Published online Mar 18, 2024. doi: 10.13105/wjma.v12.i1.87026
Peer-review started: July 19, 2023
First decision: August 17, 2023
Revised: September 26, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: March 18, 2024
Processing time: 232 Days and 9.1 Hours
Lateral ankle sprains are the most common traumatic musculoskeletal injuries of the lower extremity, with an incidence rate of 15%-20%. The high incidence and prevalence highlights the economic impact of this injury. Ankle sprains lead to a high socioeconomic burden due to the combination of the high injury incidence and high medical expenses. Up to 40% of patients who suffer from an ankle sprain develop chronic ankle instability. Chronic instability can lead to prolonged periods of pain, immobility and injury recurrence. Identification of factors that influence return to work (RTW) and return to sports (RTS) after a lateral ankle sprain (LAS) may help seriously reduce healthcare costs.
To explore which factors may potentially affect RTW and RTS after sustaining an LAS.
EMBASE and PubMed were systematically searched for relevant studies published until June 2023. Inclusion criteria were as follows: (1) Injury including LAS or chronic ankle instability; (2) Described any form of treatment; (3) Assessment of RTW or RTS; (4) Studies published in English; and (5) Study designs including randomized controlled clinical trials, clinical trials or cohort studies. Exclusion criteria were: (1) Studies involving children (age < 16 year); or (2) Patients with concomitant ankle injury besides lateral ankle ligament damage. A quality assessment was performed for each of the included studies using established risk of bias tools. Additionally quality of evidence was assessed using the GRADEpro tool in cases where outcomes were included in the quantitative analysis. A best evidence synthesis was performed in cases of qualitative outcome analysis. For all studied outcomes suitable for quantitative analysis a forest plot was created to calculate the effect on RTW and RTS.
A total of 8904 patients were included in 21 studies, 10 randomized controlled trials, 7 retrospective cohort studies and 4 prospective cohort studies. Fifteen studies were eligible for meta-analysis. The overall RTS rate ranged were 80% and 83% in the all treatments pool and surgical treatments pool, respectively. The pooled mean days to RTS ranged from 23-93 d. The overall RTW rate was 89%. The pooled mean time to RTW ranged from 5.8-8.1 d. For patients with chronic ankle instability, higher preoperative motivation was the sole factor significantly and independently (P = 0.001) associated with the rate of and time to RTS following ligament repair or reconstruction. Higher body mass index was identified as a significant factor (P = 0.04) linked to not resuming sports or returning at a lower level (median 24, range 20-37), compared to those who resumed at the same or higher level (median 23, range 17-38). Patients with a history of psychological illness or brain injury, experienced a delay in their rehabilitation process for sprains with fractures and unspecified sprains. The extent of the delayed rehabilitation was directly proportional to the increased likelihood of experiencing a recurrence of the ankle sprain and the number of ankle-related medical visits. We also observed that 10% of athletes who did return to sport after lateral ankle sprain without fractures described non-ankle-related reasons for not returning.
All treatments yielded comparable results, with each treatment potentially offering unique advantages or benefits. Preoperative motivation may influence rehabilitation after LAS. Grading which factor had a greater impact was not possible due to the lack of comparability among the included patients.
Core Tip: Our findings indicated that all treatments yielded comparable results, with each treatment potentially offering unique advantages or benefits. The effect of preoperative motivation on the delay of rehabilitation after an ankle sprain can be substantial and multifaceted. Psychological factors can influence an individual’s perception of the severity of their injury and their perceived control over the recovery and can have an impact on an individual’s willingness, motivation and ability to engage in the rehabilitation process. Lack of studies and the different ways that return to sport or return to work was defined can cause potential limitations in the interpretation of the results.
- Citation: Maria PA, Vuurberg G, Kerkhoffs GM. Exploring influences and risk of bias of studies on return to sport and work after lateral ankle sprain: A systematic review and meta-analysis. World J Meta-Anal 2024; 12(1): 87026
- URL: https://www.wjgnet.com/2308-3840/full/v12/i1/87026.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i1.87026
Ankle sprains are the most common traumatic musculoskeletal injury of the lower extremities in individuals who engage in physical activity, including both athletes and non-athletes[1-3]. This is especially applicable for lateral ankle sprains (LAS), which account for 80% of ankle sprains, with an incidence rate of 15%-20%[1-4]. Sports such as basketball, soccer, running, American football and volleyball are specifically associated with an increased risk of ankle sprains[5-8]. Every day, approximately one LAS is recorded per 10000 people in Western countries[9]. Annually, approximately 1.6 million patients suffer from an LAS of which as many as 8000 are hospitalized in the United States alone[10]. In the United Kingdom the incidence at emergency departments was estimated to be 5.7 per 10000 persons per year[10,11].
The estimated incidence of ankle sprains, including both first-time and recurrent sprains, is between 2.15 and 3.29 per 1000 persons per year[12]. Up to 40% of these patients develop chronic ankle instability (CAI), which can lead to prolonged periods of pain, immobility and injury recurrence[13,14]. Yet despite the high incidence and high risk of developing CAI, only about 50% of the patients who sustained an LAS seek medical attention[15].
Ankle injuries not only inflict pain and functional restriction but also result in sick days at work and the inability to participate in sports. Additionally, LASs are directly correlated to low work efficiency in the days following ankle trauma, depending on the work-related ankle loading demands[10]. The high incidence and prevalence in combination to loss of working days and high medical expenses highlights the great socioeconomic impact of this injury. The total costs per individual for LAS management varies widely ranging from 360 to 1300 euros. In the Netherlands, approximately 187.2 million euros are spent annually on the treatment of sports-related ankle sprains alone[13,14].
The journey towards optimal recovery, particularly the return to sport (RTS) and return to work (RTW) following an ankle sprain, has been a focal point of extensive research in recent years. Authors typically advocate for nonsurgical treatments, such as immobilization, bandages, tape, braces, and balance training, as primary options for managing lateral ankle sprains.
As the understanding of the multifaceted nature of ankle sprains has evolved, so too has the emphasis on elucidating the diverse factors that influence the rehabilitation process. This meta-analysis aimed to synthesize the latest research findings pertaining to the intricate interplay of physical, psychological and biomechanical elements that contribute to RTS and RTW after LAS.
The purpose of this study was to determine the top ten factors that influence RTW and RTS in patients who sustained an LAS. In April 2023, a systematic search was conducted in EMBASE and PubMed to identify all relevant studies published until May 2023. The search consisted of the search entries: (1) Ankle sprain; (2) Return to work and return to sports; and (3) Treatments and their corresponding synonyms (Supplementary material).
Null hypothesis (H0): There is no significant influence of specific interventions, rehabilitation strategies or individual factors on the RTS and RTW after an LAS.
Alternative hypothesis (H1): Certain interventions, rehabilitation strategies or individual factors significantly influence the rate and success of RTS and RTW following an LAS.
