, Ramenghi LA, Castagnola E. Bacterial invasive infections in a neonatal intensive care unit: a 13 years microbiological report from an Italian tertiary care centre. J Prev Med Hyg. 2020;61:E162-E166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Milic M, Siljic M, Cirkovic V, Jovicevic M, Perovic V, Markovic M, Martic J, Stanojevic M, Mijac V. Colonization with Multidrug-Resistant Bacteria in the First Week of Life among Hospitalized Preterm Neonates in Serbia: Risk Factors and Outcomes. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Mir MA, Ashraf MW, Tripathi V, Mir BA. Isolation, characterization and prevention of various microbial strains in NIC unit and PIC unit. Sci Rep. 2021;11:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Omran EA, Eisa FF, Bakr WMK. Microbial Contamination of Neonatal Injectable Lipid Emulsions at 12 and 24 Hours' Infusion Time With Evaluation of Infection Control Measures. J Pediatr Pharmacol Ther. 2020;25:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 29. | Roberts T, Limmathurotsakul D, Turner P, Day NPJ, Vandepitte WP, Cooper BS. Antimicrobial-resistant Gram-negative colonization in infants from a neonatal intensive care unit in Thailand. J Hosp Infect. 2019;103:151-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Sakai AM, Iensue TNAN, Pereira KO, Silva RLD, Pegoraro LGO, Salvador MSA, Rodrigues R, Capobiango JD, Souza NAA, Pelisson M, Vespero EC, Yamauchi LM, Perugini MRE, Yamada-Ogatta SF, Rossetto EG, Kerbauy G. Colonization profile and duration by multi-resistant organisms in a prospective cohort of newborns after hospital discharge. Rev Inst Med Trop Sao Paulo. 2020;62:e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Thatrimontrichai A, Pannaraj PS, Janjindamai W, Dissaneevate S, Maneenil G, Apisarnthanarak A. Intervention to reduce carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2020;41:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Jung J, Choe PG, Choi S, Kim E, Lee HY, Kang CK, Lee J, Park WB, Lee S, Kim NJ, Choi EH, Oh M. Reduction in the acquisition rate of carbapenem-resistant Acinetobacter baumannii (CRAB) after room privatization in an intensive care unit. J Hosp Infect. 2022;121:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Meschiari M, Lòpez-Lozano JM, Di Pilato V, Gimenez-Esparza C, Vecchi E, Bacca E, Orlando G, Franceschini E, Sarti M, Pecorari M, Grottola A, Venturelli C, Busani S, Serio L, Girardis M, Rossolini GM, Gyssens IC, Monnet DL, Mussini C. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob Resist Infect Control. 2021;10:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Sultan AM, Seliem WA. Identifying Risk Factors for Healthcare-Associated Infections Caused by Carbapenem-Resistant Acinetobacter baumannii in a Neonatal Intensive Care Unit. Sultan Qaboos Univ Med J. 2018;18:e75-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Blanco N, O'Hara LM, Harris AD. Transmission pathways of multidrug-resistant organisms in the hospital setting: a scoping review. Infect Control Hosp Epidemiol. 2019;40:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Chia PY, Sengupta S, Kukreja A, S L Ponnampalavanar S, Ng OT, Marimuthu K. The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Antimicrob Resist Infect Control. 2020;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Isler B, Doi Y, Bonomo RA, Paterson DL. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 38. | Tomczyk S, Zanichelli V, Grayson ML, Twyman A, Abbas M, Pires D, Allegranzi B, Harbarth S. Control of Carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in Healthcare Facilities: A Systematic Review and Reanalysis of Quasi-experimental Studies. Clin Infect Dis. 2019;68:873-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Microbiology
Country/Territory of origin: Cameroon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lv L, China S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH