Published online Apr 18, 2023. doi: 10.13105/wjma.v11.i4.102
Peer-review started: December 28, 2022
First decision: January 30, 2023
Revised: February 9, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: April 18, 2023
Processing time: 106 Days and 17.9 Hours
Dehydroepiandrosterone sulfate (DHEAS) is a hormone produced by the zona reticularis of the adrenal gland and the ovaries. Initially considered as an inert compound merely serving as an intermediate in the conversion of cholesterol to androgens, interest in DHEA began to grow in the 1960s when it was found that DHEAS is the most abundant steroid hormone in human plasma and that its levels decline with age. In many countries, DHEA is considered a nutritional supplement. It has been used for a multitude of conditions which include sexual dysfunction, infertility, genitourinary syndrome of menopause, musculoskeletal disorders, cardiovascular diseases, ageing, neurological diseases, autoimmune conditions, adrenal insufficiency, and anorexia nervosa. We describe an overview of the historical evolution of DHEA, its physiology, and the disease states where it has been evaluated as a supplement.
Core Tip: In this review we discuss the current evidence for the nutraceutical utility of dehydroepiandrosterone sulfate (DHEAS). Initially regarded as a panacea for a multitude of human diseases, studies conducted with DHEA supplementation have yielded largely inconclusive results, with the possible exception as an alternative agent in adrenal insufficiency patients with low energy and low libido (in affected females), and genitourinary syndrome of menopause (vaginal preparation). However, with its easy availability as a relatively inexpensive over-the-counter supplement in many countries, DHEA, like vitamin D, has continued to evoke curiosity in the scientific community. Hence, the subject of DHEA supplementation requires a pragmatic approach, backed by robust evidence, with careful weighing of potential benefits (or lack thereof) and possible adverse effects.
- Citation: Jethwani P, Rastogi A, Shukla R. Dehydroepiandrosterone sulfate supplementation in health and diseases. World J Meta-Anal 2023; 11(4): 102-111
- URL: https://www.wjgnet.com/2308-3840/full/v11/i4/102.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i4.102
Dehydroepiandrosterone (DHEA) was first isolated and characterized by Adolf Butenandt in 1934, and he was subsequently awarded the Nobel Prize in 1939 for his “work on sex hormones”. The sulfated form of DHEA, dehydroepiandrosterone sulfate (DHEAS), was then isolated in 1944 by Munson, Gallagher, and Koch in 1944[1]. The hormone was named dehydroepiandrostenedione by Lieberman in 1949[1].
In the 1980s, despite a lack of human studies and information on its function, efficacy, and safety, DHEA began to be marketed as a non-prescription drug in the United States (US) for multiple indications such as anti-ageing, anti-cancer, and anti-obesity. It gained limelight as a “super hormone” and an “anti-ageing” wonder drug which led to multiple studies around its use in various conditions.
Soon thereafter in 1985, the US FDA predictably banned over-the-counter sales of DHEA considering the lack of health benefits and long-term safety data. However, in 1994, DHEA was re-introduced in the market with the dietary supplement health and education act, which allowed certain substances to be marketed as dietary supplements not requiring FDA approval. Marketing as a cure-all elixir and the lack of regulation led to skyrocketing production of DHEA and it became easily available as an over-the-counter supplement. However, research around the hormone has made rapid strides ever since with implications for the diagnosis and treatment of a host of human diseases (Table 1).
| Indication | Current evidence |
| Sexual dysfunction | Equivocal[6] |
| Infertility | Equivocal[13] |
| Genitourinary symptoms (alternative agent) | Positive[7,9] |
| Peri-menopausal and menopausal women | Equivocal[5] |
| Adrenal insufficiency (alternative agent) | Positive[17] |
| Anorexia nervosa | Equivocal[19] |
| Autoimmune diseases | Equivocal[24,27] |
| Musculoskeletal | Equivocal[43] |
| Neuropsychiatric diseases | Equivocal[48] |
| Anti-ageing agent | Negative[35,36] |
| Cardiovascular disorders | Equivocal[54,55] |
The following databases were used to identify the relevant studies: PubMed/Medline, Scopus, and Cochrane. We also applied Reference Citation Analysis (RCA) to further enhance our search results. All the databases were searched from their inception till December 10, 2022. We did a search again and the search was extended up till February 7, 2023 to look for any additional articles. Keywords used were mainly related to the topics of interest, including “DHEA,” “adrenal insufficiency”,” menopause”,” autoimmunity”, “immunity”, “cognition”, “infertility”, “sexual function”, “genitourinary”, “anorexia”, “bone”, “muscle”, “musculoskeletal” “systemic lupus erythematosus” or “SLE”, “schizophrenia”, “depression”, “cardiovascular disease”, “rheumatoid arthritis”, and “hypothyroidism”.
