Published online Feb 28, 2022. doi: 10.13105/wjma.v10.i1.25
Peer-review started: October 11, 2021
First decision: December 10, 2021
Revised: December 24, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: February 28, 2022
Processing time: 139 Days and 21.6 Hours
After cardiac and non-cardiac surgeries, elderly patients have a high probability of developing cardiac complications and postoperative delirium. Although several clinical trials have investigated whether perioperative intravenous dexmedetomidine can protect the heart and reduce postoperative complications such as delirium in elderly patients, the obtained results have been inconsistent. We conducted a meta-analysis to investigate the effects of dexmedetomidine on cardioprotection and other postoperative complications in elderly patients undergoing cardiac or non-cardiac surgery.
To investigate the effects of dexmedetomidine on cardiac complications and delirium in elderly patients undergoing cardiac or non-cardiac surgery.
The PubMed, Cochrane Library, web of science, and other sources were comprehensively searched for all randomized controlled trials published before May 2021 that investigated the efficacy of dexmedetomidine in the prevention of cardiac and postoperative delirium (POD).
In total, 18 studies involving 1025 patients were included in the meta-analysis. Intravenous dexmedetomidine significantly reduced cardiac troponin I (cTnI) and the inflammatory factor tumor necrosis factor-α (TNF-α) was comparable to the control group. Dexmedetomidine also reduced the POD and mortality rates. However, patients in the dexmedetomidine group were more likely to have a decreased heart rate (within the normal range) and hypotension during dexmedetomidine administration than those in the control group. There was no difference in the occurrence of myocardial infarction, bradycardia, or stroke between the two groups. Dexmedetomidine significantly shortened the time to extubate; however, it did not shorten the length of stay in the intensive care unit.
The administration of dexmedetomidine during cardiac and non-cardiac surgeries can provide myocardial protection by inhibiting inflammation and cTnI, which may be beneficial for the rapid recovery of patients. Meanwhile, the administration of dexmedetomidine reduced the incidence of POD and decreased mortality (in-hospital).
Core Tip: After cardiac and non-cardiac surgeries, elderly patients have a high probability of developing cardiac complications and postoperative delirium. Although several clinical trials have investigated whether perioperative intravenous dexmedetomidine can protect the heart and reduce postoperative complications such as delirium in elderly patients, the obtained results have been inconsistent. We conducted a meta-analysis to investigate the effects of dexmedetomidine on cardioprotection and other postoperative complications in elderly patients undergoing cardiac or non-cardiac surgery.
- Citation: Yang YL, Hu BJ, Yi J, Pan MZ, Xie PC, Duan HW. Effects of dexmedetomidine on cardioprotection and other postoperative complications in elderly patients after cardiac and non-cardiac surgerie. World J Meta-Anal 2022; 10(1): 25-36
- URL: https://www.wjgnet.com/2308-3840/full/v10/i1/25.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i1.25
Elderly patients (> 65 years old) have decreased organ functions and are prone to hemodynamic fluctuations and increased cardiac oxygen consumption, which induces or aggravates myocardial ischemia and hypoxia, leading to severe adverse cardiac events[1]. Postoperative delirium (POD) in elderly patients undergoing major operations, including heart surgery, is a relatively common and serious complication, which is associated with higher morbidity and mortality, cognitive dysfunction, increased length of hospital stay, and increased medical costs[2]. In addition, POD, infection, acute renal failure, major adverse cardiac events, and neurological complications, including permanent or transient stroke, coma, perioperative myocardial infarction (MI), heart block, and cardiac arrest, are major complications[3,4]. However, POD is the most common surgical complication in elderly patients aged 65 years and older[5,6]. The occurrence of delirium may significantly extend the length of hospitalization, delayed recovery, delayed cognitive dysfunction, and increased mortality[4]. Dexmedetomidine can reduce the occurrences of POD postoperative pain, nausea, and vomiting[4,7]. Meanwhile, dexmedetomidine can provide rapid and stable recovery and early extubation after surgery by maintaining the patient's hemodynamics[8].
Dexmedetomidine, a derivative of medetomidine, is a highly selective α2 adrenergic receptor agonist[9] that can inhibit the sympathetic nervous system and act on the noradrenergic glands in the locus coeruleus of the pons to inhibit the release of norepinephrine[6,10]. The dorsal vagus nucleus and suspicious nucleus are the human parasympathetic nerve center, and the central area of the parasympathetic nerve is directly controlled by the nucleus tractus solitarius. Because the nucleus tractus solitarius is abundant in α2 adrenergic receptors, the combination of dexmedetomidine and the nucleus tractus solitarius α2 adrenergic receptors can increase the activity of the vagus nerve and reduce the myocardial cAMP production and L-type calcium channel current, which slows down the heart rate, increases coronary blood flow, and plays a role in myocardial protection[1]. In addition, the anxiolytic, sedative/hypnotic, and analgesic effects of dexmedetomidine[2,11], including the intraoperative hemostatic effect, also enhance cardioprotection[12]. Regarding inflammation, dexmedetomidine can block the accumulation of inflammatory cells in the nervous system[10], reduce neuronal damage caused by immune responses, and reduce surgical complications such as stress response and postoperative cognitive impairment to exert its neuronal protection[9,10]. However, high-dose dexmedetomidine can cause adverse reactions such as hypertension, decreased reflex heart rate, decreased cardiac output, and decreased drug tolerance in elderly patients, requiring special attention to drug dosage[1].
This meta-analysis was reported according to the statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[13]. In addition, this study was not registered with the PROSPERO.
We performed this meta-analysis according to PRISMA guidelines. PRISMA is the minimum set of evidence-based items used for reporting in systematic reviews and meta-analyses, and can be adopted as the basis for systematic reviews for reporting different types of research. We searched 1025 studies from PubMed, Cochrane Library, and Web of Science that were published before May 2021; other search systems focused on 10 studies (Google) to confirm further eligible studies.
We manually screened whether the studies we included met our final research criteria, and then selected 18 of them for research. The basic search strategy included the following words: ("dexmedetomidine"[MeSH Terms] OR"dexmedetomidine"[All Fields]) AND ("cardiac"[MeSH Terms] OR"cardiac"[All Fields]) AND ("aged"[MeSH Terms] OR"aged"[All Fields] OR"elderly"[All Fields]). No language restrictions were applied during the search.
Bradycardia, hypertension, and hypotension were defined as HR < 60 bpm, SBP > 160 mmHg or 20% of baseline, and SBP < 90 mmHg or 20% of baseline, respectively.
We included the following standard studies: (1) Elderly patients (> 65 years old) undergoing cardiac or non-cardiac surgery; (2) We selected a dexmedetomidine group combined with normal saline or other anesthetics, regardless of the initial administration time, dose, duration, or dose; (3) The research content type was randomized controlled clinical trial (RCT); and (4) The research results included hypertension, hypotension, heart rate, cardiac status, complications, POD, stroke, mortality, extubation time, intensive care unit (ICU) time, and cardiac enzyme markers.
