Published online Jun 18, 2023. doi: 10.13105/wjma.v11.i5.167
Peer-review started: January 28, 2023
First decision: March 28, 2023
Revised: April 9, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: June 18, 2023
Processing time: 138 Days and 21.5 Hours
As of 31 December 2022, there were over 6.6 million coronavirus disease 2019 (COVID-19) deaths and over 651 million cases across 200 countries worldwide. Despite the increase in vaccinations and booster shots, COVID-19 cases and deaths continue to remain high. While the effectiveness of these vaccines has already been established by different manufacturers, the fact remains that these vaccines were created quickly for global emergency use, tested under controlled clinical conditions from voluntary subjects and age groups whose general characteristics may differ from the actual general population.
To conduct a systematic review to determine the real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the predominance of Delta and Omicron variants in preventing COVID-19 related infection, hospital, intensive care unit (ICU) admission and intubation, and death.
A combination of Medical Subject Headings and non–Medical Subject Headings was carried out to identify all relevant research articles that meets the inclusion and exclusion criteria from PubMed, Cochrane, CINAHL, Scopus, ProQuest, EMBASE, Web of Science, and Google Scholar databases, as well as qualified research studies from pre–print servers using medRxiv and Research Square, published from January 1, 2021 - December 31, 2022.
As per the inclusion and exclusion criteria, the effectiveness of Pfizer-BioNTech and Moderna vaccines were evaluated from an estimated total study population of 26535692 using infection, hospital, ICU admission and intubation, and death as outcome measures from studies published between 2021 and 2022, conducted in New York, Finland, Canada, Costa Rica, Qatar, Greece, and Brazil. The risk of bias was evaluated using risk of bias in nonrandomized studies of interventions (ROBINS-I) tool for cohort, case-control, and cross-sectional studies. While clinical trial data on Pfizer-BioNTech and Moderna vaccines demonstrated 94% vaccine effectiveness in the elderly, the results in this study showed that vaccine effectiveness in real-world settings is marginally lower against infection (40%-89%), hospitalization (92%), ICU admission and intubation (98%-85%), and death (77%-87%) with an indication of diminished effectiveness of vaccine over time. Furthermore, 2 doses of mRNA vaccines are inadequate and only provides interim protection.
Because of the natural diminishing effectiveness of the vaccine, the need for booster dose to restore its efficacy is vital. From a research perspective, the use of highly heterogeneous outcome measures inhibits the comparison, contrast, and integration of the results which makes data pooling across different studies problematic. While pharmaceutical intervention like vaccination is important to fight an epidemic, utilizing common outcome measurements or carrying out studies with minimal heterogeneity in outcome measurements, is equally crucial to better understand and respond to an international health crisis.
Core Tip: This systematic review investigates the real-world effectiveness of mRNA coronavirus disease 2019 (COVID-19) vaccines in reducing morbidity and mortality in the elderly during the predominance of Delta and Omicron variants. This study found that the effectiveness of mRNA COVID-19 vaccines in the elderly against the Delta and Omicron variants is marginally lower than what was suggested in clinical trial data. Vaccine efficacy also diminishes over time, indicating the need for a booster dose to restore its effectiveness. Furthermore, to better understand and respond to an epidemic, studies should utilize common outcome measurements or minimize heterogeneity in outcome measures to facilitate data comparison and integration of results.
- Citation: Palalay H, Vyas R, Tafuto B. Real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the Delta and Omicron variants: Systematic review. World J Meta-Anal 2023; 11(5): 167-180
- URL: https://www.wjgnet.com/2308-3840/full/v11/i5/167.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i5.167
As of December 31, 2022, there were over 6.6 million coronavirus disease 2019 (COVID-19) deaths and over 651 million cases across 200 countries worldwide[1]. During the second half of 2021, COVID-19 cases and deaths were predominantly influenced by the Delta and Omicron variants, wreaking havoc even in countries with tough COVID-19 restrictions[2,3]. Epidemiological studies have shown that the contagious and highly transmissible nature of the Delta and Omicron variants has even put the elderly population in a more disadvantaged position, accounting roughly 14% of all COVID-19 cases and 70% of all COVID-19 deaths as of December 31, 2022[4-7]. While there is no broad consensus on the age at which a person can be considered elderly, the approved cutoff age as per the United Nations is 60+ years[8].
As a response to the extensive impact of COVID-19, which has become a public health concern and an international health crisis, the Centers for Disease Control and Prevention rolled out a global strategy response framework which outlined a combination of non-pharmaceutical and pharmaceutical interventions[9-11]. While the primary method of epidemic control has been non-pharmaceutical measures, pharmaceutical intervention, like vaccine, is expected to be the only effective, long-term defense against infection and death[12,13]. Vaccination is critical since the epidemic is still challenging to control due to the dormant symptoms and contagious nature of the virus especially during the incubation period which triggers late detection of infection[12,13].
Of the 356 vaccine candidates, over 12 billion vaccine doses have been administered by 34 different vaccines approved under Emergency Use Authorization[1,14]. Despite the increase in vaccinations and booster shots, COVID-19 cases and deaths continue to remain high[1]. While the effectiveness of these vaccines has already been established by different manufacturers, the fact remains that these vaccines were created quickly for global emergency use, tested under controlled clinical conditions from voluntary subjects and age groups whose general characteristics may differ from the actual general population[15-17]. In spite of the many observational studies providing data on the effectiveness of vaccination in various populations, this study aims to compile the disparate data through systematic review[18-29]. This study carefully examines the effectiveness of COVID-19 vaccines in real-world settings in the elderly during the predominance of Delta and Omicron variants.
The systematic review was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards to ensure a comprehensive and methodical approach[30].
The review searched for qualified studies using a combination of Medical Subject Headings (MeSH) and non–Medical Subject Headings from PubMed, Cochrane, CINAHL, Scopus, ProQuest, EMBASE, Web of Science, and Google Scholar databases, as well as qualified research studies from pre–print servers using medRxiv and Research Square, published from January 1, 2021 – December 31, 2022. The search was independently performed by a single researcher using the following keywords and search terms (Supplementary Table 1: Keywords and Search Terms using PICO): (1) Covid-19; covid 19; covid19; SARS CoV 2*; SARS-CoV-2*; SARS Coronavirus 2 Infection; sars virus; 2019 Novel Coronavirus*; nCoV; 2019-nCoV*; COVID-19 Pandemic*; COVID-19 Virus*; Coronavirus; Coronavirus Disease*; Severe Acute Respiratory Syndrome Coronavirus 2 Infection; CV-19; CV19; (2) covid 19 vaccine*; covid-19 vaccine*; Pfizer-BioNTech vaccine; Comirnaty; BNT162b2; Bnt-162b2; Bnt162b2; Tozinameran; Tozinameran [INN]; UNII-5085ZFP6SJ; Moderna vaccine; mRNA-1273; MRNA-1273; Spikevax; CX-024414; Elasomeran; Elasomeran [INN]; M-1273; Moderna covid-19 vaccine rna; TAK-919; UNII-EPK39PL4R4; Covid 19 booster; Covid-19 booster; SARS-CoV-2 vaccine; SARS-CoV-2 booster; vaccinated; inoculat*; immuni*; post-vaccination; antibody; protected; (3) unvaccinated; uninoculated; uninoculated; unimmunized; unprotected; susceptible; and (4) reduce incidence*; reduce admission*; reduce infection*; reduce hospitalization*; reduce morbidity*; reduce mortality*; reduce death*; lessen infection*; lessen admission*; lessen hospitalization*; lessen morbidity*; lessen mortality*; lessen death*; prevent incidence*; prevent infection*; prevent admission*; prevent hospitalization*; prevent morbidity*; prevent mortality*; prevent death*; minimize incidence*; minimize admission*; minimize infection*; minimize hospitalization*; minimize morbidity*; minimize mortality*; minimize death*; control incidence*; control admission*; control infection*; control hospitalization*; control morbidity*; control mortality*; control death*; combat incidence*; combat admission*; combat infection*; combat hospitalization*; combat morbidity*; combat mortality*; combat death*; eliminate incidence*; eliminate admission*; eliminate infection*; eliminate hospitalization*; eliminate morbidity*; eliminate mortality*; eliminate death*; diminish incidence*; diminish admission*; diminish infection*; diminish hospitalization*; diminish morbidity*; diminish mortality*; diminish death*; solve incidence*; solve admission*; solve infection*; solve hospitalization*; solve morbidity*; solve mortality*; solve death*.