There was no restriction for study design and language (where English language translation was available). All articles related to DHEA supplementation were reviewed and relevant articles were considered for inclusion in this scoping review.
About 75%-90% of DHEA is produced by the zona reticularis of the adrenal gland while the rest is produced in the ovaries and the brain. Its sulfated form, DHEAS, is exclusively synthesized by the adrenals. DHEA has a shorter half-life and is secreted in a pulsatile manner, mirroring the circadian rhythm of corticotrophin. In contrast, DHEAS has a longer half-life and relatively more stable levels across the day, providing a continuous reservoir of DHEA.
DHEA and DHEAS start increasing in boys and girls around the age of six to eight years, and this increase in adrenal androgens is known as adrenarche and the concomitant clinical appearance of pubic hair is known as pubarche. The levels rise steadily and peak in the second to third decade of life. Thereafter there is a progressive decline by around 2%-5% each year with advancing age, such that levels decrease by 80%-90% in the eighth to ninth decade of life[2].
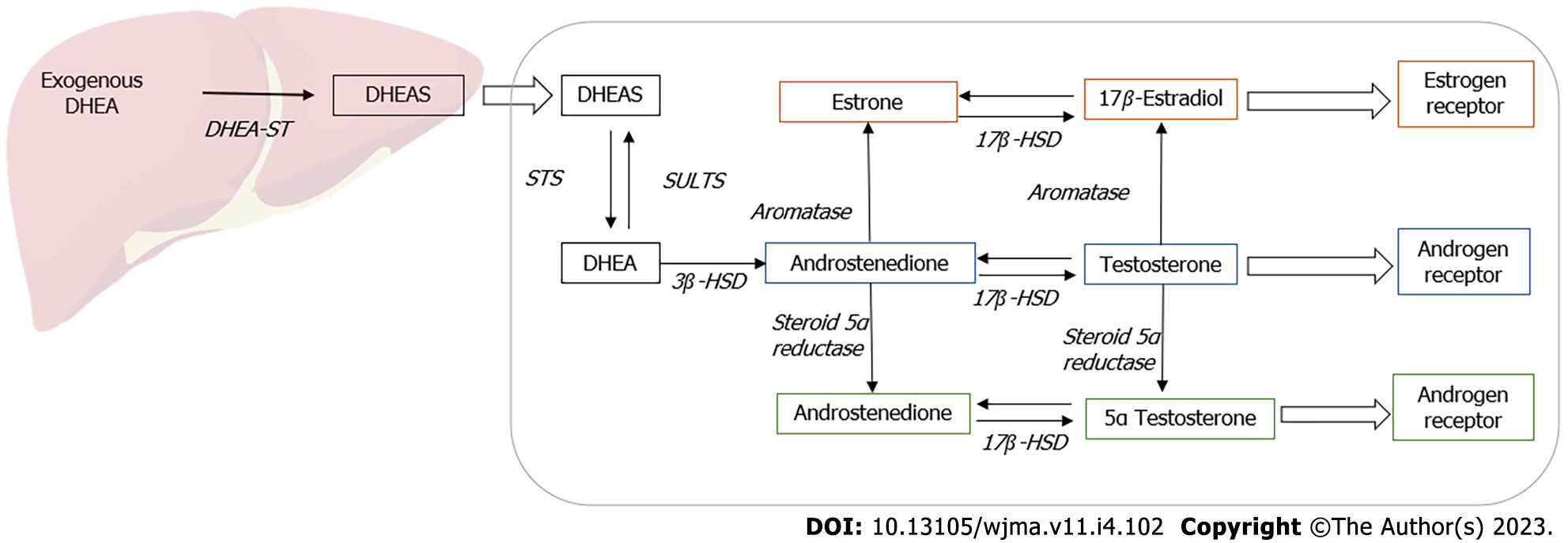
The exact mechanism of action of DHEA remains uncertain with some evidence suggesting that it has pleiotropic effects. As DHEA has minor steroidogenic activity, it acts predominantly by conversion to androgens and estrogens in peripheral target tissues (Figure 1). It also functions as a neurosteroid and acts via receptors for N methyl-D aspartate receptors (NMDA) and gamma amino butyric acid alpha (GABAα), peroxisome proliferator-activated receptor α (PPARα), or receptors for pregnane X, androstanol, and estrogen receptor β[3].