Non-randomized controlled trials, case reports, meeting abstracts, and comments were excluded.
Including non-elderly surgery and no results of our study were also excluded.
Two authors (Yang and Hu) independently conducted qualified research selection and data extraction. Disagreements between the two authors were discussed with the third author (Duan) to arrive at the final solution. The extracted data were as follows: first author, annual publication, type of surgery, number of patients, dose of dexmedetomidine, method of anesthesia, hypotension, hypertension, heart rate, myocardial infarction, bradycardia, delirium, stroke, mortality, extubation time, ICU duration, and myocardial enzyme markers.
Two examiners (Yang and Hu) independently used version 2 of the Cochrane tool to assess RCT deviation risk for methodological quality assessment. When the two examiners disagreed, the disagremments were resolved via discussions with a third examiner (Duan).
We employed a Review Manager 5.0 software (Cochrane Collaboration Company) for rigorous statistical analysis. Risk ratios (RRs) with 95% confidence intervals (CIs) and the Mantel-Haenszel method (fixed or random model) were adopted to analyze dichotomous data. For continuous outcomes, the mean differences (MD) or standardized mean differences (SMD) with 95%CIs were calculated. If significant heterogeneity existed (I2 > 50%), sensitivity analysis was performed, each study was ignored separately, and a random effects model was selected.
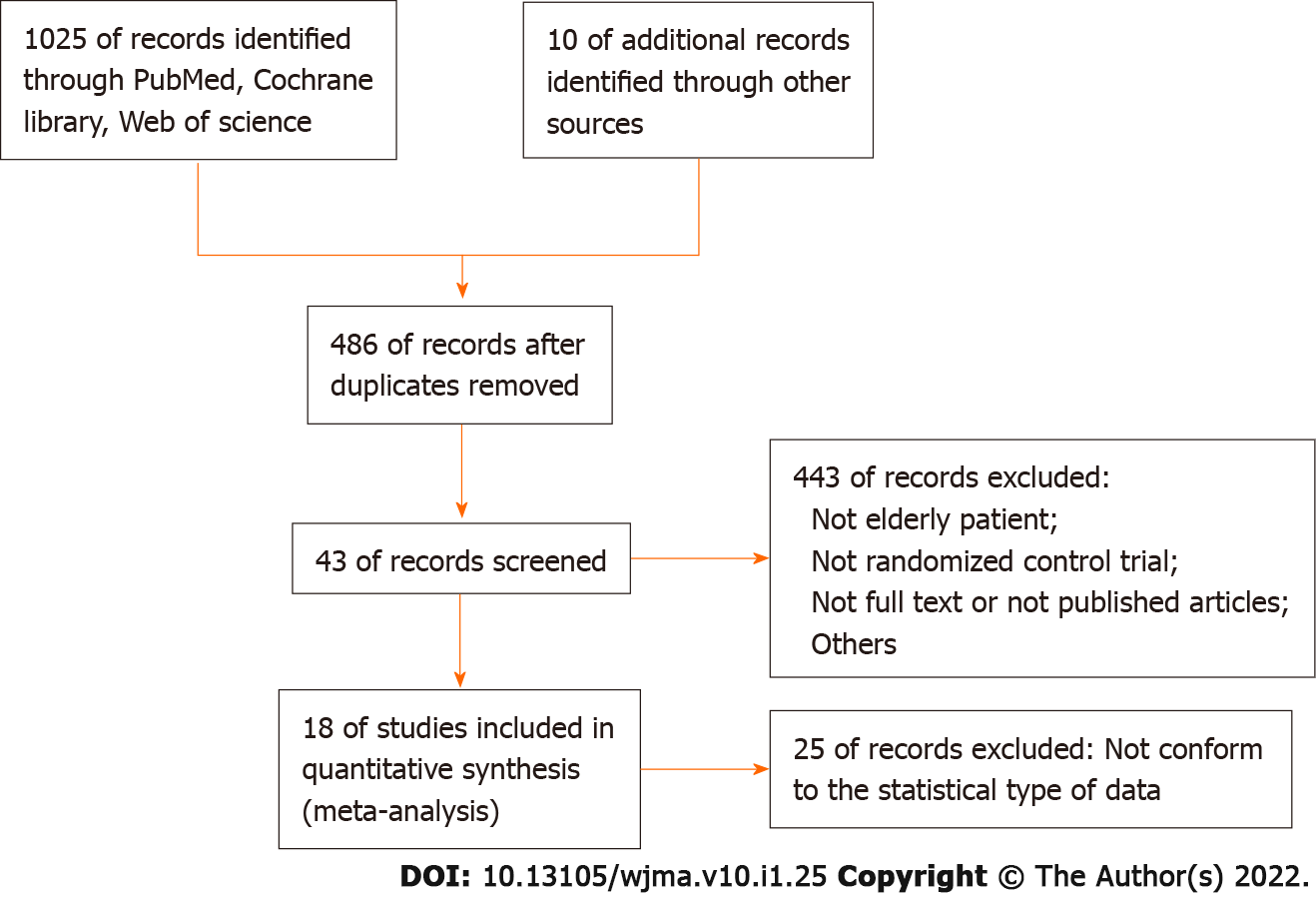
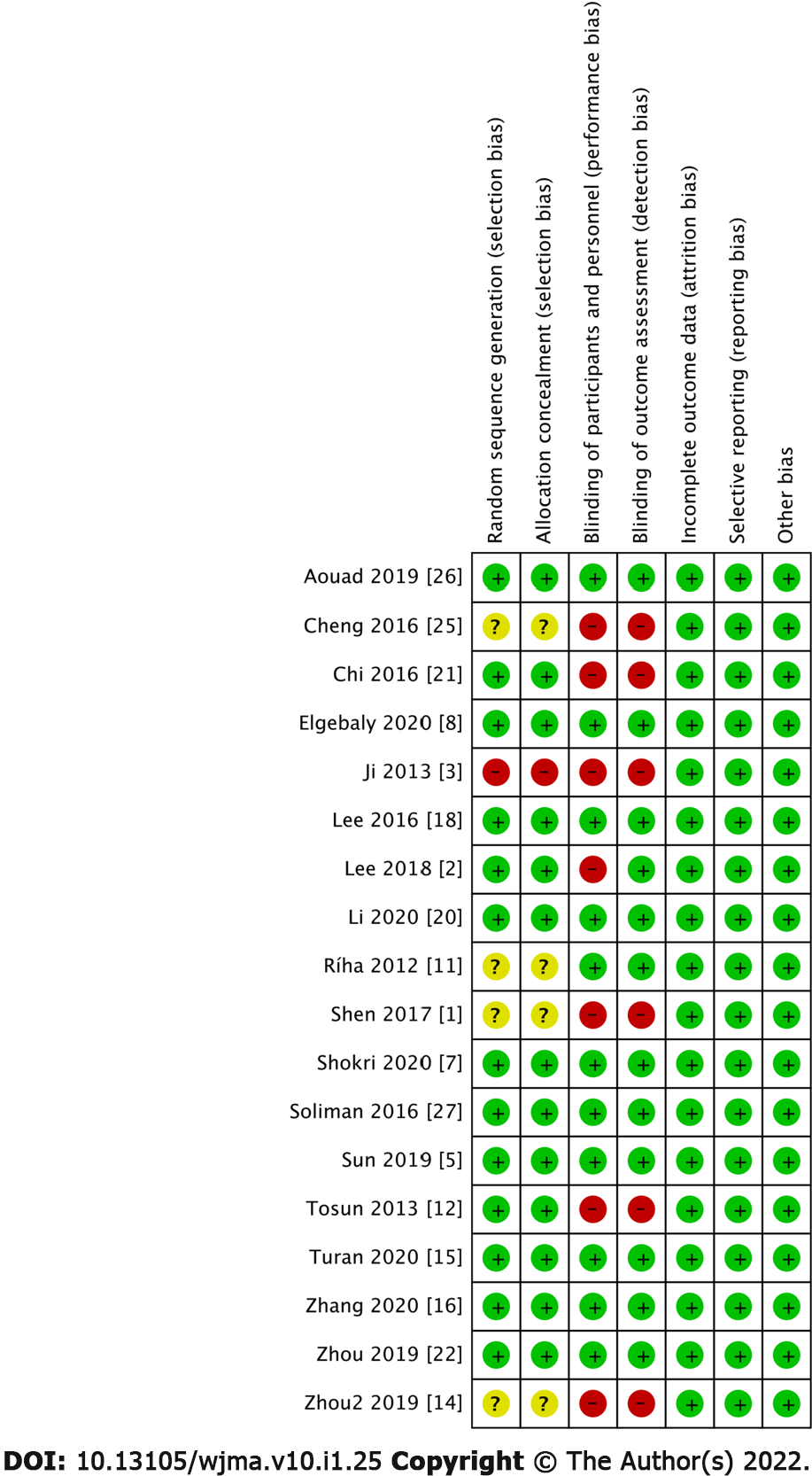