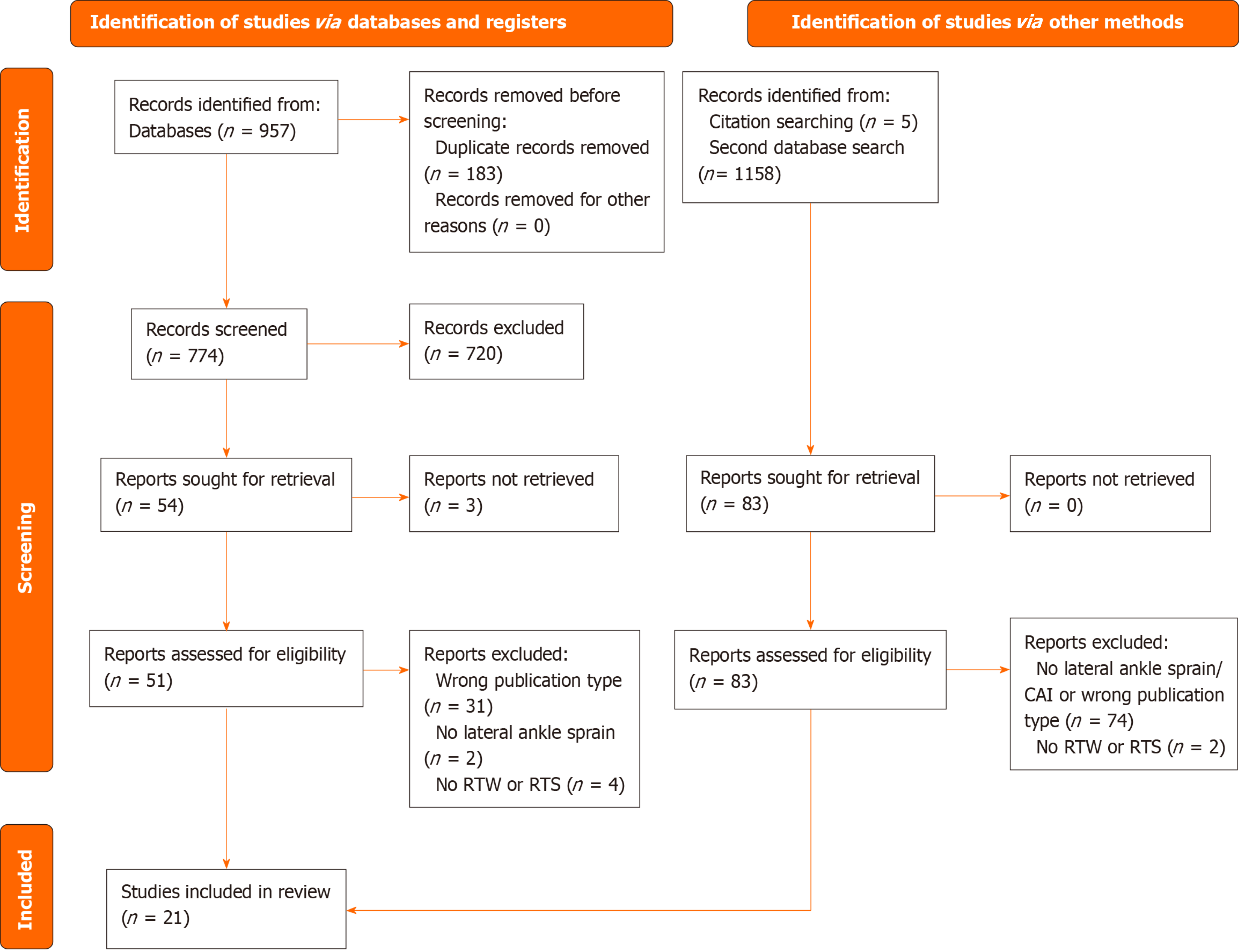
Two authors independently screened the identified studies for relevance based on titles and abstracts using Rayyan QCRI[16] as data management software. If there was any disagreement the studies were openly discussed to reach consensus on initial inclusion or exclusion. Full texts of all potentially relevant studies were screened and selected based on the predefined inclusion and exclusion criteria. The selection process is shown in the PRISMA Flow Diagram in Figure 1[17].
Studies with the following criteria were included: (1) Patients who sustained an LAS or suffered from CAI; (2) Patients received any form of treatment; (3) They assessed RTW or RTS; (4) Studies were published in English; and (5) The study designs included randomized controlled clinical trials (RCTs), clinical trials or cohort studies. Exclusion criteria were: (1) Studies involving children (age < 16 year); or (2) Patients with solely concomitant ankle injury besides lateral ankle ligament damage.
A comprehensive quality assessment was conducted by scoring the risk of bias of each of the included studies using established tools and scales depending on each study design.
For the cohort studies and clinical trials, the Risk of Bias in Non-randomized Studies-Interventions[18] was used to assess the risk of bias in non-randomized therapy studies. The risk was scored as ‘low’, ‘moderate’, ‘serious’ or ‘critical risk’. The lowest scored category was decisive for the overall risk of bias. For RCTs, the Cochrane Risk of Bias Tool[19] was used to assess the risk of bias. The risk of bias for the RCTs was scored as ‘low’, ‘high’ or ‘unclear’ per key domain. Overall low risk of bias was only assigned to studies that scored low risk of bias for all key domains. When one or more key domains were scored as high risk of bias, the overall risk of bias was scored as being high. All other scenarios were scored ‘unclear’ for the overall risk of bias.
Additionally, quality of evidence was assessed per outcome. The quality of summarized evidence in the quantitative analysis was assessed using the GRADEpro GDT tool[20]. Quality of evidence was scored as ‘high’, ‘substantial’, ‘moderate’, ‘low’ or ‘very low.’ In cases where < 3 studied the same outcome and a meta-analysis could not be performed, outcomes were included in the qualitative assessment. The quality of these outcomes was scored using a best evidence synthesis[21] (Table 1).
| Level of evidence | Study design |
| Level 1 | Systematic Review or multiple RCTs |
| Level 2 | One RCT or multiple comparative studies |
| Level 3 | One comparative study or non-comparative research |
| Level 4 | Expert opinion |
Study characteristics were collected including the first author, year of publication and study design. Then, baseline characteristics of each study were extracted including number of included patients, patient characteristics (including age, sex and weight), duration of follow-up, treatment type, studied outcomes (minimally including RTW and RTS) and injury recurrence. If reported whether patients represented a specific group within the population (i.e. athletes or military) this was also recorded.
A quantitative and qualitative analysis was performed. For inclusion in the quantitative analysis, at least three studies reporting the same outcome variable were required. In cases where less than three studies reported the specific outcome measure, or heterogeneity was considerable (≥ 75%), studies were included in the qualitative analysis.
To determine the degree of heterogeneity the I2 was used. The I2 was interpreted as 0%-40% representing no important heterogeneity, 30%-49% representing moderate heterogeneity, 50%-90% representing substantial heterogeneity and 75%-100% meaning considerable heterogeneity[22]. For the analysis and calculation of the effect, correction for heterogeneity was performed using a fixed model (I2 < 50%) or a random effects model (I2 ≥ 50%).
To calculate the effect of each studied outcome (i.e. factor) on RTW and RTS a forest plot was created. Using RevMan (V.5.4, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) the mean difference or odds ratio and corresponding 95% confidence interval (95%CI) were calculated. A P value of < 0.05 was considered significant.
The initial search identified 957 potential studies. After removing 183 duplicates and screening of titles and abstracts, 59 were selected for full-text review. A secondary search yielded 1158 potential studies. Of these, 78 studies remained after removal of duplicates, exclusion for not meeting eligibility criteria or being previously reviewed in the initial search. After screening the remaining 78 records, 12 studies were selected for full-text assessment. Lastly, a reference search yielded five additional studies. Fifty-nine studies from the initial search, twelve studies from the secondary search and five studies from the reference search remained for full-text assessment. Resulting in a final selection of 21 eligible studies for qualitative and quantitative syntheses. The inclusion process of studies is illustrated by the PRISMA flowchart in Figure 1. All included studies were published in English. Publication dates of included studies ranged from 1995 to 2023.