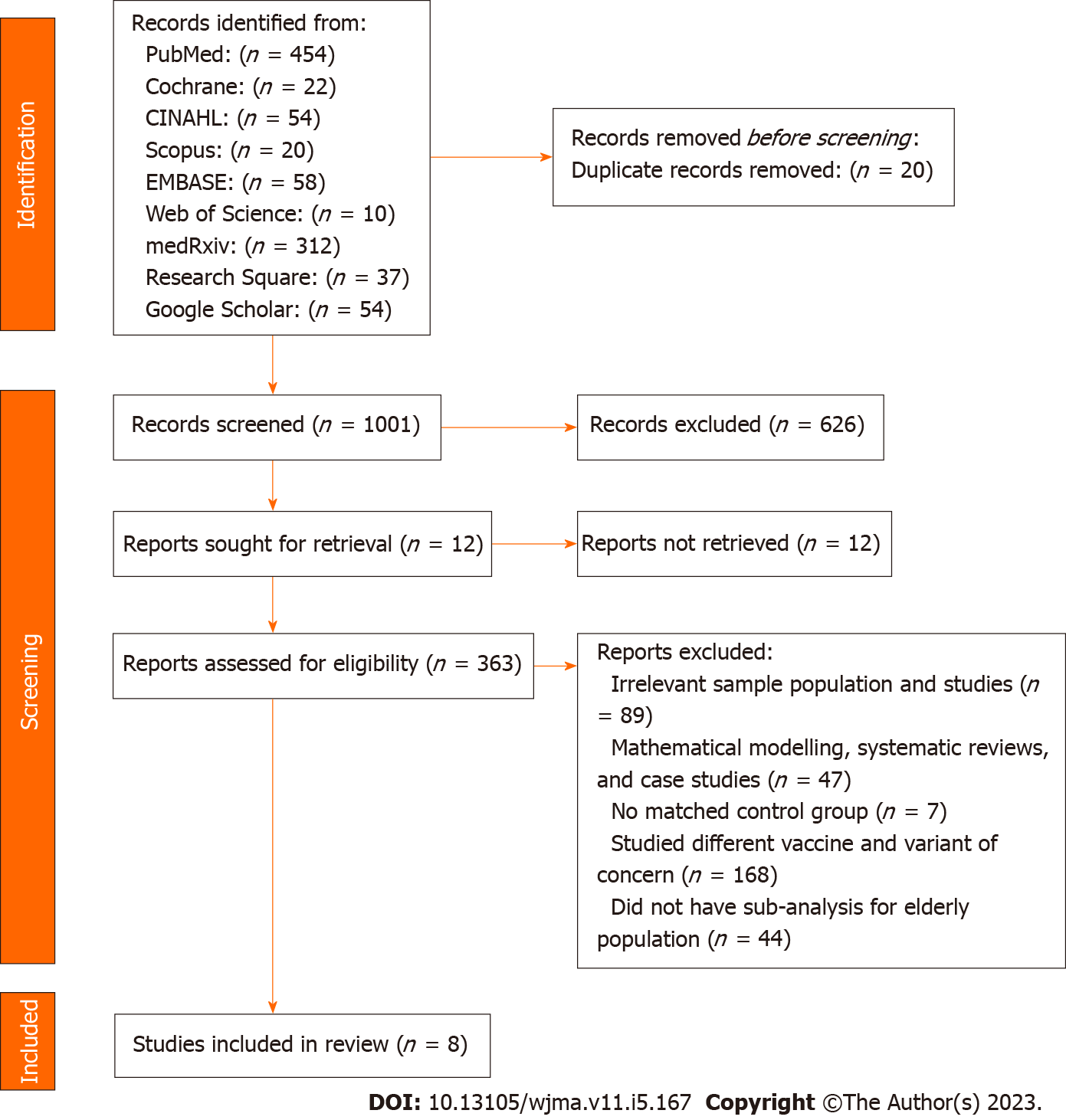
In accordance to the inclusion criteria, the systematic review identified relevant English-published observational studies, which examined the effectiveness of COVID-19 vaccines among the: (1) Elderly populations who were ≥ 60 years old; (2) recipient of at least 2 doses of mRNA (Pfizer-BioNTech and Moderna) vaccines; (3) during the predominance of Delta (B.1.617.2) or Omicron (B.1.1.529/BA); and (4) studies which examined subjects as COVID-19 positive based on a positive Reverse Transcription Polymerase Chain Reaction (RT-PCR or PCR) tests as well as studies which compared and examined the incidence of COVID-19, infection, hospitalization, admission to intensive care unit (ICU), intubation, and death. This systematic review, however, will not include: (1) Systematic review and meta-analysis studies, case reports, case series, reviews, editorials, conference papers, letters, and correspondence; (2) studies on animals; (3) studies with mathematical modelling analysis; (4) studies with insufficient data to calculate the prevention rate of COVID-19; (5) studies with immunocompromised subjects; (6) studies which did not have an unvaccinated subjects to compare; (7) studies that did not use SARS-CoV-2 vaccination as the exposure; (8) duplicate studies or studies with overlapping participants; and (9) studies that did not explain how COVID-19 subjects were determined (Figure 1).
The review process underwent 4 stages: (1) All the papers found within the identified databases were examined and the publication year, study titles, authors, and abstracts were imported into an Excel spreadsheet; (2) the records were managed, screened and duplicates were eliminated manually by assessing the study title, authors, and abstract for inclusion; (3) only those with titles and abstracts that match the inclusion criteria were retrieved and carefully evaluated for full text review; and (4) using a separate Excel spreadsheet, a data extraction sheet was developed to independently extract the general study characteristics (author and publication year, study design, location, purpose, study population, including age of study population, variant of concern, vaccine type, number of doses received, outcome measures, vaccine effectiveness, and results). All qualified studies for systematic reviews were imported, stored, and managed in EndNote20.