As illustrated in Figure 1. We initially identified 1035 studies by searching the database. After screening out duplicate studies, 486 studies entered the next step of screening: 443 studies were excluded because the focused on non-elderly patients, were non-RCT, were unable to obtain full-text qualifications or not published, etc. (Google search); 25 excluded studies did not comply with the statistical data in this article. Finally, we included 18 studies that met all eligibility criteria (Figure 1). The basic characteristics of the enrolled studies are presented in Table 1. A summary of the risk of bias is presented in Figure 2. Regarding the blindness of patients, researchers, and evaluators, 11 trials were rated as double-blind low-risk trials, and seven trials were rated as high-risk or unclear-risk trials because related articles were not clear about blindness.
| Ref. | No. of patients in study | DEX dose | Surgical procedure | Anesthesia | Outcome parameters | ||
| DEX | Control | Loading dose (μg.kg-1) | Infusion rate (μg. | ||||
| Aouad et al[26], 2019 | 48 | 50 | 1 | N/A | Elective surgery | GA | Hypotension; HR |
| Cheng et al[25], 2016 | 222 | 283 | N/A | 0.24-0.6 | Cardiac Surgery | GA | Delirium; MI; Mortality; Stroke |
| Chi et al[21], 2016 | 34 | 33 | 1 | 0.6 | Off-pump coronary artery bypass graft surgery | GA | Hypertension; ICU time |
| Elgebaly et al[8], 2020 | 30 | 30 | N/A | 0.4 | Open-heart surgery | GA | HR; cTnI; ET; Length of ICU stay |
| Ji et al[3], 2013 | 568 | 566 | N/A | 0.24-0.6 | CABG or valve surgery or CABG or valve surgery combined with other procedures. patients excluded were those undergoing emergency surgery, off-pump or robotic surgery, surgery requiring deep hypothermic circulatory arrest, or surgery involving the thoracic aorta | GA | Delirium; Mortality stroke; MI |
| Lee et al[18], 2016 | 20 | 20 | 1 | 0.5 | Orthopedic surgery in supine position | GA | HR; Hypertension |