A total of 8904 patients were included in these 21 studies, 10 randomized controlled trials, 7 retrospective cohort studies and 4 prospective cohort studies. Of the studies that provided information on age, the mean age of included patients was 28 years. Of the studies that reported on sex, a total of 957 (36%) were female and 1682 (65%) were male. Eleven studies reported on RTS. Eight studies reported on RTW. These patients received surgical treatment, functional treatment or no treatment. One study reported solely on costs. Within these studies no significant difference was found in age, sex, laterality or between team or individual sports. The follow-up period ranged from 1.5-84.0 mo. Eleven (52%) studies assessed surgical interventions, whereas 10 studies (48%) assessed nonsurgical interventions, such as physical therapy, brace, tape or external support bandage (Table 2).
| Ref. | Study design | RTS or RTW | Sample size, n | Sex, F:M | Age, yr (± SD) | Patient population | Follow-up, month (± SD) | Loss to follow-up |
| Avci et al[25], 1998 | RCT | RTW | 57 (64 enrolled) | 20:37 | Mean = 28.9 (NR) | General | 1.5 | 7 (11) |
| Bouveau et al[32], 2022 | RCS | RTS | 40 | 17:23 | Median = 32.9 (range: 15.6-59.9) | Active population | Median 28.8 | NA |
| Cooke et al[13], 2009 | RCT | RTW | 584 | 247:337 | Mean = 30 (± 10.8) | General | 9 | 34 (6) |
| Eiff et al[35], 1994 | RCT | RTW | 82 | NR | Range = 16-50 (mean/median: NR) | Military | 12 | 6 (7) |
| Hong et al[40], 2022 | PCS | RTS | 147 | 16:131 | Mean = 24.4 (± 4.9) | Athletes | 24 | 0 |
| Hou et al[30], 2022 | RCT | RTS | 70 | 36:34 | Arthroscopic: Mean = 28.3 (± 5.4); open surgery mean = 28.6 (± 4.8) | Active population | 24 | 10 (13) |
| Hupperets et al[27], 2009 | RCT | RTS | 552 | 248:274 | Mean = 28 (± 11.7) | Athletes | 12 | 14 (3) |
| Leanderson et al[38], 1995 | RCT | RTW | 73 | 25:48 | Mean = 28 (NR) | General | 2.5 | NR |
| Lee et al[41], 2019 | PCS | RTW | 18 | 9:9 | Mean = 19.3 (± 3.0) | Athletes | Mean = 28.8 (± 4.3) | 0 |
| Lee et al[42], 2020 | RCS | RTW | 125 | 35:90 | Mean = 32 (± 7) | Military | Min 12, Mean 84 (NR) | NR |
| Liu et al[43], 2022 | RCS | RTS | 64 | 20:44 | Mean one anchor = 30.5 (± 9.5); mean two anchor = 29.6 (± 8.0) | Active population | 24 | NR |
| May et al [42], 2022 | RCS | RTS | 59 | 21:20a | Mean returners = 27.2 (± 9.3); mean non-returners = 27.1 (± 7.7) | Active population | Min 24 | 18 (30) |
| Melton et al[33], 2018 | RCS | RTW | 127 | 10:117 | Mean = 30.4 (± 6) | Military | 1 | 0 |
| O’Connor et al[26], 2020 | RCT | RTW | 60 | 20:40 | Mean = 29.5 (NR) | General | 1 | 10 (17%) |
| Punt et al[28], 2015 | RCT | RTS | 90 | 39:51 | Wii Fit™ mean = 34.3 (± 10.7); physical therapy mean = 34.7 (± 11.3); no therapy mean = 33.5 (± 9.5) | General | 1.5 | 2 (2%) |
| Razzano et al[29], 2019 | RCT | RTS | 61 | 28:33 | Mean = 23 (NR) | Athletes | 4 | 0 |
| Rhon et al[36], 2021 | RCS | RTS | 6150 | 8818: | Median = 31.75 (range: NR)b | Military | 12 | NA |
| Slatyer et al[24], 1997 | RCT | RTW | 364 | 54:310 | Range = 18-35 (median: NR) | Military | 6 | 0 |
| Takao et al[31], 2020 | PCS | RTS | 93 | 65:28 | Mean = 22.2 (± 12.5) | Athletes | 12 | NR |
| Wang et al[34], 2023 | RCT | RTS and RTW | 64 | 39:22 | Open mean = 28.6 (± 8.1); arthroscopic mean = 27.1 (± 7.7) | Generalized joint laxity patients | 24 | 3 (4.7) |
| White et al[23], 2016 | PCS | RTS | 42 | 5:37 | Median = 22 (range: NR) | Elite athletes | Median = 44 (range: NR) | 0 |
All 18 included studies were assessed for study quality using corresponding risk of bias tools depending on the study design. Of the 14 included studies a total of 5 studies were cohort studies. From the studies assessed according to the Risk of Bias in Non-randomized Studies-Interventions scale, 100% of studies had an overall moderate risk of bias. The moderate risk of bias was mainly due to moderate assessment of confounders (n = 3), uncertainties in the patient selection process (n = 3) and missing data (Table 3). The lowest risk of bias was seen in the cohort study by White et al[23], which only scored moderate risk of bias for patient selection.
| Ref. | Confounding | Selection of participants | Classification of interventions | Deviation from intended interventions | Missing data | Measurements of outcomes | Selection of the reported results | Overall risk of bias |
| Bouveau et al[32], 2022 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Hong et al[40], 2022 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Lee et al[41], 2019 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Lee et al[42], 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Liu et al[43], 2022 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| May et al[44], 2022 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Melton et al[33], 2018 | Moderate | Low | Low | Low | Moderate | low | low | Moderate |
| Rhon et al[36], 2021 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Takao et al[31], 2020 | Low | Moderate | Low | Low | Moderate | Moderate | Low | Moderate |
| White et al[23], 2016 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
The remaining nine studies (64%) included RCTs. Only Slatyer et al[24] scored an overall low risk of bias for study quality. This high risk of bias of the remaining eight studies was explained by unclarity or missing information regarding the blinding of patients and personnel. Two studies, Avci et al[25] and O’Connor et al[26] additionally scored a high risk of bias for outcome blinding. Despite the overall high risk of bias, Hupperets et al[27], Punt et al[28] and Razzano et al[29] scored well on five out of six key domains (Table 4).