The studies included in the review were assessed based on: (1) Age of study population; (2) variant of concern; (3) type of vaccine used; and (4) effectiveness of vaccines based on outcome measures. The effectiveness of COVID-19 mRNA vaccines in reducing morbidity and mortality were examined by comparing the following outcomes amongst the selected studies:
(1) Effectiveness of COVID-19 mRNA vaccines to reduce morbidity in terms of infections, hospitalization, admission to ICU and intubation;
And (2) effectiveness of COVID-19 mRNA vaccines to reduce mortality or deaths.
This study did not require the approval of an ethical committee or an Institutional Review Board since data collection and synthesis were gathered from already published studies in which proper consent or approvals would have been obtained by the researchers.
The methodological quality of these observational studies was assessed through the risk of bias using ROBINS-I tool (risk of bias in non-randomized studies of interventions) and were analyzed using a narrative synthesis method which gathered the information from several sources and employed words and text to summarize and explain the findings since meta-analysis is not practical due to significant heterogeneity between the studies[31,32].
After searching 9 different databases, 1,021 studies were identified from PubMed (n = 454), Cochrane (n = 22), CINAHL (n = 54), Scopus (n = 20), EMBASE (n = 58), Web of Science (n = 10), medRxiv (n = 312), Research Square (n = 37), and Google Scholar (n = 54). From the preliminary review, 20 duplicates, 626 unrelated, and 12 unretrieved studies were excluded, leaving 363 studies were moved for title and abstract screening. As per the inclusion and exclusion conditions in the eligibility criteria, 354 studies were excluded for the following reasons: (1) Irrelevant sample population and studies (n = 89); (2) mathematical modelling, systematic reviews, and case studies (n = 47); (3) no matched control group (n = 7); (4) studied different vaccine and variant of concern (n = 168); (4) did not have sub-analysis for elderly population (n = 43); and (5) overlap in study population (n = 1). As a result, only 8 studies were included for systematic review[33-40]. PRISMA Flow Diagram summarized the literature selection process (Figure 1).
Among these studies, 3 were published and 5 were published on the pre-print platforms[33-40]. All of the 8 studies used observational study designs such as cohort, case control, and cross-sectional studies[33-40]. These studies reported the effectiveness of Pfizer-BioNTech (n = 8) and Moderna (n = 4) vaccines, with 7 studies examining 2 doses, 2 studies examining 2nd and booster doses, and 1 study examining booster dose in reducing COVID-19 morbidity and mortality during the prevalence of Delta (B.1.617.2) and Omicron (B.1.1.529/BA) variants[33-40]. Study locations were in New York, Finland, Canada, Costa Rica, Qatar, Greece, and Brazil published between 2021 (n = 3) and 2022 (n = 5)[33-40]. The studies compared an estimated total sample size of 8740562 vaccinated elderly people and 9658245 unvaccinated elderly cohorts from an estimated total study population of 26535692 which evaluated the effectiveness of mRNA vaccines of an adult population including elderly cohorts who were 50 years old and older[33-40]. Although the goal of the study is to focus on elderly subjects who were 60 years old and older, some of the selected studies in this review, grouped the elderly subjects from 50 years old to include 60 years old and older subjects[35-38]. The largest sample size of vaccinated elderly people was 3479102 and 8138482 unvaccinated elderly cohorts while the smallest sample size of vaccinated and unvaccinated elderly people was 45345 and 1272, respectively[34,35,39]. The outcome measures used by the selected studies defined morbidity as infection (n = 5), hospitalization (n = 6), admission to ICU and intubation (n = 2) and mortality or death (n = 4) as outcome measurements[33-40]. The characteristics of included studies are shown in Tables 1 and 2.
| Ref. | Study design | Location | Purpose | Age of study group | Vaccine type | Number of doses received | Variant of concern |
| Baum et al[33], 2022 | Cohort study | Finland | To estimate VE against severe COVID-19 among the elderly | Adult population including ≥ 70 yr old | Pfizer-BioNTech | 2 doses | Omicron |
| Grewal et al[34], 2022 | Case control design | Canada | To estimate vaccine effectiveness of mRNA vaccines among aged ≥ 60 yr who were tested for SARS-CoV-2 | ≥ 60 yr old | Pfizer-BioNTech and Moderna | Booster | Omicron |
| Rosenberg et al[35], 2021 | Cohort study | USA (NY) | To describe vaccine efficacy in NY | Adult population including ≥ 50 yr old | Pfizer-BioNTech and Moderna | 2 doses | Delta |
| Rosero-Bixby[36], 2021 | Cross-sectional study | Costa Rica | To estimate the dose-dependent effectiveness of coronavirus disease (COVID-19) vaccines to prevent severe illness in real-world conditions | Adult population including ≥ 58 yr old | Pfizer-BioNTech | 2 doses | Delta |
| Rane et al[37], 2022 | Case control study | USA (NY) | To monitor changes in vaccine effectiveness against COVID-19 outcomes for various vaccine products in different population subgroups | Adult population including ≥ 50 yr old | Pfizer-BioNTech | 2 doses | Delta |
| Chemaitelly et al[38], 2021 | Case control study | Qatar | To estimate vaccine effectiveness against any SARS-CoV-2 infection and against any severe, critical, or fatal case of COVID-19 | Adult population including ≥ 50 yr old | Pfizer-BioNTech | 2 doses | Delta |
| Lytras et al[39], 2022 | Cohort study | Greece | To estimate COVID-19 effectiveness against disease and death | Adult population including ≥ 60 yr old | Pfizer-BioNTech and Moderna | 2 doses and booster | Delta |
| Ranzani et al[40], 2022 | Case control study | Brazil | To evaluate vaccine effectiveness against symptomatic COVID-19 and severe COVID-19 (hospital admission or deaths) | Adult population including ≥ 70 yr old | Pfizer-BioNTech | 2 doses and booster | Omicron |
| Ref. | Study population (n) | Age of study population | mRNA vaccinated elderly participants with 2 doses | mRNA vaccinated participants with 2 doses according to vaccine type | mRNA vaccinated elderly participants with 2 doses according to age | Unvaccinated elderly participants | Unvaccinated elderly participants according to age | ||
| Pfizer-BioNTech | Moderna | Pfizer-BioNTech | Moderna | ||||||
| Baum et al[33], 2022 | 897932 | Adult population including ≥ 70 yr old | 241630 | - | - | 70-79 yr old: 171816; 80-89 yr old: 57024; 90-115 yr old: 12790 | 747486 | 70-79 yr old: 480532; 80-89 yr old: 223267; 90-115 yr old: 43687 | |
| Grewal et al[34], 2022 | 46849 | ≥ 60 yr old | 45345 | - | - | - | - | 1272 | - |
| Rosenberg et al[35], 2021 | 8834604 | Adult population including ≥ 50 yr old | 3479102 | - | - | 50-64 yr old: 846664; ≥ 65 yr old: 984464 | 50-64 yr old: 624226; ≥ 65 yr old: 1023748 | 976536 | 50-64 yr old: 606411; ≥ 65 yr old: 370125 |
| Rosero-Bixby[36], 2021 | 3670000 | Adult population including ≥ 58 yr old | 741474 | - | - | 741474 | 58887 | 58887 | |
| Rane et al[37], 2022 | 1058493 | Adult population including ≥ 50 yr old | 143104 | - | - | 50-59 yr old: 64806; 60-69 yr old: 48260; 70-79 yr old: 22997; ≥ 80 yr old: 7041 | 27362 | 50-59 yr old: 14936; 60-69 yr old: 8352; 70-79 yr old: 3066; ≥ 80 yr old: 1008 | |
| Chemaitelly et al[38], 2021 | - | Adult population including ≥ 50 yr old | 1402622 | 907763 | 494859 | - | - | 8043 | 50-59 yr old: 6350; 60-69 yr old: 1326; ≥ 70 yr old: 367 |
| Lytras et al[39], 2022 | 9200000 | Adult population including ≥ 60 yr old | 2380402 | 2128913 | 251492 | - | - | 8138482 | |
| Ranzani et al[40], 2022 | 1417149 | Adult population including ≥ 60 yr old | 306883 | - | - | 60-79 yr old: 258306; ≥ 80 yr old: 48577 | 306588 | 60-79 yr old: 265073; ≥ 80 yr old: 41515 | |
The risk of bias was evaluated by following ROBINS-I tool (risk of bias in non-randomized studies of interventions)[31]. All of the 8 observational studies were rated to have moderate risk of bias mainly due to lack of control for confounders such as comorbidities or socioeconomic status like age and occupation, outbreak data such as location and time of test, and other risk-taking behavior modification[33-40]. Due to the dependence in surveillance data which were subject to incomplete information, 5 studies received a moderate risk of bias score because of missing data, while 3 studies due to misclassification of measurement of outcomes, were rated with moderate bias[35-40]. Table 3 shows the results of ROBINS-I risk of bias assessment of observational studies.