| Lee et al[2], 2018 | 95 | 109 | 1 | 0.2-0.7 | Laparoscopic major non-cardiac surgery | GA | Incidence of delirium |
| Li et al[20], 2020 | 309 | 310 | 0.6 | 0.5 | Major non-cardiac surgery | GA | Delirium: Length of ICU stay (h) |
| Ríha et al[11], 2012 | 17 | 21 | 1 | 0.5-1.5 | Elective CABG procedures with the use of cardiopulmonary bypass to treat coronary artery disease | GA | Length of ICU stay; MI; ET |
| Shen et al[1], 2017 | 30 | 30 | 0.5 | 0.5 | Coronary heart disease and underwent gastric cancer operation | GA | Hypotension; cTnI; MI; Bradycardia |
| Shokri et al[7], 2020 | 144 | 142 | N/A | 0.7-1.2 | Coronary artery bypass grafting | GA | Delirium; Hypotension; HR; Bradycardia; ET; Mortality |
| Soliman et al[27], 2016 | 75 | 75 | 1 | 0.3 | Aortic vascular surgery | GA | Hypertension; HR; MI; Bradycardia; Mortality |
| Sun et al[5], 2019 | 281 | 276 | N/A | 0.1 | Major elective noncardiac surgery | GA | Delirium; Hypotension; Hypertension; Mortality |
| Tosun et al[12], 2013 | 18 | 20 | 0.5 | 0.5 | Coronary artery bypass graft surgery | GA | HR |
| Turan et al[15], 2020 | 398 | 396 | 0.1 | 0.2 | Cardiac surgery | GA | Hypotension; MI; Bradycardia; mortality; ICU time; Stroke |
| Zhang et al[16], 2020 | 120 | 120 | 0.5 | 0.3 | Hip Fracture Operation | GA | Delirium; Hypotension; Hypertension; Bradycardia |
| Zhou et al[22], 2019 | 14 | 14 | 0.5 | 0.5 | Valve replacement surgery | GA | cTnI |
| Zhou et al[14], 2019 | 53 | 47 | N/A | 0.2-0.7 | Cardiac surgery | GA | HR; ET; cTnI |
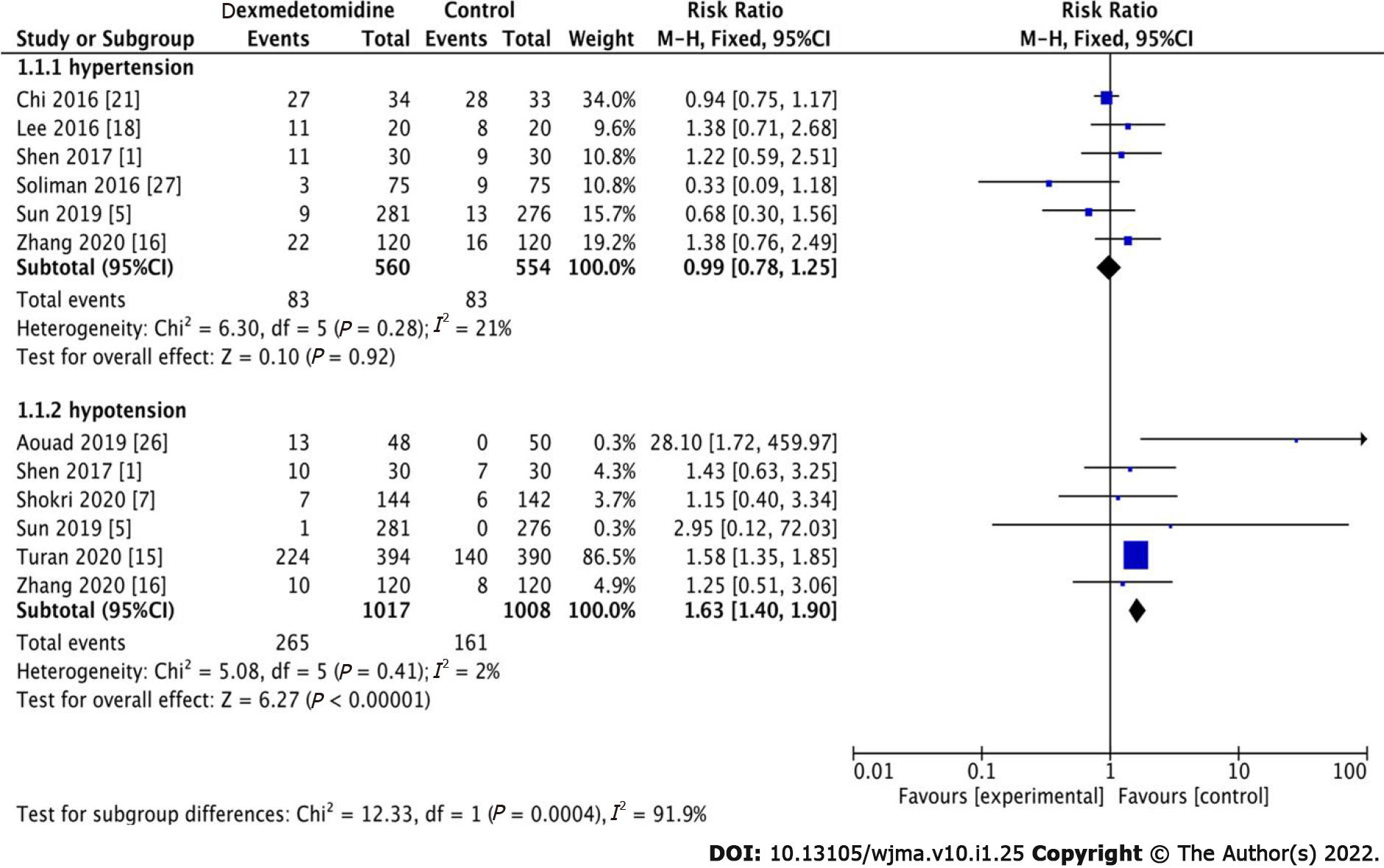