Serum levels of DHEA and DHEAS start declining from the third decade, leading to decreased androgen levels. In a randomized, double-blind, placebo-controlled trial by Panjari et al[4], 93 post-menopausal women with low libido were included and the effect of DHEA on sexual function was assessed. They observed that there was no significant improvement in sexual function with regard to the primary outcome measures which included the change in total satisfying sexual events and the Sabbatsberg Sexual Self-Rating Scale total score. There was no significant change in secondary outcome measures as well, which included measures of well-being and quality of life.
In a systematic review and meta-analysis of 28 studies of DHEA therapy in 1273 post-menopausal women, DHEA therapy did not improve sexual function, quality of life, or menopausal symptoms and was associated with androgenic side effects[5]. It is to be noted that these studies had a duration less than 3 mo. Also, oral DHEA was used in all of these, and there are no studies on local DHEA (see below). Currently, the endocrine society guidelines recommend against the use of DHEA for sexual dysfunction and other related indications because of a lack of long-term safety efficacy data[6].
Genitourinary syndrome of menopause (GSM), a term first introduced in 2014, is a relatively common entity with a prevalence ranging from 27% to 82%. It encompasses symptoms ranging from vulvovaginal dryness and dyspareunia to urinary urgency and dysuria, and leads to significant impairment in quality of life and sexual function. In a randomized prospective double-blind placebo-controlled trial by Labrie et al[7], the efficacy of 0.5% intravaginal DHEA in women with GSM was assessed. They observed that vaginal DHEA (Prasterone) significantly relieved dyspareunia with improvement in vaginal secretions and epithelial integrity. Prasterone was first approved by the FDA in 2016 for the treatment of dyspareunia due to GSM[8]. Recent guidelines suggest vaginal DHEA as an alternative agent in individuals with GSM symptoms after the initial use of non-hormonal agents[9].
DHEA has been found to improve ovarian steroidogenesis and also leads to an increase in IGF-1 which is speculated to have a favorable effect on oocyte quality and follicular development[10,11]. In a randomized prospective study by Wiser et al[12], they enrolled 33 women with poor ovarian reserve (17 in the DHEA group and 16 in the control group) and observed the effects of DHEA supplementation on in vitro fertilization. Patients in the DHEA group had a significantly higher live birth rate as compared to controls. In a meta-analysis by Qin et al[13], they included nine studies and observed that clinical pregnancy rates were significantly increased in women with diminished ovarian reserve supplemented with DHEA. However, when the analysis was restricted to randomized control trials, there was no significant difference in pregnancy rates. In a recent meta-analysis by Schwarze et al[14], they included five studies with a total of 910 individuals with diminished ovarian reserve, of which 413 received DHEA. They observed that DHEA supplementation was associated with significantly improved pregnancy rates and decreased abortion frequency. There was no effect on the number of oocytes retrieved. Hence, the results have been largely conflicting and it is difficult to draw any conclusions as the definitions for diminished ovarian reserve, stimulation protocols used, and dosing and duration of DHEA varied notably between studies. Taken together, it implies that DHEA may have a role in the peri-implantation period but have less impact on ovulation induction/ooscytes retrieval. Future randomised controlled trials planned with primary endpoints of implantation success in such group of subjects, may yield a favorable role for DHEA supplementation.