| Ref. | Confounding | Selection of participants | Classification of interventions | Deviations from interventions | Missing data | Measurement of outcomes | Reported result | Overall bias |
| Baum et al[33], 2022 | Moderate | Low | Moderate | Moderate | Low | Low | Low | Moderate |
| Grewal et al[34], 2022 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Rosenberg et al[35], 2021 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Rosero-Bixby[36], 2021 | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Rane et al[37], 2022 | Low | Low | Low | Low | Moderate | Low | Low | Low |
| Chemaitelly et al[38], 2021 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Lytras et al[39], 2022 | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Ranzani et al[40], 2022 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
Vaccine effectiveness against infection: 5 of the 8 studies (36%) reported the effectiveness of vaccines using infection as an outcome measure[34,35,37,38,40]. Among these, 2 of the studies used booster dose to evaluate vaccine effectiveness against asymptomatic and symptomatic infections while 3 studies assessed the effectiveness of 2 doses of vaccines[34,35,37,38,40]. The findings from these studies revealed that 2 doses of mRNA vaccines offer 83%-89% protection against infection, while other studies revealed vaccine’s protection level against infection at 40%-63%, marginally lower for 65 years old when compared to Moderna, Pfizer-BioNTech vaccine was reported to have slightly lower efficacy against infections with indications of declining protection over a period of time[34,35,37,38,40].
Vaccine effectiveness against hospitalization: 3 of the 8 studies (21%) reported the effectiveness of vaccines using hospitalization as an outcome measure which demonstrated 92% efficacy for older people[33,35,36]. Similarly, when compared to Moderna, Pfizer-BioNTech vaccine have lower marginal protection against hospitalization with indication of waning effectiveness against hospitalization, 6 months after the 2nd dose[33,35].
Vaccine effectiveness against ICU admission and intubation: 2 out of the 8 studies (14%) reported the effectiveness of vaccines using admission to ICU and intubation as outcome measures[33,39]. The study on ICU admissions revealed that Pfizer-BioNTech vaccine’s protection waned from 98% down to 85% after 6 months among 70 years old and older[33]. Similar findings was observed when intubation was used as an outcome measure, which revealed diminished vaccine effectiveness from 96.9% down to 86%, in 6 months among 60 years old and older populations but was restored at 97.6% by booster dose[39].
Vaccine effectiveness against death: 4 out of the 8 studies (29%) reported the effectiveness of vaccines using death with hospitalization as outcome measures[34,38-40]. The findings showed that although 2 doses of mRNA vaccine can prevent death, it offers a marginally limited protection against death among 75 years old and older with indications of diminishing protection which was only restored by a booster dose[38-40]. Additionally, the finding showed that Pfizer-BioNTech has marginally higher protection level against death at 87% when compared to Moderna at 77%[34]. The outcomes of included studies for vaccine effectiveness are shown in Table 4.
| Ref. | Outcome measurements | Vaccine effectiveness | ||||
| Infection (n/%) | Hospitalization (n/%) | ICU admission (n/%) | Intubation | Death (n/%) | ||
| Baum et al[33], 2022 | - | 2 doses of Pfizer (within 3 mo): 30/5.64%; 2 doses of Pfizer (within 6 mo): 193/36.28%; 2 doses of Pfizer (≥ 6 mo): 148/27.82%; 3 doses of Pfizer (within 3 mo): 95/17.86%; 3 doses of Pfizer (≥ 6 mo): 66/12.41% | 2 doses of Pfizer (within 3 mo): 5/8.33%; 2 doses of Pfizer (within 6 mo): 24/40%; 2 doses of Pfizer (≥ 6 mo): 14/23.33%; 3 doses of Pfizer (within 3 mo): 9/15%; 3 doses of Pfizer (≥ 6 mo): 8/13.33% | - | - | Hospitalization: 2 doses of Pfizer (within 3 mo): 90%; 2 doses of Pfizer (within 6 mo): 85%; 2 doses of Pfizer (≥ 6 mo): 72%; 3 doses of Pfizer (within 3 mo): 95%; 3 doses of Pfizer (≥ 6 mo): 88%; ICU Admission: 2 doses of Pfizer (within 3 mo): 98%; 2 doses of Pfizer (within 6 mo): 95%; 2 doses of Pfizer (≥ 6 mo): 82%; 3 doses of Pfizer (within 3 mo): 98%; 3 doses of Pfizer (≥ 6 mo): 85% |
| Grewal et al[34], 2022 | Infection: 3 doses of Pfizer: 2691/42.43%; 3 doses of Moderna: 3651/57.57%; Symptomatic Infection: 3 doses of Pfizer: 395/38.69%; 3 doses of Moderna: 626/61.31% | 3 doses of Pfizer: 214/58.79%; 3 doses of Moderna: 150/41.21% | - | - | 3 doses of Pfizer: 214/58.79%; 3 doses of Moderna: 150/41.21% | Infection: 3 doses of Pfizer: 31%; 3 doses of Moderna: 51%; Symptomatic Infection: 3 doses of Pfizer: 61%; 3 doses of Moderna: 73%; Hospitalization or Death: 3 doses of Pfizer: 87%; 3 doses of Moderna: 77% |
| Rosenberg et al[35], 2021 | ≥ 65 yr old (2 doses of Pfizer): 5302/61.70%; ≥ 65 yr old (2 doses of Moderna): 3291/38.30% | ≥ 65 yr old (2 doses of Pfizer): 972/64.07%; ≥ 65 yr old (2 doses of Moderna): 545/35.93% | - | - | - | Infection: ≥ 65 yr old (2 doses of Pfizer): 83.0%; ≥ 65 yr old (2 doses of Moderna): 89.2%; Hospitalization: ≥ 65 yr old (2 doses of Pfizer): 91.9%; ≥ 65 yr old (2 doses of Moderna): 95.7% |
| Rosero-Bixby[36], 2021 | 40-57 yr old: 37/0.006%; ≥ 58 yr old: 65/0.009% | - | - | - | Hospitalization: 40-57 yr old: 94%; ≥ 58 yr old: 92% | |
| Rane et al[37], 2022 | 60-69 yr old: 3232/5.8%; 70-79 yr old: 1221/2.2%; ≥ 80 yr old: 468/0.8% | - | - | - | - | Infection: 51-64 yr old: 60%; 65 80 yr old: 55%; ≥ 80 yr old: 51% |