We determined that there was no significant difference in hypertension between the dexmedetomidine and control groups (Figure 3, RR: 0.99, 95%CI: 0.78-1.25, P = 0.92, I2 = 21%). However, 426 patients had hypotension among the 2025 patients, of whom 265 and 161 were in the dexmedetomidine and control groups, respectively. Therefore, it could be inferred that dexmedetomidine had a significant effect on lowering blood pressure (Figure 3, RR: 1.63, 95%CI: 1.40-1.90, P < 0.001, I2 = 2%).
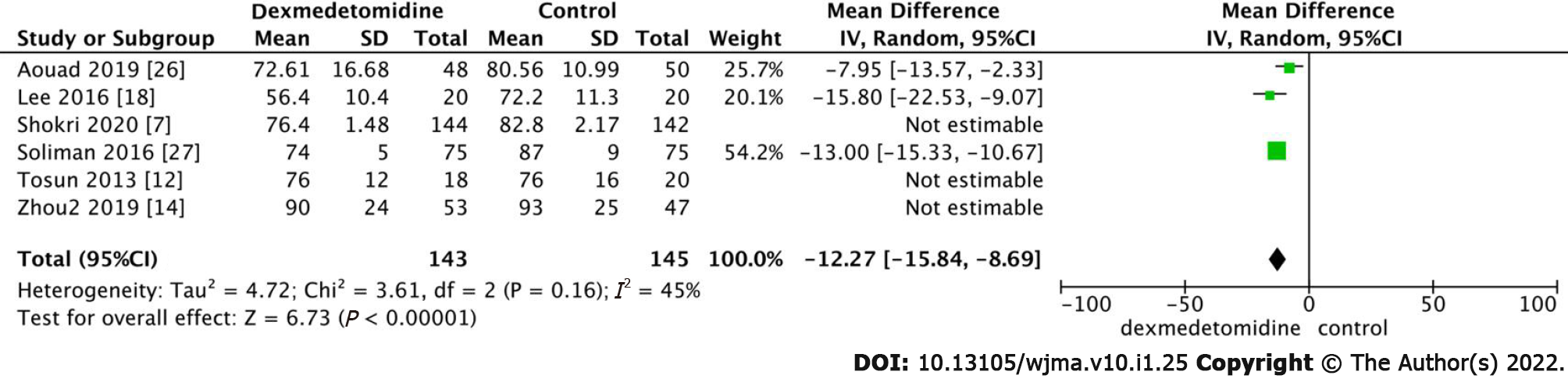
HR was significantly lower in the dexmedetomidine group than in the control group (Figure 4, MD:
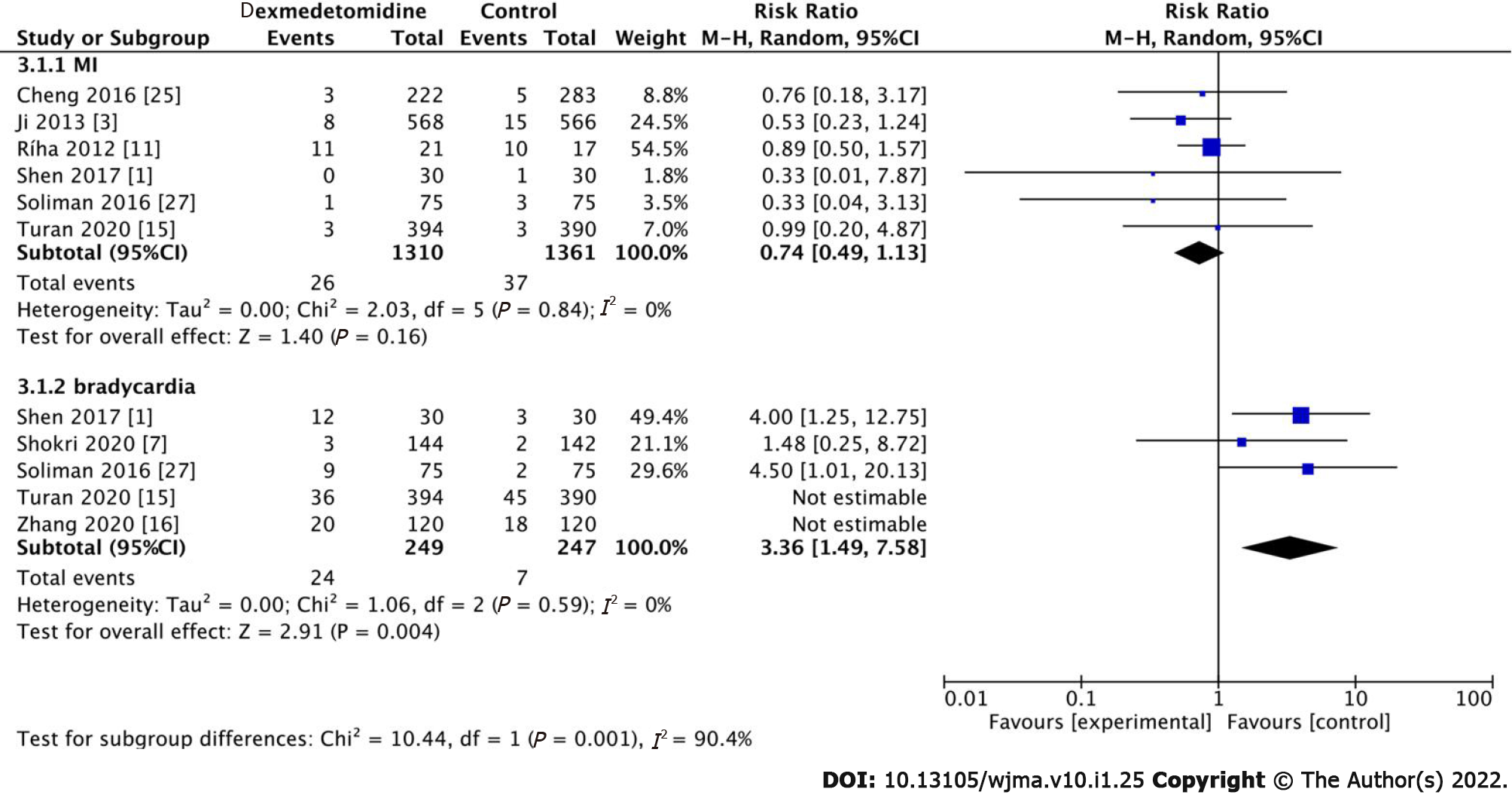
In total, six studies investigated the occurrence of MI. In these studies, 26 patients developed MI in the dexmedetomidine group (Figure 5, RR: 0.74, 95%CI: 0.49-1.13, P = 0.16, I2 = 0%). However, there was no statistically significant difference between the dexmedetomidine and control groups. In addition, five studies participated in the study of bradycardia, and there was no significant statistical difference between the dexmedetomidine and control groups (Figure 5, RR: 1.51, 95%CI: 0.79-2.89, P = 0.21, I2 = 63%). However, we performed a sensitivity analysis by excluding Turan 2020 and Zhang 2020, and the heterogeneity decreased to zero (P = 0.004).
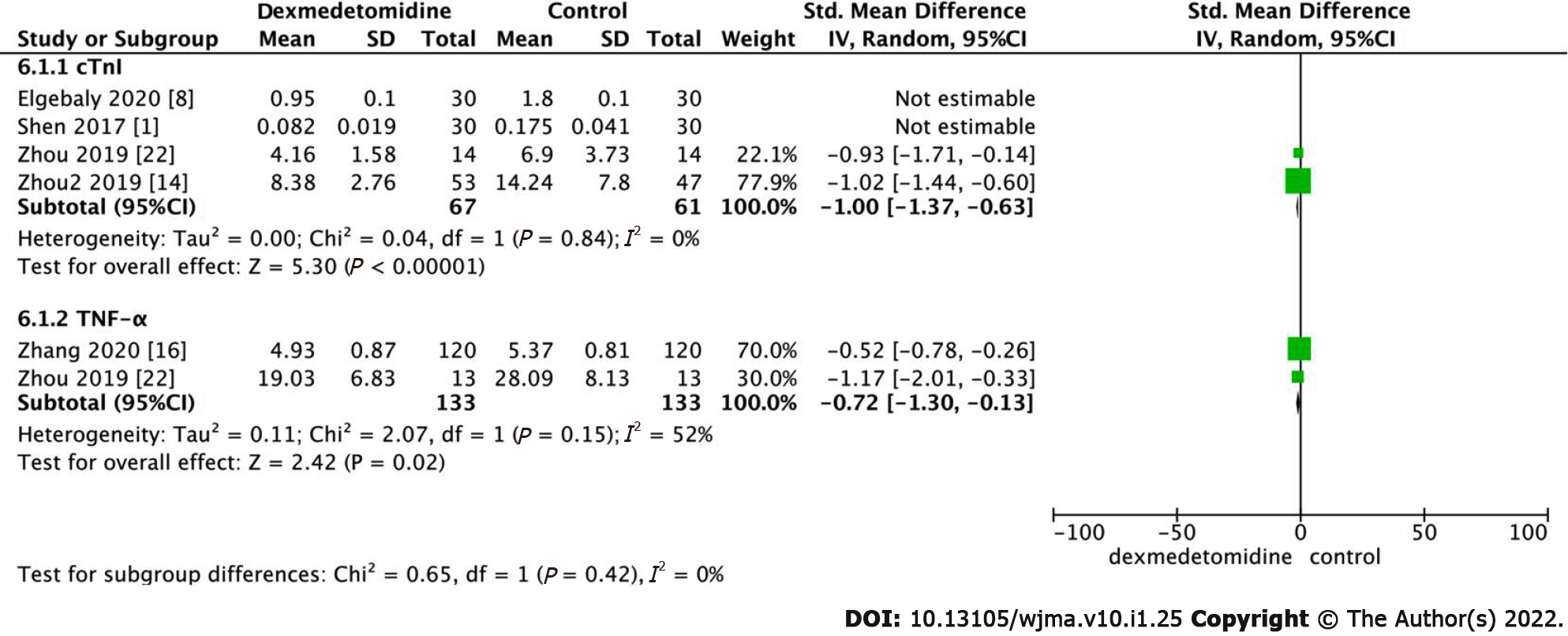
The level of cardiac troponin I (cTnI) in elderly patients after surgery was analyzed. The obtained results indicated that the level of cTnI in the dexmedetomidine group was lower than that in the control group after surgery (Figure 6, SMD: -3.14, 95%CI: -5.16 to -1.11, P = 0.002, I2 = 97%). Statistical heterogeneity was absent when the studies conducted by Elgebaly 2020 and Shen 2017 were excluded, and the obtained results were statistically significant (SMD: -1.00, 95%CI: -1.37 to -0.63, P < 0.001, I2 = 0%).