| Ref. | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Overall risk |
| Avci et al[25], 1998 | Low | Low | High | High | Low | Low | High |
| Cooke et al[13], 2009 | Low | Low | High | Unclear | Low | Low | High |
| Eiff et al[35], 1994 | Low | Unclear | High | Unclear | Low | Low | High |
| Hou et al[30], 2022 | Low | High | High | Unclear | Low | Low | High |
| Hupperts et al[27], 2009 | Low | Low | High | Low | Low | Low | High |
| Leanderson et al[38], 1995 | Low | Unclear | High | Unclear | Low | Low | High |
| O’Connor et al[26], 2010[26] | Low | Low | High | High | Low | Low | High |
| Punt et al[28], 2015 | Low | Low | High | Low | Low | Low | High |
| Razzano et al[29], 2019 | Low | Low | High | Low | Low | Low | High |
| Wang et al[34], 2023 | High | High | High | Unclear | Low | Low | High |
| Slatyer et al[24], 1997 | Low | Low | Low | Low | Low | Low | Low |
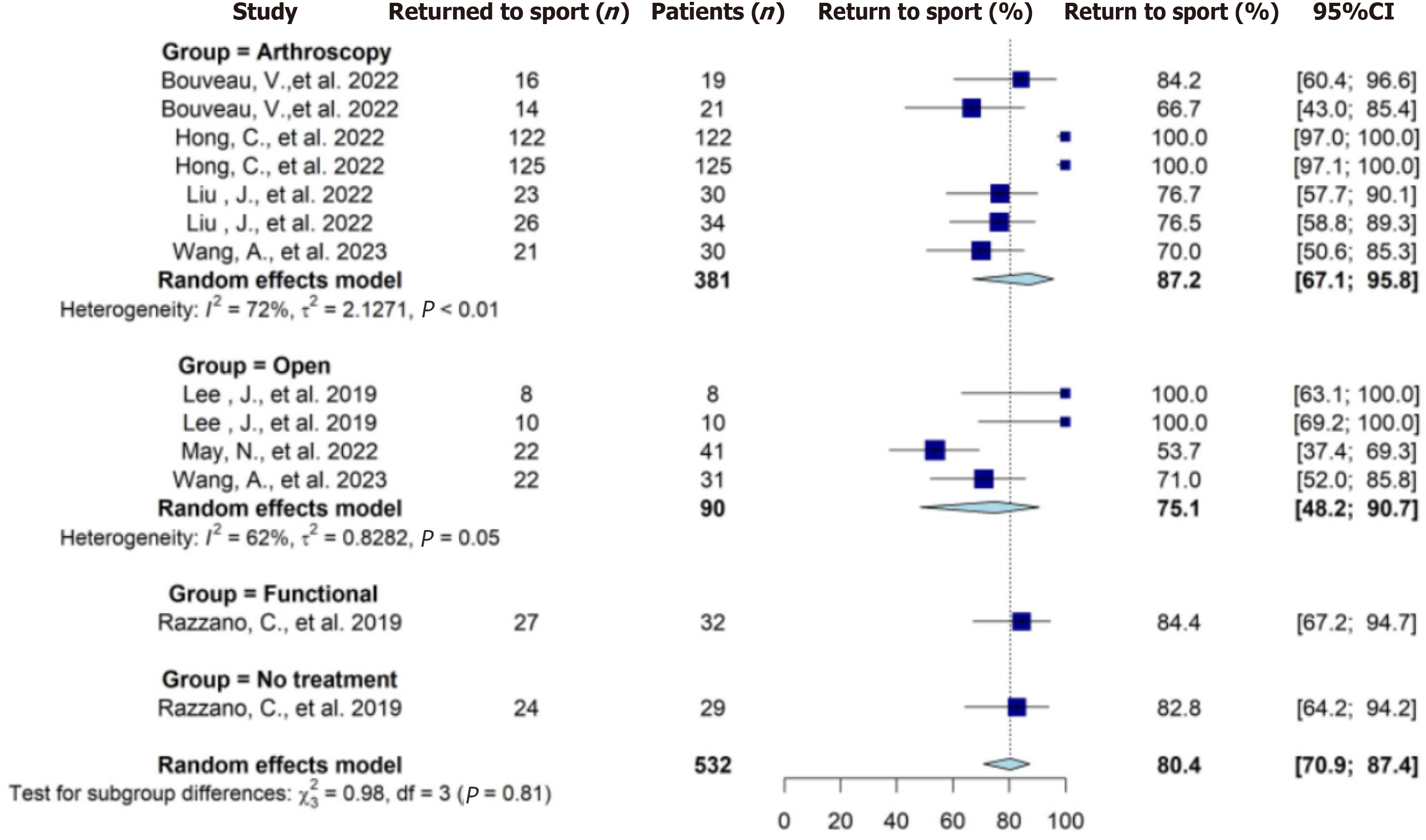
Seven studies reported on the rate of RTS on a total of 532 patients (Table 5). Overall, a total of 460 patients were reported to RTS at the final follow-up. The pooled RTS rate from meta-analysis was 80% (95%CI: 71%-87%). The pooled RTS rate for operative treatments from meta-analysis was 83% (95%CI: 69%-92%). No significant difference was found (level 1 of evidence) in the rate of RTS between these four treatment groups (Figure 2). Seven studies reported on time to RTS on a total number of 609 patients (Table 5). The pooled mean days to RTS ranged from 23-70 d (Table 6). The RTS results from the meta-analysis are categorized as low quality of evidence.
| Ref. | Intervention + patients (n) | Rate of RTS | Time to RTS, d (± SD) | P value |
| Bouveau et al[32], 2022 | Arthroscopic repair (19) | 16 (76.2) | NR | NS |
| Arthroscopic reconstruction (21) | 14 (67.7) | NR | ||
| Hong et al[40], 2022 | Arthroscopic Broström with isolated injury (122) | 122 (100) | Mean = 68.6 (range: 58-105) | P = 0.004 |
| Arthroscopic Broström with associated injury (125) | 125 (100) | Mean = 82.8 (range: 65-132) | ||
| Hou et al[30], 2022 | Arthroscopic Broström (36) | NR | Mean = 13.2 (± 2.4) | P = 0.023 |
| Open Bröstrom (34) | NR | Mean = 18.7 (± 3.1) | ||
| Lee et al[41], 2019 | Open Broström early return (8) | 8 (100) | Mean = 88.16 (± 9.12) | NR |
| Open Broström late return (10) | 10 (100) | Mean = 145.92 (± 39.52) | ||
| Liu et al[43], 2022 | Arthroscopic one anchor suture (30) | 23 (76.7) | NR | NS |
| Arthroscopic two anchor suture (34) | 26 (76.5) | NR | ||
| May et al[44], 2022 | Modified Broström (41) | 22 (53.6) | NR | NR |
| Punt et al[28], 2015 | Wii Fit™ (30) | NR | Mean = 27.4 (± 20.3) | NS |
| Physical Therapy 930) | NR | Mean = 39.7 (± 24.9) | ||
| No treatment (30) | NR | Mean = 23.0 d (± 15.5) | ||
| Razzano et al[29], 2019 | Electric Therapy (32) | 2 month = 23 (71.9); 4 month = 27 (84.3) | NR | 2 month, P = 0.029; 4 month, NS |
| No treatment (29) | 2 mo = 16 (55.2); 4 mo = 24 (82.7) | NR | ||
| Takao et al[31], 2020 | A1: Unilateral arthroscopic repair (43) | NR | Mean = 41.6 d (± 18.2) | Group A vs group B, P < 0.001; group A1 vs group A2, NS; group B1 vs group B2, P = 0.001 |
| A2: Bilateral arthroscopic repair (16) | NR | Mean = 44.6 d (± 22.5) | ||
| B1: Arthroscopic repair + ankle stabilization + postop nonweight bearing (22) | NR | Mean = 70.7 d (± 23.1) | ||
| B2: Arthroscopic repair + ankle stabilization + postop weight bearing (12) | NR | Mean = 45.0 d (± 13.7) | ||
| Wang et al[34], 2023 | Arthroscopic Broström (30) | 21 (70.0) | Mean = 15.1 wk (± 7.8 wk) | NS |
| Open Broström (31) | 22 (71.0) | Mean = 17.2 wk (± 9.3 wk) | ||
| White et al[23], 2016 | Modified Broström, isolated injury | NR | Median = 77 (range: 56-127) | P < 0.001 |
| Modified Broström, associated injuries | NR | Median = 105 (range: 82-178) |
| Intervention type | Mean d to RTS (± SD) | Pooled mean d to RTS (± SD) |
| Arthroscopic surgery | 13.2 (± 2.4)[30]; 105.7 (± 54.6)[34] | 60 (± 46) |
| Open surgery | 18.7 (± 3.1)[30]; 120.4 (± 65.1)[34] | 70 (± 51) |
| All surgery | 13.2 (± 2.4)[30]; 18.7 (± 3.1)[30]; 105.7 (± 54.6)[34]; 120.4 (± 65.1)[34] | 65 (± 49) |
| Functional treatment | 27.4 (± 20.3)[28]; 39.7 (± 24.9)[28] | 34 (± 6.2) |
| No treatment | 23 (± 15.5)[28] | 23 (± 16) |
A higher percentage (72%) (P = 0.029) of athletes with grade I or II ankle sprain treated with electrotherapy were observed to RTS at the 2-mo mark when compared to a sham treatment (55%). However, by the 4-mo mark, the numbers in both groups were similar (84% and 83%, respectively) without significant difference. The Visual Analog Scale at 30 d after treatment (P = 0.043) favored the electrotherapy treatment group (1) over the sham device group (1.7)[29] (evidence level 2). In another study on patients with grade III CAI, Visual Analog Scale during walking was lower (P = 0.018) at the 3-mo follow-up in favor of arthroscopic treatment (mean ± SD: 2.3 ± 2.5), which had quicker (P = 0.023) RTS when compared to open surgery (mean ± SD: 4.9 ± 2.5)[30] (evidence level 2).