| Chemaitelly et al[38], 2021 | 2 doses of Pfizer (within 1 mo): 2915/2.51%; 2 doses of Pfizer (within 2 mo): 1450/1.28%; 2 doses of Pfizer (within 3 mo): 800/0.71%; 2 doses of Pfizer (within 4 mo): 492/0.44%; 2 doses of Pfizer (within 5 mo): 548/0.49%; 2 doses of Pfizer (within 6 mo): 460/0.41%; 2 doses of Pfizer (≥ 7 mo): 135/0.12% | 2 doses of Pfizer (within 1 mo): 32/0.78%; 2 doses of Pfizer (within 2 mo): 23/0.56%; 2 doses of Pfizer (within 3 mo): 17/0.42%; 2 doses of Pfizer (within 4 mo): 10/0.25%; 2 doses of Pfizer (within 5 mo): 0/0; 2 doses of Pfizer (within 6 mo): 8/0.20%; 2 doses of Pfizer (≥ 7 mo): 6/0.15% | 2 doses of Pfizer (within 1 mo): 32/0.78%; 2 doses of Pfizer (within 2 mo): 23/0.56%; 2 doses of Pfizer (within 3 mo): 17/0.42%; 2 doses of Pfizer (within 4 mo): 10/0.25%; 2 doses of Pfizer (within 5 mo): 0/0; 2 doses of Pfizer (within 6 mo): 8/0.20%; 2 doses of Pfizer (≥ 7 mo): 6/0.15% | Infection: 2 doses of Pfizer (within 1 mo): 75.8%; 2 doses of Pfizer (within 2 mo): 69.7%; 2 doses of Pfizer (within 3 mo): 63.7%; 2 doses of Pfizer (within 4 mo): 39.1%; 2 doses of Pfizer (within 5 mo): 11.4%; 2 doses of Pfizer (within 6 mo): 9.2%; 2 doses of Pfizer (≥ 7 mo): -4.4%; Hospitalization and Death: 2 doses of Pfizer (within 1 mo): 95.9%; 2 doses of Pfizer (within 2 mo): 96.3%; 2 doses of Pfizer (within 3 mo): 93.4%; 2 doses of Pfizer (within 4 mo): 80.8%; 2 doses of Pfizer (within 5 mo): 100%; 2 doses of Pfizer (within 6 mo): 81.8%; 2 doses of Pfizer (≥ 7 mo): 44.1% | ||
| Lytras et al[39], 2022 | 2 doses of Pfizer: 548/90.28%; 2 doses of Moderna: 35/5.77%; 3 doses of Pfizer: 24/0.04% | 2 doses of Pfizer: 1629/64.60%; 2 doses of Moderna: 42/2.44%; 3 doses of Pfizer: 51/5.96% | Intubation: 60-79 yr old (2 doses): 96.9%; ≥ 80 yr old (2 doses): 94.4%; ≥ 80 yr old (2 doses, within 6 mo): 86.0%; ≥ 80 yr old (3 doses): 97.6%; Death: 60-79 yr old (2 doses): 94.6%; ≥ 80 yr old (2 doses): 91.0%; ≥ 80 yr old (2 doses, within 6 mo): 84.1%; ≥ 80 yr old (3 doses): 98.4% | |||
| Ranzani et al[40], 2022 | Symptomatic Infection: 60-74 yr old (2 doses): 34077/86.93%; ≥ 75 yr old (2 doses): 15539/79.84%; 60-74 yr old (3 doses, within 2 mo): 15053/64%; 60-74 yr old (3 doses, after 2 mo): 3273/66.36%; ≥ 75 yr old (3 doses, within 2 mo): 116955/89.86%; ≥ 75 yr old (3 doses, after 2 mo): 64495/99.36% | 60-74 yr old (2 doses): 2035/63.3%; ≥ 75 yr old (2 doses): 2750/52.81%; 60-74 yr old (3 doses, within 2 mo): 50/68.50%; 60-74 yr old (3 doses, after 2 mo): 51/96.01%; ≥ 75 yr old (3 doses, within 2 mo): 511/90.41%; ≥ 75 yr old (3 doses, after 2 mo): 2964/72.40% | Infection: 60-74 yr old (2 doses): 63.4%; ≥ 75 yr old (2 doses): 40.7%; 60-74 yr old (3 doses, within 2 mo): 88.4%; 60-74 yr old (3 doses, after 2 mo): 90.4%; ≥ 75 yr old (3 doses, within 2 mo): 77.3%; ≥ 75 yr old (3 doses, after 2 mo): 78.5%; Hospitalization or death: 60-74 yr old (2 doses): 63.4%; ≥ 75 yr old (2 doses): 40.7%; 60-74 yr old (3 doses, within 2 mo): 88.4%; 60-74 yr old (3 doses, after 2 mo): 90.4%; ≥ 75 yr old (3 doses, within 2 mo): 77.3%; ≥ 75 yr old (3 doses, after 2 mo): 78.5% | |||
While clinical trial data on Pfizer-BioNTech and Moderna vaccines demonstrated 94% effectiveness among the elderly, the results in this study showed that the effectiveness of mRNA vaccines in real-world settings is marginally lower against COVID-19 infection, hospitalization, ICU admission and intubation, and deaths during the predominance of Delta and Omicron variants[33-40].
The results in this systematic review further strengthen and supplement the increasing evidence on the real-world effectiveness of mRNA vaccines. While the inclusion and exclusion criteria of this review limits a variety of similar studies in the data analysis, for discussion purposes these studies echoed similar findings. A study conducted in United Kingdom revealed that vaccine effectiveness for ≥ 60 years old is 42.3%[41]. The same observed pattern is reported for ≥ 75 years old in a study conducted in Israel, in a case-control study conducted among US military personnel and in a test-negative design study conducted in Malaysia[42-44]. Using random-effects model on 15 observational studies to estimate the pooled vaccine effectiveness (VE) with 95% confidence intervals for each vaccine type against each variant, the systematic review and meta-analysis conducted by Zhang et al[45] revealed a limited vaccine effectiveness among ≥ 65 years old. The result in this study also align with the result in our study and with the findings of other studies focusing on vaccine effectiveness in the elderly during the predominance of Delta and Omicron variants[46-48]. Utilizing the same research model, a contrasting result was reported by Li et al[49] when they evaluated the effectiveness of vaccine in over 30000 participants aged 60 years and older. This systematic review and meta-analysis however, largely focused on randomized controlled trials which may have skewed the outcomes. Given that clinical trials on COVID-19 vaccines are conducted under controlled clinical conditions from volunteer subjects of targeted age groups, these studies are not able to take into account the abilities of COVID-19 to mutate and evade the vaccine[46,47,50-53]. Therefore, the tangible effect of vaccines can be substantially different from the real-world which may not necessarily illustrate the authentic effectiveness of vaccines. Furthermore, although interventional studies such as clinical trials are more methodologically sound, observational studies are more reliable since they produce practical and realistic results that are grounded from real-world experiences.