Adrenal insufficiency is associated with reduced androgen levels which have been suggested to have multiple effects including loss of libido, reduced energy, and consequently decreased quality of life despite optimal glucocorticoid replacement. DHEA therapy has been suggested as a potential therapy to mitigate these effects. In a randomized double-blind placebo-controlled study by Binder et al[15], they included 23 young women with secondary hypoadrenalism and observed significant improvements in pubic hair growth and psychological well-being. In a subsequent meta-analysis by Alkatib et al[16] which included 10 studies in women with either primary or secondary adrenal insufficiency, DHEA supplementation lead to minor improvements in quality of life. However, there was no effect on sexual function or anxiety. Currently, the guidelines suggest that DHEA replacement (25–50 mg as a single oral dose in the morning) may be considered in individuals with low energy and in women with reduced libido despite optimized glucocorticoid and mineralocorticoid replacement[17]. Monitoring is done by clinical and biochemical markers such as measurement of DHEAS, testosterone, androstenedione, and sex hormone-binding globulin (SHBG) 24 h after the last DHEA dose. If the patient fails to report a sustained, beneficial effect of replacement after 6 mo, the treatment should be discontinued.
DHEA levels have also been implicated to play a role in low bone mass in anorexia nervosa. In a randomized control trial by Gordon et al[18] which compared the effects of DHEA vs conventional hormone replacement therapy in young women with anorexia nervosa, they observed that while hip bone mineral density (BMD) increased significantly with both therapies, DHEA therapy was associated with increased bone formation markers. DHEA therapy in addition was associated with significant improvement in psychological parameters. However, in a recent systematic review and meta-analysis, DHEA treatment was not found to be associated with improvement in BMD compared with placebo after adjustment for weight gain[19]. Therefore, while DHEA does play a role in the bone pathology in anorexia nervosa, evidence with treatment remains sparse and further randomized trials are needed.
DHEA has been found to modulate inflammatory responses by blunting the production of pro-inflammatory cytokines, downregulating complement activation via the generation of C1 inhibitor, and enhancing T-cell and NK cell cytotoxicity[20]. In accordance, DHEAS levels have also been found to be decreased in multiple autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, autoimmune hypothyroidism, fibromyalgia, and polymyalgia rheumatica[21-23].
In a randomized double-blind placebo-controlled study by Nordmark et al[24], they included 41 women with SLE on steroids and assessed the efficacy of DHEA supplementation. They observed significant improvement in some domains of health-related quality of outcome measures which included an improvement in mental health. There was also an improvement in sexual well-being while there was no improvement in other domains such as physical function, general health, or vitality.
Similarly, DHEA levels have also been found to be low in Sjogren’s syndrome, which has been hypothesized as a potential cause of fatigue in these individuals. In a multicenter randomized controlled trial by Virkki et al[25], they included 107 individuals with primary Sjogren’s syndrome and assessed the efficacy of DHEA administration on several measures of fatigue. They observed that DHEA supplementation at a dose of 50 mg significantly improved measures of fatigue but a similar improvement was observed with placebo as well. Their results were similar to an earlier study by Hartkamp et al[26], and hence the authors suggested cognitive behavioral interventions in these individuals. In autoimmune hypothyroidism, Shukla et al[27] investigated the relationship between DHEAS levels and arthralgias in individuals with primary hypothyroidism. They assessed 73 individuals with subclinical hypoth
Ageing is associated with declining DHEA levels and deterioration in cognition. Several studies have explored the relationship of DHEA supplementation with cognitive outcomes. DHEA is synthesized in the brain and is the most abundant neurosteroid in humans. DHEA and DHEAS have multiple actions including neuroprotection, acting via AMPA and NMDA receptors, neuronal differentiation and apoptosis via tyrosine kinase receptors, inhibition of 11-β HSD1 activity, and anti-oxidant and anti-inflammatory actions[28,29]. In a study by Wolf et al[30], the authors studied the effect of DHEA supplementation on cognition in healthy elderly men and women. This was a double-blind placebo-controlled study and they observed that DHEA supplementation at a dose of 50 mg had no effect on cognitive abilities in these individuals. In a double-blind placebo-controlled study by Alhaj et al[31], they studied the effects of DHEA administration on episodic memory in 24 healthy young men and observed that DHEA was associated with both subjective and objective improvements in memory when given at a dose of 150 mg over a period of 7 d. In a Cochrane review by Grimley Evans et al[32] that included five trials, they observed that DHEA supplementation was not associated with any beneficial effects on cognitive outcomes in healthy individuals over 50 years of age. Subsequently, in a double-blind placebo-controlled cross-over study by Merritt et al[33], 50 mg of oral DHEA supplementation did not improve short-term memory in post-menopausal women. Hence, although DHEA does seem to play a role in cognitive function, there is little evidence to support its role as therapy for the same. Therefore, large-scale clinical studies are needed to assess whether DHEA could be used as a diagnostic and therapeutic tool for clinical implications.