Arthroscopic ligament repair and simultaneous procedures for other pathologies delayed (P < 0.001) mean days to RTS (mean ± SD: 42 ± 19 d, range 1-58) when compared to ligament repair alone without concurrent procedures (mean ± SD: 61 ± 23 d, range 32-123). Ankle stabilization surgery with concurrent procedures allowing weight bearing (mean ± SD: 45 ± 14 d, range 32-75) showed a shorter time to RTS than ankle stabilization surgery with simultaneous procedures that required nonweight bearing after surgery (P < 0.001, mean ± SD: 71 ± 23 d, range 35-123)[31] (evidence level 3). A delayed (P < 0.001) RTS was also seen when associated injuries (median 105 d, range 82-178) were present compared to isolated injuries (median 72, range 56-127) in patients with grade I or II ankle sprain[23] (evidence level 3).
In a single study, it was found that individuals with CAI who had a higher body mass index (BMI) (median 24, range 20-37) were more likely (P = 0.04) to refrain from resuming sports or to RTS at a lower level in contrast to those with a similar or lower BMI (median 23, range 17-38) who were more inclined to resume their sports activities at the same or higher level. In this study higher preoperative motivation emerged as the sole factor significantly and independently (P = 0.001) associated with both rate of and time to RTS following ligament repair or ligament reconstruction[32] (evidence level 3).
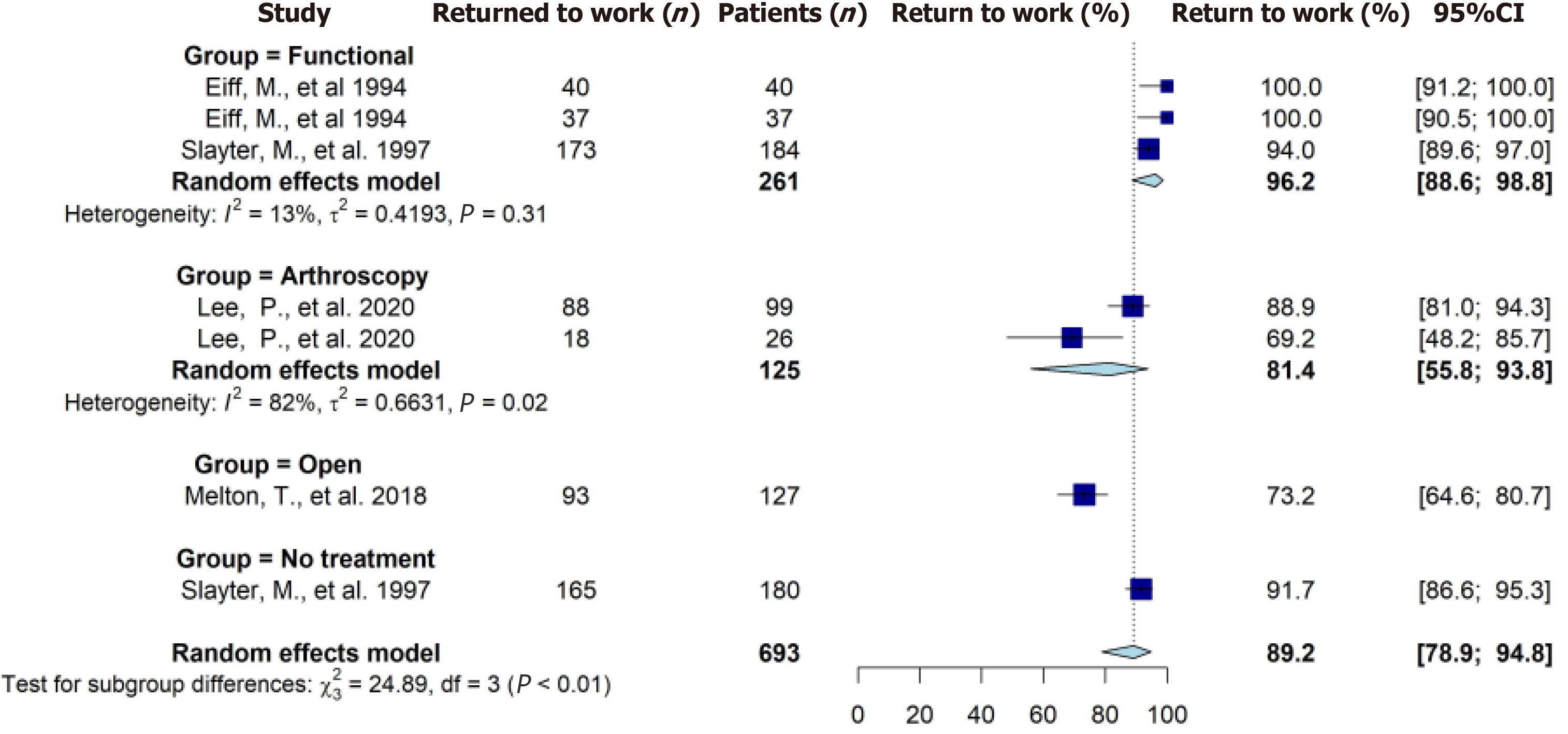
Five studies reported on the rate of RTW on a total of 693 patients (Table 7). Overall, a total of 614 patients were reported to have RTW at the last follow-up. The pooled RTW rate from meta-analysis was 89% (95%CI: 79%-95%) (Figure 3). Seven studies reported on time to RTW on a total of 1284 patients (Table 7). The pooled mean time to RTW ranged from 5.8-8.1 d (Table 8). The RTW results from the meta-analysis were categorized as low quality of evidence.