By focusing on Delta and Omicron variants, we hypothesize that much of the previous research on vaccine effectiveness only included earlier variants which may have skewed the results for newer and more dominant variants like Delta and Omicron. We also aim to provide value in understanding the effectiveness of mRNA vaccines by comparing their effectiveness in real-world settings. While the results in this study reported a marginal difference in effectiveness between Moderna and Pfizer-BioNTech vaccines, the minor difference on an absolute scale can be significant when considering world-wide population for vaccination[54].
Furthermore, the observed waning effectiveness of vaccine in this study supports the findings of other studies which suggested that the diminishing effectiveness of vaccine is due to the extensive abilities of COVID-19 virus to evolve and generate new variants which allow them to avoid the effects of the vaccines[51-53]. The ability of Delta and Omicron variants to elude sensitivity to antibody neutralization was observed to decline over time making the vaccine less effective[41,55-58]. Consistent to the findings of this study, this imply that 2 doses of mRNA vaccines is inadequate and only provides interim protection against COVID-19 infection, hospitalization, ICU admission and intubation, and deaths[58-61]. Because of the vaccine’s natural diminishing effectiveness, the importance of booster dose to restore its efficacy is vital in providing additional protection against emerging variants[33,34,39,40,49,62-64]. This position is in line with the study conducted in an elderly long-term care facility where the effectiveness of vaccine was observed to only have improved after the second dose, along with other studies pointing out that booster dose can provide significant protection and is the most effective approach to COVID-19 prevention[59,63,65].
This study provides useful information on the effectiveness of mRNA vaccines in the real-world settings which are not under a regulated condition of clinical trials. Specifically, the strengths of this study made use of an inexpensive design that is reproducible since it rests on an organized search strategy, strong procedure with the inclusion of literature from pre-print servers. It also included a broad range of possible outcome measures to include as many studies as possible that can provide relevant information on the topic. Furthermore, this systematic review included research studies from different parts of the world that have relatively large representatives of elderly population with longer follow up which is useful in minimizing selection bias.
The findings of this study should be cautiously interpreted due to certain limitations. First, the included literature were observational studies which have restrictions in statistical power. Second, since there is limitation to access the data used by the research studies, this study has a risk in information bias. Third, due to the missing data and estimation of some studies, a degree of misclassification further delimits this study. Fourth, in exchange of large sample sizes, this study sits on a potential bias of unmeasured confounder such as comorbidities or socioeconomic status like age and occupation, outbreak data such as location and time of test, and other risk-taking behavior modification. Finally, the use of heterogenous outcome measures creates limitation due to potential classification errors.
As a response to the rapidly evolving COVID-19 outbreak, many research studies were organized and carried out resulting in highly heterogeneous outcome measurements. From a research perspective, this heterogeneity inhibits the comparison, contrast, and integration of the results which makes data pooling across different studies problematic. Therefore, this systematic review suggests that, while pharmaceutical intervention like vaccination is important to fight an epidemic, utilizing common outcome measurements or carrying out studies with minimal heterogeneity in outcome measurements, is equally crucial to better understand and respond to an international health crisis. Notwithstanding these limitations, the consistent findings of this review indicated waning of vaccine effectiveness over time, implying that a large proportion of the vaccinated population, particularly the elderly, may lose protection unless booster doses are rolled out to restore the effectiveness of the vaccine (Supple
Although there has been a rise in the administration of vaccinations and booster shots, coronavirus disease 2019 (COVID-19) infections and fatalities continue to persist at a significant level. The effectiveness of these vaccines has been confirmed by multiple manufacturers and were rapidly developed for emergency use with testing conducted in controlled clinical conditions and on voluntary participants, whose characteristics may differ from those of the broader population.
The COVID-19 pandemic has caused an unprecedented global health crisis resulting in millions of deaths and infections worldwide. Despite the availability of COVID-19 vaccines and the administration of booster shots, the number of cases and deaths remains high. The development and clinical trials of these vaccines were conducted in controlled environments with volunteers which may not fully represent the general population. Therefore, there is a need to determine the real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the predominance of Delta and Omicron variants in preventing COVID-19-related infections, hospitalizations, intensive care unit (ICU) admission and intubation, and death.
This study aimed to conduct a systematic review of available research articles to evaluate the effectiveness of Pfizer-BioNTech and Moderna vaccines on the elderly using infection, hospitalization, ICU admission and intubation, and death as outcome measures.
The study utilized a combination of Medical Subject Headings (MeSH) and non-MeSH to identify relevant research articles from various databases and pre-print servers.
While clinical trial data on Pfizer-BioNTech and Moderna vaccines demonstrated high vaccine effectiveness in the elderly, the results of this study showed that vaccine effectiveness in real-world settings is marginally lower against infection, hospitalization, ICU admission and intubation, and death, with an indication of diminished effectiveness of the vaccine over time. Furthermore, 2 doses of mRNA vaccines are inadequate and only provide interim protection, emphasizing the need for booster doses to restore its efficacy.
Continued monitoring and research to improve the effectiveness of vaccines and combat the virus effectively is important to evaluate vaccine efficacy in real-world settings, especially as new variants emerge. In addition, the use of highly heterogeneous outcome measures poses a challenge in comparing and integrating the results, and standardized outcome measures or minimal heterogeneity in outcome measurements are essential to better understand and respond to a global health crisis.