Concomitant to its potential multiple actions on well-being, sexual function, and cognition, early interest in DHEA came about with it being promoted as the “fountain of youth hormone.” In the DHEAge study by Baulieu and colleagues, they observed the effects of DHEA supplementation at 50 mg daily for a year in 280 older men and women (age 60-79, 140 each). While there was some improvement in some parameters such as sexual function in women over 70 years of age, BMD at the femoral neck, and skin indices, there was no difference in libido, BMD, or sexual function in men[34]. Moreover, there was no difference in body composition or muscle strength in women. Thereafter, in a double-blind randomized placebo-controlled study by Nair et al[35], they investigated the effects of DHEA administration in 87 elderly men with low levels of DHEAS and bioavailable testosterone and 57 elderly women with low DHEAS levels. There was no improvement in body composition, quality of life, physical performance, or insulin supplementation with DHEA supplementation. Similarly, there have been studies that have found no effects of DHEA supplementation on sexual function or well-being parameters[36,37]. Taken together, studies have argued against the use of DHEA as a cure-all elixir and the lack of long-term safety data does not justify the use of DHEA in healthy elderly individuals.
As age-related decline in androgens and estrogens is said to contribute to the loss of muscle mass and bone mineral density in older adults, DHEA has been suggested as a potential agent for minimizing these losses. Moreover, the peak and nadir of BMD mirror the rise and fall in levels of DHEA, respectively, and this has led to multiple studies of DHEA supplementation for bone health.
In bone, DHEA has been postulated to have a dual, pro-anabolic, and anti-catabolic effect. The anabolic effect of DHEA comes from its ability to increase the activity of osteoblasts secondary to raised IGF-1 levels via the GH/IGF-1 pathway. The anti-catabolic action involves its ability to inhibit the overall function of osteoclasts via direct and indirect actions on the estrogen receptor. DHEA also results in increased osteoprotegerin levels, which contributed to reduced resorption by osteoclasts.
In a recent pooled analysis of four double-blind randomized control trials by Jankowski et al[38], they examined the efficacy of DHEA in 295 women and 290 men aged 55 years or elder given DHEA or placebo daily for 12 mo. They observed that men had a significant increase in DHEAS, estradiol, and IGF-1 while women in addition had a significant increase in testosterone levels as well. There was no effect of DHEA on BMD in men, while there was a small increase in lumbar spine (1.0% ± 3.4%) and trochanter (0.5% ± 3.8%) with maintained hip BMD in women. This modest increase in BMD is less than that found with other anti-osteoporotic agents including bisphosphonates, denosumab, and teriparatide. However, these trials did not primarily involve women with osteoporosis which may explain these findings.
DHEA is also said to contribute to muscle growth and strength through an anabolic effect aug
Scattered studies have found some positive effects of DHEA on muscle strength, muscle mass, and mobility, as well as physical function[41,42]. However, in a systematic review by Baker et al[43], they included eight randomized control trials and observed that the effects of DHEA on muscle strength and physical performance were inconclusive.
DHEA has also been found to play a role in the pathophysiology of schizophrenia. It has been suggested to modulate neuronal differentiation and synaptogenesis. Additionally, it also interacts with multiple hormone receptor systems including gamma-aminobutyric acid, glutamate, and dopamine[44]. In a systematic review and meta-analysis by Misiak et al[45] which included 19 studies, DHEAS levels were found to be significantly elevated in individuals with schizophrenia.
DHEA being a neurosteroid has also been evaluated in depression on account of its multiple actions. Its direct actions involve its ability to regulate neuronal excitability by interactions with neurotransmitter receptors known to modulate mood. Other indirect actions involve its ability to regulate cortisol levels and its potential to increase IGF-1 levels.