| Ref. | Intervention + patients (n) | Rate of RTW, n (%) | Time to RTW in mean d (± SD) | P value |
| Avci et al[25], 1998 | Soft cast tape (31) | NR | 2.5 (NR) | P < 0.001 |
| Scotch plus tape (26) | NR | 6.3 (NR) | ||
| Cooke et al[13], 2009 | Below knee cast (142) | NR | 7.7 (NR) | NR |
| Aircast (149) | NR | 9.6 (NR) | ||
| Bledsoe brace (149) | NR | 6.9 (NR) | ||
| Tubular bandage (144) | NR | 7.7 (NR) | ||
| Eiff et al[35], 1994 | Early mobilization (40) | 10 d = 22: (54); 3 wk = 30 (75); 6 wk = 36 (97); 3 month = 40 (100); 6 month = 40 (100) | 4.3 (NR) | 10 d, P < 0.001 |
| Immobilized (37) | 10 d = 4 (13); 3 wk = 29 (79); 6 wk = 36 (96); 3 month = 37 (100); 6 month = 37 (100) | 4.7 (NR) | ||
| Leanderson et al[38], 1995 | Air-Stirrup ankle brace (39) | NR | 5.3 (range: 0-26) | P < 0.05 |
| Compression bandage (34) | NR | 9.1 (range: 0-21) | ||
| Lee et al[42], 2020 | Isolated ankle stabilization with fibular periosteum augment (99) | 88 (88.9) | NR | NS |
| Isolated ankle stabilization without fibular periosteum augment (26) | 18 (69.2) | NR | ||
| Melton et al[33], 2018 | Modified Broström (127) | 93 (73.2) | NR | NA |
| O’Connor et al[29], 2020 | Tubigrip (18) | NR | 5.2 (± 4.9) | NS |
| Elastoplast (20) | NR | 3.7 (± 3.5) | ||
| No Support (16) | NR | 5.8 (± 4.7) | ||
| Slatyer et al[24], 1997 | Piroxicam (184) | 173 | 2.74 (NR) | P < 0.001 |
| Placebo (180) | 167 | 8.57 (NR) | ||
| Wang et al[34], 2023 | Arthroscopic Broström (30) | NR | 6.8 (± 2.1) | P = 0.006 |
| Open Broström (31) | NR | 8.1 (± 2.4) |
| Intervention type | Means d to RTW (± SD) | Pooled mean d to RTW (± SD) |
| Arthroscopic surgery | 6.8 (± 2.1)[34] | 6.8 (± 2.1) |
| Open surgery | 8.1 (± 2.4)[34] | 8.1 (± 2.4) |
| All surgery | 6.8 (± 2.1)[34]; 8.1 (± 2.4)[34] | 7.5 (± 0.7) |
| Functional treatment | 6.3 (NR)[25]; 2.5 (NR)[253]; 7.7 (NR)[13]; 9.6 (NR)[13]; 6.9 (NR)[13]; 7.7 (NR)[13]; 4.3 (NR)[35]; 4.7 (NR)[35]; 9.1 (range 0-21)[38]; 5.3 (range: 0-26)[238]; 5.2 (± 4.9)[26]; 3.7 (± 3.5)[265]; 2.74 (NR)[249] | 5.8 (± 2.2) |
| No treatment | 5.8 (± 4.7)[265]; 8.57 (NR)[24] | 7.2 (± 1.4) |
The open modified Broström procedure allowed the majority of patients (73%) in an examined military population with CAI (complaints for > 6 mo) to return to active duty[33] (evidence level 3). For patients with grade III CAI (complaints for > 6 mo), arthroscopic surgery showed quicker RTW (mean ± SD: 6.8 ± 2.1 d) compared to patients in the open surgery group (mean ± SD: 8.1 ± 2.4 d, P = 0.006)[34] (evidence level 2).
When comparing immobilization of 3 d to immobilization of 10 d, 53% (P < 0.001) of the early mobilized patients reported RTW after 10 d compared to 13% of the immobilized patients. A lower percentage of patients in the early mobilization group (57%) reported levels of pain compared to the immobilization group (87%, P < 0.05). After 3 wk, all patients in both groups were able to return to any type of work, and after 3 mo, they were able to resume full work[35] (evidence level 2). Comparing two different forms of immobilization for grade III ankle sprain, the Soft Cast® (2.5 d) demonstrated significantly better results (P < 0.001) in terms of days away from work compared to Scotchcast Plus® (6.3 d)[25] (evidence level 2).
One study observed patients who suffered from an ankle sprain with fracture. They found that those individuals with a previous history of traumatic brain injury, depression, anxiety or substance abuse experienced a delay in their rehabilitation process. In the case of patients diagnosed with post-traumatic stress disorder (PTSD), the impact of the delay was observed in unspecified ankle sprains[36] (evidence level 2).
This meta-analysis sought to consolidate recent research findings on the complex interactions among physical, psychological and biomechanical factors influencing the process of RTS and RTW following LAS. Preoperative motivation, psychological factors, mobilization and weight bearing were factors associated with a faster RTS or RTW. Absent ligament structures and associated injuries were factors that negatively influenced RTS or RTW. The effect of psychological factors on the delay of rehabilitation after an ankle sprain can be substantial and multifaceted. Psychological factors can influence an individual’s perception of the severity of their injury and their perceived control over the recovery process. Depression, anxiety, PTSD and substance abuse can have a significant impact on an individual’s willingness, motivation and ability to engage in the rehabilitation process. Conditions like depression and anxiety may lead to a lack of interest or reduced commitment to the recovery process, resulting in delays in attending therapy se
After sustaining an ankle sprain, simple tasks such as walking, climbing stairs or standing for extended periods may become challenging and uncomfortable. Fear of re-injury, loss of confidence and psychological distress can arise, impacting mental well-being. A patient with PTSD might be hesitant to engage in activities that remind them of the traumatic event, including activities related to rehabilitation. Fear of re-injury or the pain can also hinder progress and lead to delays. Individuals with a history of substance abuse may rely on maladaptive coping mechanisms to deal with stress and pain. These coping strategies can interfere with the recovery process and lead to setbacks. Patients with work-related injuries were observed to be at a greater risk for experiencing persistent pain. This finding suggests that occupational factors may have a significant impact on pain outcomes and delayed RTW after an ankle sprain[36]. Our results showed that patient satisfaction after modified Bröstrom surgery was very high (88%), even among athletes who were unable to return to preinjury levels. A large proportion of those athletes (46%) did not return to their preinjury activity, but only 37% reported ankle-related reasons for not returning[33].
In one study, higher BMI was found to be a significant factor associated with not returning to exercise or returning to a lower level (P = 0.04). Individuals with a higher BMI may experience challenges related to weight-bearing and joint stress during recovery, which can contribute to a longer delay in returning to sports activities. This finding underscores the importance of addressing weight management strategies and implementing appropriate rehabilitation protocols tailored to individuals with higher BMIs to optimize their recovery. Concomitant injuries or additional procedures have shown to have a negative effect on RTS or RTW. Further, it is common to treat an ankle sprain nonsurgically unless chronic complaints persist[37].
While certain studies have indicated a potential connection between psychological factors and delays in RTW, it is important to recognize that additional variables, such as proprioceptive disturbance, may also contribute to these delays. The high failure rate observed in ankle sprain treatments could also be attributed to neglected associated injuries, such as syndesmosis or cartilage injuries. Another contributing factor might be inadequate treatment that does not align with the specific injury grades and healing phases[1].
Although patient burden is our primary concern, insights into the costs indicate the need for cost-efficiency to minimize the socioeconomic burden. One study found that the extent of the delayed rehabilitation was directly proportional to the increased likelihood of experiencing a recurrence of the ankle sprain and the number of ankle-related medical visits. Patients who received delayed care had up to ten additional visits. Costs were also 1.13 times more likely to be greater (up to $1400 per episode), with a linear relationship noted with each day rehabilitation care was delayed. The total financial burden (adjusted for inflation) of ankle sprain ranges from $11.7-$90.9 million per year, and costs in military and civilian settings are similar[38]. Home-based proprioception training adjacent to physical therapy showed costs 4 times lower than physical therapy alone[28]. Higher grade ankle sprains also increased the cost per patient (P < 0.001)[39]. Our results showed that the extent of the delayed rehabilitation was directly proportional to the increasing likelihood of experiencing a recurrence of the ankle sprain and the number of ankle-related medical visits. If taking into account these psychological factors improving rehabilitation, considering these factors will also lead to lower healthcare costs per patient.
While we aimed to comprehensively identify and analyze the factors influencing RTW and RTS after an LAS, there is a possibility that certain relevant factors were not considered or included in our analysis due to lack of published data. The complex nature of ankle sprain recovery may involve additional factors that were not captured in our study. When identified, these new factors may improve our current protocols and knowledge in treatment of ankle sprains.