As illustrated in Figure 6, the level of Factor-α (TNF-α) in the dexmedetomidine group was lower (SMD: -0.72, 95%CI: -1.30 to -0.13, P = 0.02, I2 = 52%) after surgery than that of the control group. Sensitivity analysis and subgroup analysis failed to eliminate the heterogeneity; therefore, a random-effects model was adopted.
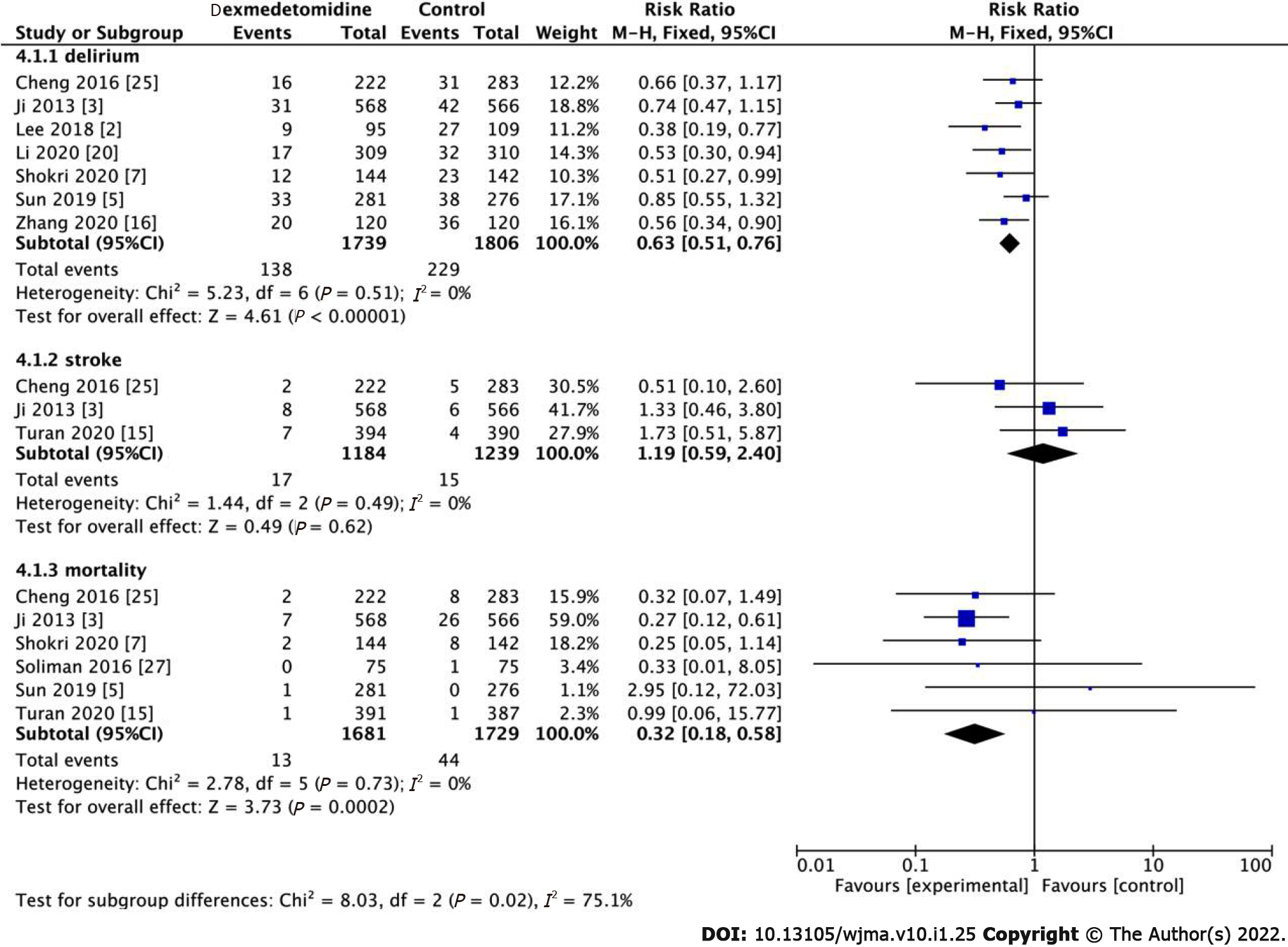
Seven studies have demonstrated that the use of dexmedetomidine use is associated with POD. The occurrence of POD was reported in all the RCTs. Notably, the occurrence of POD in the dexmedetomidine group was significantly lower than that in the control group (Figure 7, RR: 0.63, 95%CI: 0.51-0.76, P < 0.001, I2 = 0%). Low heterogeneity was observed, which we rated as high-quality evidence. We conclude that dexmedetomidine may significantly reduce delirium.
Among the 18 high-quality studies, three studies demonstrated that there was no correlation between the use of dexmedetomidine and postoperative stroke (Figure 7, RR: 1.19, 95%CI: 0.59-2.40, P = 0.62, I2 = 0%). In addition, regarding mortality, the results indicated that the dexmedetomidine group had lower postoperative mortality than the control group (Figure 7, RR: 0.32, 95%CI: 0.18-0.58, P = 0.0002). There was low heterogeneity among the studies regarding mortality outcomes (I2 = 0).