In one of the initial studies by Wolkowitz et al[46], they included 22 individuals with major depression and observed that DHEA was associated with a significant improvement in depressive score compared to placebo. Thereafter in 2005, Schmidt et al[47] evaluated the efficacy of DHEA in 46 individuals (23 men and 23 women) aged 45 to 65 years with depression in a randomized double-blind placebo-controlled study. They observed that 6 wk of DHEA supplementation was associated with a significant improvement in measures of depression. In a recent meta-analysis by Peixoto et al[48] which included 14 studies, DHEA was associated with a beneficial effect on depressive symptoms compared to placebo. However, the quality of evidence was low due to high clinical heterogeneity in clinical studies. Hence, although DHEA has been found to have some beneficial effect on depressive symptoms, the results should be interpreted with caution and further well-designed larger clinical trials will help in assessing these findings.
DHEAS levels have also been found to be decreased in cardiovascular disease in a few studies, suggesting a possible therapeutic role in atherosclerosis and coronary artery disease[49,50]. The mechanisms suggested to explain these effects include inhibition of platelet aggregation, smooth muscle cell proliferation and plasminogen activator inhibitor-1 generation, increased nitric oxide generation, and vasodilation[51,52]. In the Women’s Ischemia Syndrome Evaluation study, lower DHEAS levels were associated with higher cardiovascular mortality and all-cause mortality and this was independent of other major cardiovascular risk factors. However, when adjusted for the presence or severity of obstructive coronary artery disease, the risk became non-significant[49]. DHEAS levels have also been found to be associated with arrhythmias. In the Rotterdam study which involved 1180 individuals without atrial fibrillation at baseline, after a mean follow-up of 12.3 years, DHEAS levels were found to be inversely associated with the risk of atrial fibrillation[53]. However, several studies have also failed to show an association between DHEA levels and cardiovascular disease[54,55]. In a case-control study by Golden et al[54], they assessed the correlation between DHEAS levels and atherosclerosis in 364 post-menopausal women and observed that DHEAS levels were not associated with the risk of atherosclerosis which was assessed by carotid artery intimal medial thickness. In a recent study by Zhao et al[56], they observed that while increased testosterone levels were associated with an increased risk of cardiovascular diseases, DHEAS levels were not associated with these outcomes. In fact, some studies have found DHEA supplementation to be associated with increased cardiovascular risk. DHEA supplementation has been found to be associated with a pro-atherogenic state via upregulation of lipoprotein processing genes leading to macrophage foam cell formation[57]. Similarly, DHEA supplementation has also been found to be associated with deranged lipid profiles[58,59]. In a double-blind randomized cross-over study by Srinivasan et al[58], they assessed the effect of 50 mg DHEA supplementation for 3 mo on lipid parameters, they observed significantly decreased levels of high-density lipoprotein (HDL) in women supplemented with DHEA. Hence, the association of lower DHEAS levels with increased cardiovascular risk remains uncertain. On the contrary, evidence suggests a cautionary approach in using DHEA supplementation in view of a possible association with adverse cardiovascular profile.
The major concerns with DHEA revolve around its ability to convert to androgen and estrogen metabolites. Reported androgenic side effects involve mild acne, facial hair growth, and seborrhea[60]. DHEA has also been associated with a pro-atherogenic state with decreased HDL levels[57,58]. It has also been seen that DHEA leads to proliferation of breast cancer cells via stimulation of estrogen receptors[61]. Hence, there have been concerns with the use of DHEA in hormone dependent cancers including breast cancer, endometrial cancer, and prostate cancer[62,63]. Currently, there is limited evidence with a paucity of long-term safety data and caution needs to exercised.