This study represented a first attempt to comprehend which factors may influence RTS and RTW using a meta-analysis. While our analysis provided valuable insights into which factors may potentially be influential, it is essential to acknowledge that grading which factor has a greater or lesser impact is not possible due to the lack of comparability among the included patients regarding the grading of ankle ligament injury and concomitant injuries. In some studies, patients received surgical treatment in acute situations[40-43]. Therefore, any surgical treatment performed earlier is highly debatable. Despite our efforts to include high-quality studies, many were listed as high or moderate risk due to patients or personnel physically not being able to be blinded in order for a patient to receive treatment. Other limitations were variations in terms of methodology, small sample size, and short follow-up time. There was inconsistency in how RTS was defined within orthopedic sports medicine literature. While many studies defined RTS as the athlete’s return to competitive play, other variations include returning to practice, training or meeting specific competition levels and objectives. The studies in our review employed different outcome measures to assess RTS and RTW, making it cha
As shown in Tables 3 and 4, certain studies exhibited a moderate or high risk of bias. For instance, when evaluating the risk of bias in Table 4, some studies were assigned a high risk due to the absence of blinding for participants and personnel or the lack of blinding in outcome assessment. In the case of our included studies, implementing blinding was deemed physically impossible, which could lead the risk of bias assessment tool to assign a lower score to a study that is, in qualitative terms, superior.
Overall, our findings indicated that all treatments yielded comparable results, with each treatment potentially offering unique advantages or benefits. Given the variety of factors that affect RTS and RTW after LAS, tailored interventions targeting these psychological and physical factors have the great potential to improve recovery and accelerate return to normal activities. For example, Scotchcast Plus® had a quicker (P < 0.001) RTW compared to the Soft Cast®. The range of motion (ROM) measurements were also better (P = 0.001) in the Scotchcast Plus® group (43°, range: 35-55°) compared to the Soft Cast® group (54°, range: 35-65°)[23].
Another study found that following 3-5 d of functional treatment with either air-stirrup or compression bandage, females experienced a greater restriction in ROM in their injured ankle compared to males (P < 0.05)[29]. In current literature males are approximately 1.5 times more likely than females to return to either their previous level of sport or competitive sport after obtaining an LAS[44]. Future research should be conducted on possible cofactors such as ROM affecting the differences in outcomes between males and females. Future studies should also assess the weight of concomitant injuries and psychological factors in RTS and RTW.
Consideration of simultaneous procedures for other medical conditions should be factored in while evaluating the timeframe for resuming work or sports activities. Treatment plans that address physical, psychological and social aspects of recovery may aid regaining mobility, overall well-being and returning to preinjury level of activities after an LAS.
Collectively, the findings derived from these studies provided valuable insights into the various treatment approaches employed and their associated outcomes. However, it is important to note that the current body of literature does not provide sufficient comparative data to draw definitive conclusions on what factors work in favor of or negatively influence RTS or RTW. Further research with larger sample sizes and standardized methodologies is warranted to establish more robust evidence regarding treatment approaches and their effectiveness in facilitating the RTW and RTS after LAS.
This systematic review and meta-analysis identified several factors that were observed to influence the RTS or RTW after LAS. In random order, these factors included: (1) Factors favoring RTS or RTW: (a) Preoperative Motivation and Psychological factors. In patients with CAI, higher preoperative motivation was the primary factor associated with a faster RTS following ligament repair or reconstruction. However, patients with a history of psychological illness or brain injury experienced delays in their rehabilitation for ankle sprains with fractures and unspecified sprains. Among athletes who RTS after LAS without fractures, 10% cited non-ankle related reasons for not returning. The evidence for these findings was categorized as level 2; (b) Early mobilization. Immobilization of 3 d was found to have a positive impact on the RTS or RTW when compared to immobilization of 10 d (Categorized as evidence level 3); and (c) Postoperative weight bearing. Ankle stabilization surgery with concurrent weight-bearing procedures resulted in a shorter time to RTS compared to ankle stabilization surgery with simultaneous non-weight-bearing procedures (Categorized as evidence level 3); and (2) Factors negatively influencing return to sport or work: (a) Absent ligament structures. An additional procedure such as ankle stabilization surgery due to other pathologies than ligament rupture may result in a longer time to return to work (categorized as evidence level 3); and (b) Associated injuries. The presence of associated injuries along with the ankle sprain negatively impacted the return to sport or work (Categorized as evidence level 3).
It is important to consider these factors when developing personalized treatment plans and interventions for individuals recovering from LAS to optimize their chances of successful RTS and/or RTW.
Lateral ankle sprains (LAS) are a highly prevalent musculoskeletal injury affecting both athletes and non-athletes, constituting 80% of all ankle sprains with an incidence of 15%-20%. Up to 40% of LAS cases progress to chronic ankle instability, causing prolonged pain and reduced mobility. This interplay of high incidence, prevalence, workdays lost and substantial medical expenses underscores the profound socioeconomic impact of LAS.
The consequences of ankle sprains extend beyond pain and functional impairment. Despite the alarmingly high incidence and chronic ankle instability risk, only around half of LAS patients seek medical attention.
In light of these compelling circumstances, the primary objective of this systematic review and meta-analysis was to comprehensively investigate the factors that may exert an influence on return to work (RTW) and return to sport (RTS) following LAS.
EMBASE and PubMed were systematically searched for relevant studies published until June 2023. Quality assessments were carried out for each study using established risk of bias tools, and the quality of evidence was evaluated using the GRADEpro tool for quantitative outcomes. Qualitative outcome analysis was subjected to a best evidence synthesis, and for outcomes amenable to quantitative analysis, forest plots were generated to ascertain the impact on RTW and RTS.
The RTS rates were 80% and 83% in both the all treatments and surgical treatments groups, respectively. Mean RTS times ranged from 23-93 d, with an overall RTS rate of 89%. The average time to RTW ranged from 5.8-8.1 d. Preoperative motivation, early mobilization and postoperative weight bearing resulted in a shorter time to RTS.
Overall, our findings indicated that all treatments yielded comparable results. Given the variety of factors that affected RTW and RTS after LAS, tailored interventions targeting both psychological and physical factors have the great potential to improve recovery and accelerate return to normal activities.
Future studies should assess the weight of psychological factors in RTS and RTW. Treatment plans that address physical, psychological and social aspects of recovery may aid regaining mobility, overall well-being and returning to preinjury levels of activities after LAS.