Future research should continue to evaluate the real-world effectiveness of COVID-19 vaccines, including the efficacy of booster shots and the effectiveness of vaccines against new variants. Additionally, efforts should be made to standardize outcome measures to enable better comparisons across studies and facilitate the integration of findings. Ultimately, such research will be crucial in guiding public health policies and interventions aimed at controlling the spread of COVID-19 and in mitigating its impact on public health.
| 1. | WHO Coronavirus Disease (COVID-19) Dashboard Data. World Health Organization. Accessed December 31, 2022. Available from: https://covid19.who.int/. |
| 2. | Vokó Z, Kiss Z, Surján G, Surján O, Barcza Z, Wittmann I, Molnár GA, Nagy D, Müller V, Bogos K, Nagy P, Kenessey I, Wéber A, Polivka L, Pálosi M, Szlávik J, Rokszin G, Müller C, Szekanecz Z, Kásler M. Effectiveness and Waning of Protection With Different SARS-CoV-2 Primary and Booster Vaccines During the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 Study). Front Immunol. 2022;13:919408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Duong BV, Larpruenrudee P, Fang T, Hossain SI, Saha SC, Gu Y, Islam MS. Is the SARS CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 533] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 5. | Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee SS. All Nations Must Prioritize the COVID-19 Vaccination Program for Elderly Adults Urgently. Aging Dis. 2021;12:688-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9403] [Article Influence: 1567.2] [Reference Citation Analysis (0)] |
| 7. | Rates of COVID-19 Cases or Deaths by Age Group and Vaccination Status. Centers for Disease Control and Prevention Accessed December 31, 2022. Available from: https://data.cdc.gov/Public-Health-Surveillance/Rates-of-COVID-19-Cases-or-Deaths-by-Age-Group-and/3rge-nu2a/data. |
| 8. | Kowal P, Dowd J. Definition of an older person. Proposed working definition of an older person in Africa for the MDS Project. 2001. [DOI] [Full Text] |
| 9. | Mallah SI, Ghorab OK, Al-Salmi S, Abdellatif OS, Tharmaratnam T, Iskandar MA, Sefen JAN, Sidhu P, Atallah B, El-Lababidi R, Al-Qahtani M. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. 2021;20:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | CDC strategy for global response to COVID-19 (2020-2023). Pamphlet (or booklet). 15/2021. |
| 11. | Perra N. Non-pharmaceutical interventions during the COVID-19 pandemic: A review. Phys Rep. 2021;913:1-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 12. | Shrotri M, Swinnen T, Kampmann B, Parker EPK. An interactive website tracking COVID-19 vaccine development. Lancet Glob Health. 2021;9:e590-e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Dhouib W, Maatoug J, Ayouni I, Zammit N, Ghammem R, Fredj SB, Ghannem H. The incubation period during the pandemic of COVID-19: a systematic review and meta-analysis. Syst Rev. 2021;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Coronavirus (COVID-19) Vaccinations. Our World in Data. Accessed December 31, 2022. Available from: https://ourworldindata.org/covid-vaccinations. |
| 15. | Fleming TR, Krause PR, Nason M, Longini IM, Henao-Restrepo AM. COVID-19 vaccine trials: The use of active controls and non-inferiority studies. Clin Trials. 2021;18:335-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Krause PR, Gruber MF. Emergency Use Authorization of Covid Vaccines - Safety and Efficacy Follow-up Considerations. N Engl J Med. 2020;383:e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon CS, Inouye SK. The Exclusion of Older Persons From Vaccine and Treatment Trials for Coronavirus Disease 2019-Missing the Target. JAMA Intern Med. 2020;180:1546-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Tang P, Hasan MR, Coyle P, Yassine HM, Al-Khatib HA, Smatti MK, Al-Kanaani Z, Al-Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul-Rahim HF, Nasrallah GK, Al-Kuwari MG, Butt AA, Al-Romaihi HE, Al-Thani MH, Al-Khal A, Bertollini R. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 boosters against SARS-CoV-2 Omicron (B.1.1.529) infection in Qatar. medRxiv. 2022:2022.01.18.22269452. [DOI] [Full Text] |
| 19. | Alali WQ, Ali LA, AlSeaidan M, Al-Rashidi M. Effectiveness of BNT162b2 and ChAdOx1 vaccines against symptomatic COVID-19 among Healthcare Workers in Kuwait: A retrospective cohort study. medRxiv. 2021:2021.07.25.21261083. [DOI] [Full Text] |
| 20. | Alencar CH, Cavalcanti LPG, Almeida MM, Barbosa PPL, Cavalcante KKS, Melo DN, de Brito Alves BCF, Heukelbach J. High Effectiveness of SARS-CoV-2 Vaccines in Reducing COVID-19-Related Deaths in over 75-Year-Olds, Ceará State, Brazil. Trop Med Infect Dis. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Amirlak L, Haddad R, Hardy JD, Khaled NS, Chung MH, Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Andrejko KL, Pry J, Myers JF, Jewell NP, Openshaw J, Watt J, Jain S, Lewnard JA; California COVID-19 Case-Control Study Team. Prevention of Coronavirus Disease 2019 (COVID-19) by mRNA-Based Vaccines Within the General Population of California. Clin Infect Dis. 2022;74:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 275] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 24. | Bello-Chavolla OY, Antonio-Villa NE, Valdés-Ferrer SI, Fermín-Martínez CA, Fernández-Chirino L, Ramírez-García D, Mancilla-Galindo J, Kammar-García A, Ávila-Funes JA, Zúñiga-Gil CH, García-Grimshaw M, Ceballos-Liceaga SE, Carbajal-Sandoval G, Montes-González JA, Zaragoza-Jiménez CA, García-Rodríguez G, Cortés-Alcalá R, Reyes-Terán G, López-Gatell H, Gutiérrez-Robledo LM. Effectiveness of a nation-wide COVID-19 vaccination program in Mexico. medRxiv. 2022:2022.04.04.22273330. [DOI] [Full Text] |
| 25. | Chin ET, Leidner D, Zhang Y, Long E, Prince L, Schrag SJ, Verani JR, Wiegand RE, Alarid-Escudero F, Goldhaber-Fiebert JD, Studdert DM, Andrews JR, Salomon JA. Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines Among Incarcerated People in California State Prisons: Retrospective Cohort Study. Clin Infect Dis. 2022;75:e838-e845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, Soda E, Derado G, Verani JR, Schrag SJ, Jernigan JA, Leung VH, Parikh S. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks - Connecticut, December 2020-February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:396-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Giansante C, Stivanello E, Perlangeli V, Ferretti F, Marzaroli P, Musti MA, Pizzi L, Resi D, Saraceni S, Pandolfi P. COVID-19 vaccine effectiveness among the staff of the Bologna Health Trust, Italy, December 2020-April 2021. Acta Biomed. 2021;92:e2021270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Hammerman A, Sergienko R, Friger M, Beckenstein T, Peretz A, Netzer D, Yaron S, Arbel R. Effectiveness of the BNT162b2 Vaccine after Recovery from Covid-19. N Engl J Med. 2022;386:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 29. | Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1377] [Cited by in RCA: 1172] [Article Influence: 234.4] [Reference Citation Analysis (0)] |
| 30. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51475] [Article Influence: 10295.0] [Reference Citation Analysis (2)] |
| 31. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12554] [Article Influence: 1255.4] [Reference Citation Analysis (2)] |
| 32. | Popay J, Roberts H, Sowden A, Petticrew M. Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC Methods Programme. 2006. [DOI] [Full Text] |
| 33. | Baum U, Poukka E, Leino T, Kilpi T, Nohynek H, Palmu AA. High vaccine effectiveness against severe Covid-19 in the elderly in Finland before and after the emergence of Omicron. medRxiv. 2022:2022.03.11.22272140. [DOI] [Full Text] |
| 34. | Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, Kwong JC. Effectiveness of a Fourth Dose of COVID-19 Vaccine among Long-Term Care Residents in Ontario, Canada: Test-Negative Design Study. medRxiv. 2022:2022.04.15.22273846. [DOI] [Full Text] |