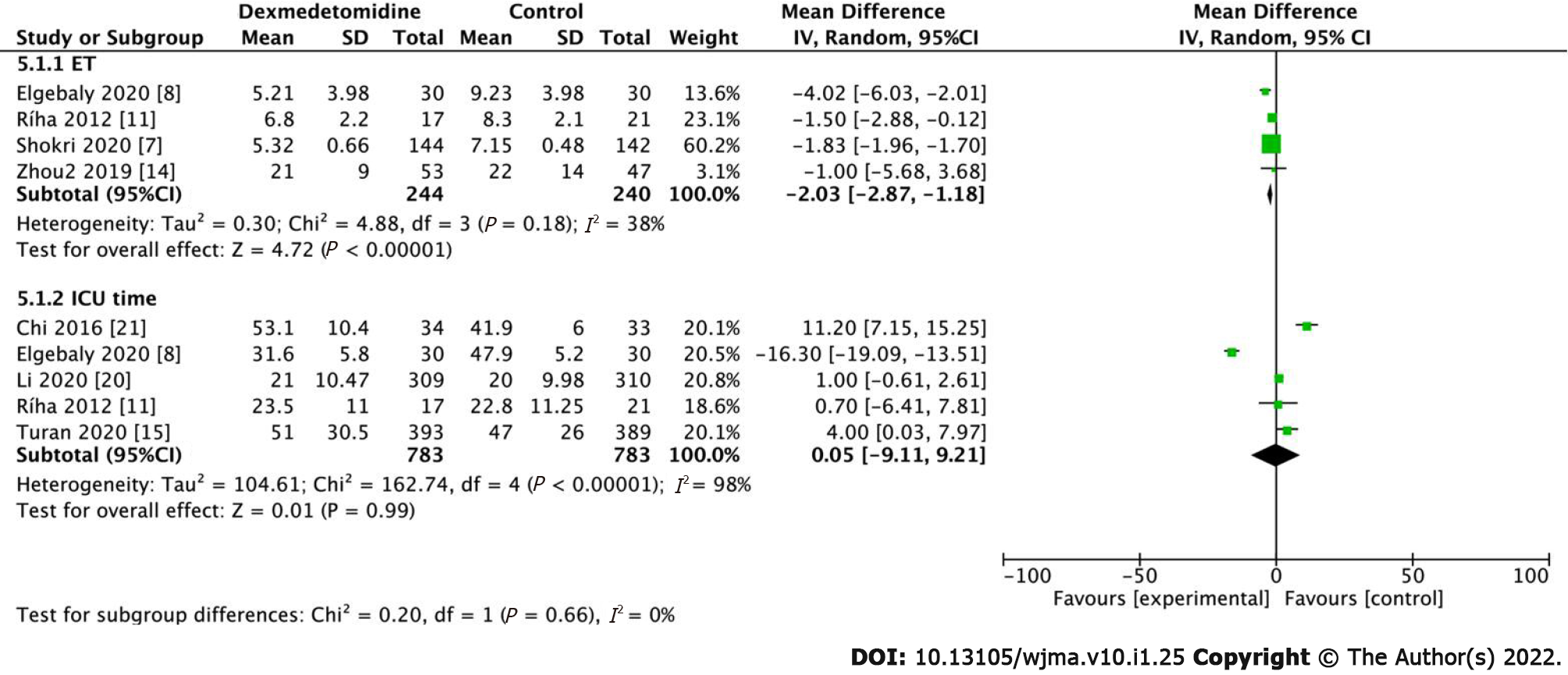
Extubation time (ET) was recorded in 4 studies including 484 patients. The results demonstrated that the use of dexmedetomidine in the perioperative period can shorten ET in patients compared to control patients (Figure 8, MD: -2.03, 95%CI: -2.87 to -1.18, P < 0.001). With low heterogeneity (I2 = 38%), it can be deduced that dexmedetomidine shortens extubation time after surgery without subgroup analysis. However, regarding ICU length of stay, there was no statistical difference between the dexmedetomidine and control groups (Figure 8, MD: 0.05, 95%CI: -9.11 to -9.21, P = 0.99), and heterogeneity I2 was also higher at 98%.
Dexmedetomidine is a highly selective and effective α2-adrenergic receptor agonist that can provide dose-dependent sedation, anti-anxiety, and moderate analgesia[15], with minimal inhibition of respiratory functions. The central sympathetic nervous system reduces the systemic inflammatory response after surgery and regulates the immune system[5,16,17].
First, we compared the occurrence of intraoperative hypertension and hypotension, and determined that dexmedetomidine could increase the risk of intraoperative hypotension; however, it could also lower than the HR within the normal range. This was attributed to the fact that dexmedetomidine stimulates vascular smooth muscle α2 receptors, thereby resulting in a transient increase in blood pressure and a decrease in HR[18]. Studies have shown that dexmedetomidine has an effective sedative effect in heart and vascular surgeries, minimizes the variability of heart rate and blood pressure, and reduces the response of tachycardia to painful stimulation[6,8,19].
Second, we explored cardiac complications and inferred that dexmedetomidine did not increase the occurrence of myocardial infarction and bradycardia[20]; however, it reduced the expression of cTnI and improved the protection of the myocardium. Studies have demonstrated that more bradycardia was observed in dexmedetomidine, which was the expected result of α2-adrenergic receptor agonists; however, considering that the occurrence of bradycardia was transient, intervention was required, and the proportion of patients was low[21]. In clinical practice, postoperative administration of small doses of dexmedetomidine might be an acceptable and safe strategy for patients in general surgery wards[5].
cTnI is a highly sensitive and specific marker that is adopted as the gold standard diagnostic marker for myocardial infarction in coronary artery bypass grafting (CABG); cTnI is also a significant predictor of the prognosis of patients with heart diseases[14]. The low postoperative cardiac biomarker cTnI exhibited cardioprotective effects of dexmedetomidine[8]. Regarding inflammation, our study determined that dexmedetomidine reduced the expression of TNF-α compared to the control group. Dexmedetomidine provides end-organ protection via anti-inflammatory, antioxidant, and anti-apoptotic effects[15]. With several basic effects such as surgical intervention, systemic inflammation, and oxidative stress, reperfusion after myocardial ischemia increases myocardial injury and leads to myocardial cell apoptosis[22]. The most well-known mechanisms by which dexmedetomidine reduces inflammation include the NF-kB pathway, Toll-like receptors, and several inflammatory mediators[22], which in turn reduce the demand for opioids and benzodiazepines[15]. Several studies have shown that postoperative inflammation may be the cause of POD[2,15]. We also determined that the occurrence of POD reduced more significantly in the dexmedetomidine group than in control group after surgery. In addition, POD occurs within 3 d after the operation and is affected by memory loss and impaired comprehension[6,23]. Delirium is a frequent postoperative complication in elderly patients after non-cardiac surgery that caused by several stressors, including neurotransmitter imbalance (especially cholinergic deficiency), inflammation, and electrolyte or metabolic disorders[7,15,24]. It has been reported that the incidence of POD in patients undergoing cardiac surgery is 20%-50%, especially in elderly patients admitted to the ICU and patients undergoing orthopedic surgery. This is associated with higher morbidity and mortality, while the longer length of hospital stay is related to ICU time, increased economic burden, and hospital-acquired complications[6,23]. This study also demonstrated that although the use of dexmedetomidine could reduce the postoperative mortality of patients, it did not reduce the risk of postoperative stroke. Studies have also shown that the mortality rate of patients receiving dexmedetomidine in the hospital, 30 d and 1 year later, was significantly reduced[3]. Stroke is a devastating complication of cardiac surgery. Among patients undergoing different cardiac surgeries, the prevalence of stroke is 1.6%-5.25%[25]; however, the exact cause of postoperative stroke remains unclear. Finally, we found that although the patients receiving dexmedetomidine had a significantly shorter postoperative extubation time than control patients, dexmedetomidine did not significantly shorten the time spent in the ICU. Dexmedetomidine can shorten the time of extubation, which can promote the rapid recovery of heart function in patients[8,9,25-27], reduce the use of psychotropic drugs, and facilitate recovery from activities as soon as possible.
The administration of dexmedetomidine during cardiac and non-cardiac surgeries can provide myocardial protection by inhibiting inflammation and cTnI, which could be beneficial to the rapid recovery of patients. Furthermore, intravenous dexmedetomidine reduces POD and mortality in patients.
There was also a consistent conclusion on whether dexmedetomidine had a protective effect on the heart and improved postoperative complications, for which we conducted a meta-analysis.