Initially marketed as a magic bullet for a myriad of human diseases, its clinical utility remains limited with conflicting results across multiple studies. Nevertheless, it remains an important physiological precursor in the synthesis of androgens and estrogens. While there is considerable evidence to suggest the role of DHEA in adrenal insufficiency and GSM, its role in menopausal females, elderly individuals, and other conditions such as sexual dysfunction, infertility, autoimmunity, and neurological and cardiovascular diseases remains to be fully elucidated. The studies done till date are limited by variations in diagnostic thresholds, DHEA dosing and timing of treatment, relatively small sample size, and shorter duration. Further large-scale, multicentric, robust randomized control trials to assess the effects of DHEA supplementation going forward will help gain a foothold in this untapped research area.
| 1. | Lieberman S. An abbreviated account of some aspects of the biochemistry of DHEA, 1934-1995. Ann N Y Acad Sci. 1995;774:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, Baulieu EE. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001;98:8145-8150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. J Mol Endocrinol. 2016;56:R139-R155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Panjari M, Bell RJ, Jane F, Wolfe R, Adams J, Morrow C, Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009;6:2579-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Scheffers CS, Armstrong S, Cantineau AE, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015;1:CD011066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 7. | Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | The US Food and Drug Administration. FDA approves Intrarosa for postmenopausal women experiencing pain during sex. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-intrarosa-postmenopausal-women-experiencing-pain-during-sex. |
| 9. | The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 10. | Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol. 1994;100:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 297] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 13. | Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. J Gynecol Obstet Hum Reprod. 2017;46:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 14. | Schwarze JE, Canales J, Crosby J, Ortega-Hrepich C, Villa S, Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: A systematic review and meta-analysis. JBRA Assist Reprod. 2018;22:369-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Binder G, Weber S, Ehrismann M, Zaiser N, Meisner C, Ranke MB, Maier L, Wudy SA, Hartmann MF, Heinrich U, Bettendorf M, Doerr HG, Pfaeffle RW, Keller E; South German Working Group for Pediatric Endocrinology. Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab. 2009;94:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:3676-3681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:364-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 1136] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 18. | Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Lin J, Kao TW, Cheng YC, Fan KC, Huang YC, Liu CW. Dehydroepiandrosterone status and efficacy of dehydroepiandrosterone supplementation for bone health in anorexia nervosa: A systematic review and meta-analysis. Int J Eat Disord. 2022;55:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Prall SP, Muehlenbein MP. DHEA Modulates Immune Function: A Review of Evidence. Vitam Horm. 2018;108:125-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Nilsson E, de la Torre B, Hedman M, Goobar J, Thörner A. Blood dehydroepiandrosterone sulphate (DHEAS) levels in polymyalgia rheumatica/giant cell arteritis and primary fibromyalgia. Clin Exp Rheumatol. 1994;12:415-417. [PubMed] |
| 22. | Durcan L, Petri M. Immunomodulators in SLE: Clinical evidence and immunologic actions. J Autoimmun. 2016;74:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Vernerova L, Mravcova M, Paulikova L, Vlcek M, Marko A, Meskova M, Penesova A, Rovensky J, Wendl J, Raslova K, Vohnout B, Jochmanova I, Lazurova I, Killinger Z, Steiner G, Smolen J, Imrich R. Contribution of Genetic Factors to Lower DHEAS in Patients with Rheumatoid Arthritis. Cell Mol Neurobiol. 2018;38:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 24. | Nordmark G, Bengtsson C, Larsson A, Karlsson FA, Sturfelt G, Rönnblom L. Effects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosus. Autoimmunity. 2005;38:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 25. | Virkki LM, Porola P, Forsblad-d'Elia H, Valtysdottir S, Solovieva SA, Konttinen YT. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren's syndrome. Arthritis Care Res (Hoboken). 2010;62:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Hartkamp A, Geenen R, Godaert GL, Bootsma H, Kruize AA, Bijlsma JW, Derksen RH. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: a randomised controlled trial. Ann Rheum Dis. 2008;67:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Shukla R, Ganeshani M, Agarwal M, Jangir R, Kandel G, Sankanagoudar S, Srivastava S. Dehydroepiandrostenedione sulphate (DHEAS) levels predict high risk of rheumatoid arthritis (RA) in subclinical hypothyroidism. PLoS One. 2021;16:e0246195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Maggio M, De Vita F, Fisichella A, Colizzi E, Provenzano S, Lauretani F, Luci M, Ceresini G, Dall'Aglio E, Caffarra P, Valenti G, Ceda GP. DHEA and cognitive function in the elderly. J Steroid Biochem Mol Biol. 2015;145:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Ratner MH, Kumaresan V, Farb DH. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front Endocrinol (Lausanne). 2019;10:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl). 2006;188:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;2006:CD006221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Merritt P, Stangl B, Hirshman E, Verbalis J. Administration of dehydroepiandrosterone (DHEA) increases serum levels of androgens and estrogens but does not enhance short-term memory in post-menopausal women. Brain Res. 2012;1483:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharrière O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279-4284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 396] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 36. | Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001;86:4686-4692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, Allen S, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84:1527-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Jankowski CM, Wolfe P, Schmiege SJ, Nair KS, Khosla S, Jensen M, von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R, Weiss EP, Villareal DT, Kohrt WM. Sex-specific effects of dehydroepiandrosterone (DHEA) on bone mineral density and body composition: A pooled analysis of four clinical trials. Clin Endocrinol (Oxf). 2019;90:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 40. | Stewart CE, Pell JM. Point:Counterpoint: IGF is/is not the major physiological regulator of muscle mass. Point: IGF is the major physiological regulator of muscle mass. J Appl Physiol (1985). 2010;108:1820-1; discussion 1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Valenti G, Denti L, Maggio M, Ceda G, Volpato S, Bandinelli S, Ceresini G, Cappola A, Guralnik JM, Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011;59:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Vuksan-Ćusa B, Šagud M, Radoš I. The role of dehydroepiandrosterone (DHEA) in schizophrenia. Psychiatr Danub. 2016;28:30-33. [PubMed] |
| 45. | Misiak B, Piotrowski P, Chęć M, Samochowiec J. Cortisol and dehydroepiandrosterone sulfate in patients with schizophrenia spectrum disorders with respect to cognitive performance. Compr Psychoneuroendocrinol. 2021;6:100041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Peixoto C, José Grande A, Gomes Carrilho C, Nardi AE, Cardoso A, Barciela Veras A. Dehydroepiandrosterone for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. J Neurosci Res. 2020;98:2510-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA, Stanczyk FZ, Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health--National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab. 2010;95:4985-4992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Teixeira CJ, Veras K, de Oliveira Carvalho CR. Dehydroepiandrosterone on metabolism and the cardiovascular system in the postmenopausal period. J Mol Med (Berl). 2020;98:39-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Jesse RL, Loesser K, Eich DM, Qian YZ, Hess ML, Nestler JE. Dehydroepiandrosterone inhibits human platelet aggregation in vitro and in vivo. Ann N Y Acad Sci. 1995;774:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Martina V, Benso A, Gigliardi VR, Masha A, Origlia C, Granata R, Ghigo E. Short-term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects. Clin Endocrinol (Oxf). 2006;64:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Krijthe BP, de Jong FH, Hofman A, Franco OH, Witteman JC, Stricker BH, Heeringa J. Dehydroepiandrosterone sulfate levels and risk of atrial fibrillation: the Rotterdam Study. Eur J Prev Cardiol. 2014;21:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 55. | Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 384] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 56. | Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Lima JA, Allison MA, Shah SJ, Bertoni AG, Budoff MJ, Post WS, Michos ED. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol. 2018;71:2555-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 57. | Ng MK, Nakhla S, Baoutina A, Jessup W, Handelsman DJ, Celermajer DS. Dehydroepiandrosterone, an adrenal androgen, increases human foam cell formation: a potentially pro-atherogenic effect. J Am Coll Cardiol. 2003;42:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Srinivasan M, Irving BA, Dhatariya K, Klaus KA, Hartman SJ, McConnell JP, Nair KS. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J Clin Endocrinol Metab. 2009;94:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Qin Y, O Santos H, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Wierman ME, Kiseljak-Vassiliades K. Should Dehydroepiandrosterone Be Administered to Women? J Clin Endocrinol Metab. 2022;107:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 61. | Maggiolini M, Donzé O, Jeannin E, Andò S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999;59:4864-4869. [PubMed] |
| 62. | Stoll BA. Dietary supplements of dehydroepiandrosterone in relation to breast cancer risk. Eur J Clin Nutr. 1999;53:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Arnold JT. DHEA metabolism in prostate: For better or worse? Mol Cell Endocrinol. 2009;301:83-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Lafranceschina S, Italy S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Yu HG