| 35. | Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, Hoefer D, Wu M, Lutterloh E, Conroy MB, Greene D, Zucker HA. COVID-19 Vaccine Effectiveness by Product and Timing in New York State. medRxiv. 2021:2021.10.08.21264595. [DOI] [Full Text] |
| 36. | Rosero-Bixby L. Vaccine effectiveness of Pfizer-BioNTech and Oxford-AstraZeneca to prevent severe COVID-19 in Costa Rica by September and October 2021: A nationwide, observational study of hospitalisations prevalence. medRxiv. 2021:2021.11.08.21266087. [DOI] [Full Text] |
| 37. | Rane MS, Robertson M, Kulkarni S, Frogel D, Gainus C, Nash D. Effectiveness of Covid-19 vaccines against symptomatic and asymptomatic SARS-CoV-2 infections in an urgent care setting. medRxiv. 2022:2022.02.21.22271298. [DOI] [Full Text] |
| 38. | Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, Al Khatib HA, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Rahim HFA, Nasrallah GK, Al Kuwari MG, Al Romaihi HE, Butt AA, Al-Thani MH, Al Khal A, Bertollini R, Abu-Raddad LJ. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. medRxiv. 2021:2021.08.25.21262584. [DOI] [Full Text] |
| 39. | Lytras T, Kontopidou F, Lambrou A, Tsiodras S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J Med Virol. 2022;94:5044-5050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Ranzani OT, Hitchings MDT, Leite de Melo R, de França GVA, de Fátima R, Fernandes C, Lind ML, Torres MSS, Tsuha DH, David LCS, Said RFC, Almiron M, de Oliveira RD, Cummings DAT, Dean NE, Andrews JR, Ko AI, Croda J. Effectiveness of an Inactivated Covid-19 Vaccine with Homologous and Heterologous Boosters against Omicron in Brazil. medRxiv. 2022:2022.03.30.22273193. [DOI] [Full Text] |
| 41. | Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell C, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ramsay M. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021:2021.05.22.21257658. [RCA] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Saciuk Y, Kertes J, Mandel M, Hemo B, Shamir Stein N, Ekka Zohar A. Pfizer-BioNTech vaccine effectiveness against Sars-Cov-2 infection: Findings from a large observational study in Israel. Prev Med. 2022;155:106947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Eick-Cost AA, Ying S, Wells N. Effectiveness of mRNA-1273, BNT162b2, and JNJ-78436735 COVID-19 Vaccines Among US Military Personnel Before and During the Predominance of the Delta Variant. JAMA Netw Open. 2022;5:e228071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Suah JL, Tng BH, Tok PSK, Husin M, Thevananthan T, Peariasamy KM, Sivasampu S. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg Microbes Infect. 2022;11:1343-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 45. | Zhang J, Yang W, Huang F, Zhang K. Effectiveness of mRNA and viral-vector vaccines in epidemic period led by different SARS-CoV-2 variants: A systematic review and meta-analysis. J Med Virol. 2023;95:e28623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7937] [Article Influence: 1587.4] [Reference Citation Analysis (1)] |
| 47. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 11149] [Article Influence: 1858.2] [Reference Citation Analysis (1)] |
| 48. | Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, Brown SM, Peltan ID, Gong MN, Mohamed A, Khan A, Exline MC, Files DC, Gibbs KW, Stubblefield WB, Casey JD, Rice TW, Grijalva CG, Hager DN, Shehu A, Qadir N, Chang SY, Wilson JG, Gaglani M, Murthy K, Calhoun N, Monto AS, Martin ET, Malani A, Zimmerman RK, Silveira FP, Middleton DB, Zhu Y, Wyatt D, Stephenson M, Baughman A, Womack KN, Hart KW, Kobayashi M, Verani JR, Patel MM; IVY Network; HAIVEN Investigators. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 49. | Li Z, Liu S, Li F, Li Y, Peng P, Li S, He L, Liu T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis. Front Immunol. 2022;13:965971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 50. | Jain S, Batra H, Yadav P, Chand S. COVID-19 Vaccines Currently under Preclinical and Clinical Studies, and Associated Antiviral Immune Response. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J Clin Med Res. 2021;13:317-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 52. | Akkiz H. Implications of the Novel Mutations in the SARS-CoV-2 Genome for Transmission, Disease Severity, and the Vaccine Development. Front Med (Lausanne). 2021;8:636532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Bian L, Gao F, Zhang J, He Q, Mao Q, Xu M, Liang Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20:365-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 54. | Dickerman BA, Gerlovin H, Madenci AL, Kurgansky KE, Ferolito BR, Figueroa Muñiz MJ, Gagnon DR, Gaziano JM, Cho K, Casas JP, Hernán MA. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans. N Engl J Med. 2022;386:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 55. | Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Weaver SC, Muik A, Sahin U, Jansen KU, Xie X, Dormitzer PR, Shi PY. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021;384:1466-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 432] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 56. | Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1522] [Article Influence: 304.4] [Reference Citation Analysis (0)] |
| 57. | Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, Nutalai R, Zhou D, Mentzer AJ, Zhao Y, Duyvesteyn HME, López-Camacho C, Slon-Campos J, Walter TS, Skelly D, Johnson SA, Ritter TG, Mason C, Costa Clemens SA, Gomes Naveca F, Nascimento V, Nascimento F, Fernandes da Costa C, Resende PC, Pauvolid-Correa A, Siqueira MM, Dold C, Temperton N, Dong T, Pollard AJ, Knight JC, Crook D, Lambe T, Clutterbuck E, Bibi S, Flaxman A, Bittaye M, Belij-Rammerstorfer S, Gilbert SC, Malik T, Carroll MW, Klenerman P, Barnes E, Dunachie SJ, Baillie V, Serafin N, Ditse Z, Da Silva K, Paterson NG, Williams MA, Hall DR, Madhi S, Nunes MC, Goulder P, Fry EE, Mongkolsapaya J, Ren J, Stuart DI, Screaton GR. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220-4236.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 565] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 58. | Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, Valluri SR, Pan K, Angulo FJ, Jodar L, McLaughlin JM. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 959] [Cited by in RCA: 839] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 59. | Au WY, Cheung PP. Effectiveness of heterologous and homologous covid-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022;377:e069989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 60. | Omer SB, Malani PN. Booster Vaccination to Prevent COVID-19 in the Era of Omicron: An Effective Part of a Layered Public Health Approach. JAMA. 2022;327:628-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1078] [Article Influence: 215.6] [Reference Citation Analysis (0)] |
| 62. | Mattiuzzi C, Lippi G. Efficacy of COVID-19 vaccine booster doses in older people. Eur Geriatr Med. 2022;13:275-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Grewal R, Nguyen L, Buchan SA, Wilson SE, Nasreen S, Austin PC, Brown KA, Fell DB, Gubbay JB, Schwartz KL, Tadrous M, Wilson K, Kwong JC. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun. 2023;14:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 64. | Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O'Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1597] [Cited by in RCA: 1679] [Article Influence: 419.8] [Reference Citation Analysis (4)] |
| 65. | Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A; Working Group for the surveillance and control of COVID-19 in Spain; Working group for the surveillance and control of COVID-19 in Spain. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Public, environmental and occupational health
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masyeni S, Indonesia; Roohvand F, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yu HG