| 1. | Petersen W, Rembitzki IV, Koppenburg AG, Ellermann A, Liebau C, Brüggemann GP, Best R. Treatment of acute ankle ligament injuries: a systematic review. Arch Orthop Trauma Surg. 2013;133:1129-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Gribble PA, Bleakley CM, Caulfield BM, Docherty CL, Fourchet F, Fong DT, Hertel J, Hiller CE, Kaminski TW, McKeon PO, Refshauge KM, Verhagen EA, Vicenzino BT, Wikstrom EA, Delahunt E. 2016 consensus statement of the International Ankle Consortium: prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50:1493-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Kerkhoffs GM, van den Bekerom M, Elders LA, van Beek PA, Hullegie WA, Bloemers GM, de Heus EM, Loogman MC, Rosenbrand KC, Kuipers T, Hoogstraten JW, Dekker R, Ten Duis HJ, van Dijk CN, van Tulder MW, van der Wees PJ, de Bie RA. Diagnosis, treatment and prevention of ankle sprains: an evidence-based clinical guideline. Br J Sports Med. 2012;46:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Ferran NA, Maffulli N. Epidemiology of sprains of the lateral ankle ligament complex. Foot Ankle Clin. 2006;11:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Dubin JC, Comeau D, McClelland RI, Dubin RA, Ferrel E. Lateral and syndesmotic ankle sprain injuries: a narrative literature review. J Chiropr Med. 2011;10:204-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 6. | Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720. [PubMed] |
| 7. | Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Fong DT, Chan YY, Mok KM, Yung PS, Chan KM. Understanding acute ankle ligamentous sprain injury in sports. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Polzer H, Kanz KG, Prall WC, Haasters F, Ockert B, Mutschler W, Grote S. Diagnosis and treatment of acute ankle injuries: development of an evidence-based algorithm. Orthop Rev (Pavia). 2012;4:e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (3)] |
| 10. | Al Bimani SA, Gates LS, Warner M, Bowen C. Factors influencing return to play following conservatively treated ankle sprain: a systematic review. Phys Sportsmed. 2019;47:31-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Shah S, Thomas AC, Noone JM, Blanchette CM, Wikstrom EA. Incidence and Cost of Ankle Sprains in United States Emergency Departments. Sports Health. 2016;8:547-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 671] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 13. | Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, Szczepura A, Wilson S, Lamb SE; CAST trial group. Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial. Health Technol Assess. 2009;13:iii, ix-ix, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Verhagen EA, van Tulder M, van der Beek AJ, Bouter LM, van Mechelen W. An economic evaluation of a proprioceptive balance board training programme for the prevention of ankle sprains in volleyball. Br J Sports Med. 2005;39:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Verhagen EA, van Mechelen W, de Vente W. The effect of preventive measures on the incidence of ankle sprains. Clin J Sport Med. 2000;10:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14289] [Article Influence: 1428.9] [Reference Citation Analysis (1)] |
| 17. | Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18:e1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 1275] [Article Influence: 318.8] [Reference Citation Analysis (0)] |
| 18. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12478] [Article Influence: 1247.8] [Reference Citation Analysis (2)] |
| 19. | Minozzi S, Dwan K, Borrelli F, Filippini G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol. 2022;141:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Schünemann H BJ, Guyatt G, Oxman. GRADE handbook for grading quality of evidence and strength of recommendations: The GRADE Working Group 2013. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 881] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 21. | Group OLoEW. The Oxford 2011 Levels of evidence. Oxford centre for evidence-based medicine 2011. 2023. Available from: http://www.cebm.net/index.aspx?o=5653. |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48488] [Article Influence: 2108.2] [Reference Citation Analysis (4)] |
| 23. | White WJ, McCollum GA, Calder JD. Return to sport following acute lateral ligament repair of the ankle in professional athletes. Knee Surg Sports Traumatol Arthrosc. 2016;24:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Slatyer MA, Hensley MJ, Lopert R. A randomized controlled trial of piroxicam in the management of acute ankle sprain in Australian Regular Army recruits. The Kapooka Ankle Sprain Study. Am J Sports Med. 1997;25:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Avci S, Sayli U. Comparison of the results of short-term rigid and semi-rigid cast immobilization for the treatment of grade 3 inversion injuries of the ankle. Injury. 1998;29:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | O'Connor G, Martin AJ. Acute ankle sprain: is there a best support? Eur J Emerg Med. 2011;18:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Punt IM, Ziltener JL, Monnin D, Allet L. Wii Fit™ exercise therapy for the rehabilitation of ankle sprains: Its effect compared with physical therapy or no functional exercises at all. Scand J Med Sci Sports. 2016;26:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Razzano C, Izzo R, Savastano R, Colantuoni C, Carbone S. Noninvasive Interactive Neurostimulation Therapy for the Treatment of Low-Grade Lateral Ankle Sprain in the Professional Contact Sport Athlete Improves the Short-Term Recovery and Return to Sport: A Randomized Controlled Trial. J Foot Ankle Surg. 2019;58:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Hou ZC, Su T, Ao YF, Hu YL, Jiao C, Guo QW, Ren S, Li N, Jiang D. Arthroscopic modified Broström procedure achieves faster return to sports than open procedure for chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2022;30:3570-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Takao M, Inokuchi R, Jujo Y, Iwashita K, Okugura K, Mori Y, Hayashi K, Komesu K, Glazebrook M; Ankle Instability Group. Clinical outcomes of concurrent surgery with weight bearing after modified lasso-loop stitch arthroscopic ankle stabilization. Knee Surg Sports Traumatol Arthrosc. 2021;29:2006-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Bouveau V, Housset V, Chasset F, Bauer T, Hardy A. Return to sports: Rate and time after arthroscopic surgery for chronic lateral ankle instability. Orthop Traumatol Surg Res. 2022;108:103398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 33. | Melton TJ, Dannenbaum JH, Drayer NJ, Robbins J, Ryan PM. Postoperative Outcome of the Modified Broström Procedure in the Active Duty Military Population: A Retrospective Cohort Study. J Foot Ankle Surg. 2018;57:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Wang AH, Su T, Jiang YF, Zhu YC, Jiao C, Hu YL, Guo QW, Jiang D. Arthroscopic modified Broström procedure achieved similar favorable short term outcomes to open procedure for chronic lateral ankle instability cases with generalized joint laxity. Knee Surg Sports Traumatol Arthrosc. 2023;31:4043-4051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 35. | Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Franche RL, Cullen K, Clarke J, Irvin E, Sinclair S, Frank J; Institute for Work & Health (IWH) Workplace-Based RTW Intervention Literature Review Research Team. Workplace-based return-to-work interventions: a systematic review of the quantitative literature. J Occup Rehabil. 2005;15:607-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 423] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Rhon DI, Fraser JJ, Sorensen J, Greenlee TA, Jain T, Cook CE. Delayed Rehabilitation Is Associated With Recurrence and Higher Medical Care Use After Ankle Sprain Injuries in the United States Military Health System. J Orthop Sports Phys Ther. 2021;51:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Leanderson J, Wredmark T. Treatment of acute ankle sprain. Comparison of a semi-rigid ankle brace and compression bandage in 73 patients. Acta Orthop Scand. 1995;66:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Kondric M, Sindik J, Furjan-Mandic G, Schiefler B. Participation Motivation and Student's Physical Activity among Sport Students in Three Countries. J Sports Sci Med. 2013;12:10-18. [PubMed] |
| 40. | Hong CC, Calder J. Ability to return to sports after early lateral ligament repair of the ankle in 147 elite athletes. Knee Surg Sports Traumatol Arthrosc. 2023;31:4519-4525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Lee K, Jegal H, Chung H, Park Y. Return to Play after Modified Broström Operation for Chronic Ankle Instability in Elite Athletes. Clin Orthop Surg. 2019;11:126-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Lee C, McQuade MG, Ostrofe AA, Goldman AH, Douglas TJ. Do Mid-term Outcomes of Lateral Ankle Stabilization Procedures Differ Between Military and Civilian Populations? Clin Orthop Relat Res. 2021;479:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Liu J, Chen M, Xu T, Tian Z, Xu L, Zhou Y. Functional results of modified Mason-Allen suture versus horizontal mattress suture in the arthroscopic Broström-Gould procedure for chronic ankle instability. J Orthop Surg Res. 2022;17:459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | May NR, Driscoll M, Nguyen S, Ferkel RD. Analysis of Return to Play After Modified Broström Lateral Ankle Ligament Reconstruction. Orthop J Sports Med. 2022;10:23259671211068541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Primadhi RA, Indonesia; Sun J, Australia; Yang MW, China; Zhang Y, China; Zhou Y, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Guo X