It has guiding significance for clinical application of dexmedetomidine on the heart and postoperative complications in elderly patients undergoing cardiac or non-cardiac surgery.
Our main goal is to investigate the effects of dexmedetomidine on cardiac complications and delirium in elderly patients undergoing cardiac or non-cardiac surgery.
We collected references to randomized controlled trials examining the efficacy of dexmedetomidine in the treatment of cardiac and postoperative complications.
Dexmedetomidine significantly reduced the cardiac troponin I and he inflammatory factor tumor necrosis factor-α, and reduced the extubation time, postoperative delirium and mortality. It really brings benefits to patients.
Dexmedetomidine has cardioprotective effects and improves patient postoperative complications.
In clinical practice, we will study the effect of dexmedetomidine at an appropriate dose on the heart and postoperative complications which will have certain guiding significance.
| 1. | Shen J, Sun Y, Han D, Zhu K, Zhao W. [Effects of dexmedetomidine on perioperative cardiac adverse events in elderly patients with coronary heart disease]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Lee C, Lee CH, Lee G, Lee M, Hwang J. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: A double blind randomized controlled study. J Clin Anesth. 2018;47:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, Liu H. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Park JW, Kim EK, Lee HT, Park S, Do SH. The Effects of Propofol or Dexmedetomidine Sedation on Postoperative Recovery in Elderly Patients Receiving Lower Limb Surgery under Spinal Anesthesia: A Retrospective Propensity Score-Matched Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Sun Y, Jiang M, Ji Y, Sun Y, Liu Y, Shen W. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther. 2019;13:2911-2922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Bi X, Wei J, Zhang X. Effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients: a systematic review and meta-analysis. J Int Med Res. 2021;49:3000605211014294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Shokri H, Ali I. A randomized control trial comparing prophylactic dexmedetomidine versus clonidine on rates and duration of delirium in older adult patients undergoing coronary artery bypass grafting. J Clin Anesth. 2020;61:109622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Elgebaly AS, Fathy SM, Sallam AA, et al Cardioprotective effects of propofol-dexmedetomidine in open-heart surgery: A prospective double-blind study [J]. Ann Card Anaesth. 2020;23:134-141. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Chen M, Li X, Mu G. Myocardial protective and anti-inflammatory effects of dexmedetomidine in patients undergoing cardiovascular surgery with cardiopulmonary bypass: a systematic review and meta-analysis. J Anesth. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Lu S, Chen X, Chen Q, Cahilog Z, Hu L, Chen Y, Cao J, Ning J, Yi B, Lu K, Gu J. Effects of dexmedetomidine on the function of distal organs and oxidative stress after lower limb ischaemia-reperfusion in elderly patients undergoing unilateral knee arthroplasty. Br J Clin Pharmacol. 2021;87:4212-4220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ríha H, Kotulák T, Březina A, Hess L, Kramář P, Szárszoi O, Netuka I, Pirk J. Comparison of the effects of ketamine-dexmedetomidine and sevoflurane-sufentanil anesthesia on cardiac biomarkers after cardiac surgery: an observational study. Physiol Res. 2012;61:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Tosun Z, Baktir M, Kahraman HC, Baskol G, Guler G, Boyaci A. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? J Cardiothorac Vasc Anesth. 2013;27:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48594] [Article Influence: 2858.5] [Reference Citation Analysis (3)] |
| 14. | Zhou HM, Ling XY, Ni YJ, Wu C, Zhu ZP. Pre-cardiopulmonary bypass administration of dexmedetomidine decreases cardiac troponin I level following cardiac surgery with sevoflurane postconditioning. J Int Med Res. 2019;47:3623-3635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Turan A, Duncan A, Leung S, Karimi N, Fang J, Mao G, Hargrave J, Gillinov M, Trombetta C, Ayad S, Hassan M, Feider A, Howard-Quijano K, Ruetzler K, Sessler DI; DECADE Study Group. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. 2020;396:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Wang T, Wang G, Yang M, Zhou Y, Yuan Y. Effects of Dexmedetomidine on Postoperative Delirium and Expression of IL-1β, IL-6, and TNF-α in Elderly Patients After Hip Fracture Operation. Front Pharmacol. 2020;11:678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Sun W, Zhao J, Li C. Dexmedetomidine Provides Protection Against Hippocampal Neuron Apoptosis and Cognitive Impairment in Mice with Alzheimer's Disease by Mediating the miR-129/YAP1/JAG1 Axis. Mol Neurobiol. 2020;57:5044-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Lee SH, Na S, Kim N, Ban MG, Shin SE, Oh YJ. The Effects of Dexmedetomidine on Myocardial Function Assessed by Tissue Doppler Echocardiography During General Anesthesia in Patients With Diastolic Dysfunction: A CONSORT-Prospective, Randomized, Controlled Trial. Medicine (Baltimore). 2016;95:e2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Klinger RY, White WD, Hale B, Habib AS, Bennett-Guerrero E. Hemodynamic impact of dexmedetomidine administration in 15,656 noncardiac surgical cases. J Clin Anesth. 2012;24:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Li CJ, Wang BJ, Mu DL, Hu J, Guo C, Li XY, Ma D, Wang DX. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg. 2020;107:e123-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Chi X, Liao M, Chen X, Zhao Y, Yang L, Luo A, Yang H. Dexmedetomidine Attenuates Myocardial Injury in Off-Pump Coronary Artery Bypass Graft Surgery. J Cardiothorac Vasc Anesth. 2016;30:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Zhou H, Zhou D, Lu J, Wu C, Zhu Z. Effects of Pre-Cardiopulmonary Bypass Administration of Dexmedetomidine on Cardiac Injuries and the Inflammatory Response in Valve Replacement Surgery With a Sevoflurane Postconditioning Protocol: A Pilot Study. J Cardiovasc Pharmacol. 2019;74:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Shen QH, Li HF, Zhou XY, Yuan XZ. Dexmedetomidine in the prevention of postoperative delirium in elderly patients following non-cardiac surgery: A systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2020;47:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Pan H, Liu C, Ma X, Xu Y, Zhang M, Wang Y. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth. 2019;66:1489-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Cheng H, Li Z, Young N, Boyd D, Atkins Z, Ji F, Liu H. The Effect of Dexmedetomidine on Outcomes of Cardiac Surgery in Elderly Patients. J Cardiothorac Vasc Anesth. 2016;30:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Aouad MT, Zeeni C, Al Nawwar R, Siddik-Sayyid SM, Barakat HB, Elias S, Yazbeck Karam VG. Dexmedetomidine for Improved Quality of Emergence From General Anesthesia: A Dose-Finding Study. Anesth Analg. 2019;129:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Soliman R, Zohry G. The myocardial protective effect of dexmedetomidine in high-risk patients undergoing aortic vascular surgery. Ann Card Anaesth. 2016;19:606-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Liu Y, Raghuraman MS S-Editor: Liu JH L-Editor: A P-Editor: Liu JH