Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.107927
Revised: May 25, 2025
Accepted: August 20, 2025
Published online: March 20, 2026
Processing time: 316 Days and 0.8 Hours
Renal artery stenosis (RAS) is a vascular disorder linked to secondary hyperten
To compare ADC values in hypertensive, RAS, and healthy kidneys, assess the correlation between ADC and stenosis severity, and evaluate its relationship with split glomerular filtration rate (GFR).
This prospective observational study which included 86 patients with suspected RAS and twenty normal healthy controls underwent NC-MRA on a 3T-MR-Scanner followed by DW-MRI at b values of 0 and 1000 seconds/mm2 in the transverse plane. ADC maps were created using Functool. ADC values were measured in the cortex and medulla of each kidney's upper, middle, and lower pole, and the average ADC (ADCavg) for cortex and medulla calculated. In patients with RAS, degree of stenosis (DOS) was calculated on NC-MRA. The ADC of 212 kidneys was compared, and the relationship between DOS and ADC was established. In addition, split GFR was calculated in 30 kidneys using 99mTc-DTPA, and correlated with ADC value. The ADC values of kidneys with and without RAS were compared using the Student’s t-test. The correlation between ADC and stenosis severity was assessed by Spearman’s test, while the relationship between ADC and split GFR was evaluated using Pearson’s test. A P
RAS was detected in 58 of 86 (67.44%) hypertensive patients (81 of 172 kidneys), and the ADCavg (P = 0.044) was significantly lower in RAS kidneys than in kidneys with normal arteries and essential hypertension and healthy controls.
DW-MRI can be a useful non-invasive technique to estimate the kidney’s functional status in RAS patients. It can be used as a complementary assessment tool with NC-MRA to triage patients in need of interventional mana
Core Tip: Diffusion-weighted magnetic resonance imaging (DW-MRI) provides a non-invasive method to assess renal function in patients with renal artery stenosis (RAS). This study demonstrates that the apparent diffusion coefficient is significantly lower in RAS-affected kidneys, correlating with stenosis severity and split glomerular filtration rate. DW-MRI, in combination with non-contrast magnetic resonance angiography, offers a promising tool for early detection and triage of RAS patients, potentially guiding timely intervention and improving clinical outcomes.
- Citation: Lal H, Agarwal S, Ponmalai K, Prasad R, Bhadauria DS, Gambhir S, Mandal S, Kumar S, Yadav P, Jowel P. Apparent diffusion coefficient of kidneys with non-contrast magnetic resonance angiography for functional and anatomical assessment in renal artery stenosis. World J Methodol 2026; 16(1): 107927
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/107927.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.107927
Renal artery stenosis (RAS) is a vascular disorder of the kidney associated with secondary hypertension, chronic kidney disease (CKD), and end-stage renal failure by inducing interstitial fibrosis in the post-stenotic kidney (STK)[1]. Early diagnosis is critical, as revascularization can reverse hypertension and preserve renal function in select patients. Traditional imaging modalities, including contrast-enhanced magnetic resonance angiography (CE-MRA) and computed tomography angiography, provide anatomical assessment but require gadolinium or iodinated contrast agents, posing risks of nephrogenic systemic fibrosis (NSF) or contrast-induced nephropathy in patients with CKD. Non-contrast magnetic resonance angiography (NC-MRA) has emerged as a safe alternative for anatomical evaluation of RAS, avoiding these risks while maintaining diagnostic accuracy[2,3]. However, anatomical imaging alone cannot quantify the functional impact of stenosis, necessitating complementary techniques to assess parenchymal injury[4-7].
Diffusion-weighted imaging (DWI), a non-contrast magnetic resonance (MR) technique, quantifies water molecule mobility in tissues through the apparent diffusion coefficient (ADC) and has emerged as a powerful technique for functional study of abdominal organs including kidneys[8]. Reduced ADC values correlate with microstructural changes such as fibrosis and tubular atrophy, offering a functional biomarker of renal injury. DWI is independent of exogenous contrast agents and is desirable for kidney injury assessment in CKD patients at increased risk of gadolinium associated NSF on CE-MRA[9,10]. Structural changes such as interstitial fibrosis or tubular atrophy lead to decreased ADC, which correlates well with renal function[11-14]. Several previously published works have already assessed Diffusion-weighted MR imaging (DW-MRI) of the kidney using different technical approaches[15,16]. Thoeny et al[17] and Li et al[18] have shown the reproducibility of DW-MRI of kidneys in healthy volunteers. However, ADC’s flow-dependency and coexistence of structural and hemodynamic alterations in STK complicate the interpretation of results[19].
There is a lack of data on the effect of RAS and hypertension (HT) on the ADC value, and only a few studies have been performed in RAS patients[11,20,21]. All these studies had correlated kidneys' ADC values with degree of stenosis (DOS) calculated from CE-MRA. Many patients with RAS who have CKD are omitted from CE-MRA due to the risk of NSF. Additionally, no study has correlated ADC with split glomerular filtration rate (GFR)—a critical measure of unilateral renal dysfunction in RAS—which surpasses global estimated GFR (eGFR) in detecting asymmetric functional loss.
The present study addresses these gaps by integrating NC-MRA for anatomical stenosis grading and DWI for functional assessment in a gadolinium-free protocol. We hypothesize that RAS-induced ischemia and subsequent fibrosis reduce renal ADC values, correlating with stenosis severity and split GFR. By evaluating ADC in RAS-affected kidneys, contralateral kidneys, essential hypertension, and healthy controls, we aim to establish DWI as a non-invasive tool for stratifying functional impairment in RAS, particularly in patients contraindicated for contrast-enhanced imaging. This study had the following objectives: (1) To compare ADC values of kidneys in patients with hypertension, RAS, and normal controls diagnosed with NC-MRA; (2) To identify any correlation between the DOS calculated using NC-MRA and ADC values using DWI in RAS; and (3) To correlate ADC values of kidneys with the split GFR in patients with RAS.
This study was carried out with the Institutional ethics committee’s approval (Bioethics cell; IEC code: 2018-110-MD-EXP; PGI/BE/424/2018) in India's tertiary care medical institution between September 2018 and March 2020.
Hypertensive group: Patients aged 17-70 years with suspected RAS, diagnosed per ACC/AHA guidelines (systolic/diastolic blood pressure ≥ 130/80 mmHg on three consecutive measurements using a validated oscillometric device after 5 minutes of rest).
Healthy controls: Normotensive renal donors with normal renal function (eGFR > 90 mL/min/1.73 m², no proteinuria).
Hydration status confirmed by urine-specific gravity (1.005-1.020).
(1) Prior renal artery stenting, renal neoplasms, or infections; and (2) Contraindications to MR imaging (e.g., claustrophobia, metallic implants). Antihypertensive medications were withheld 48 hours before imaging.
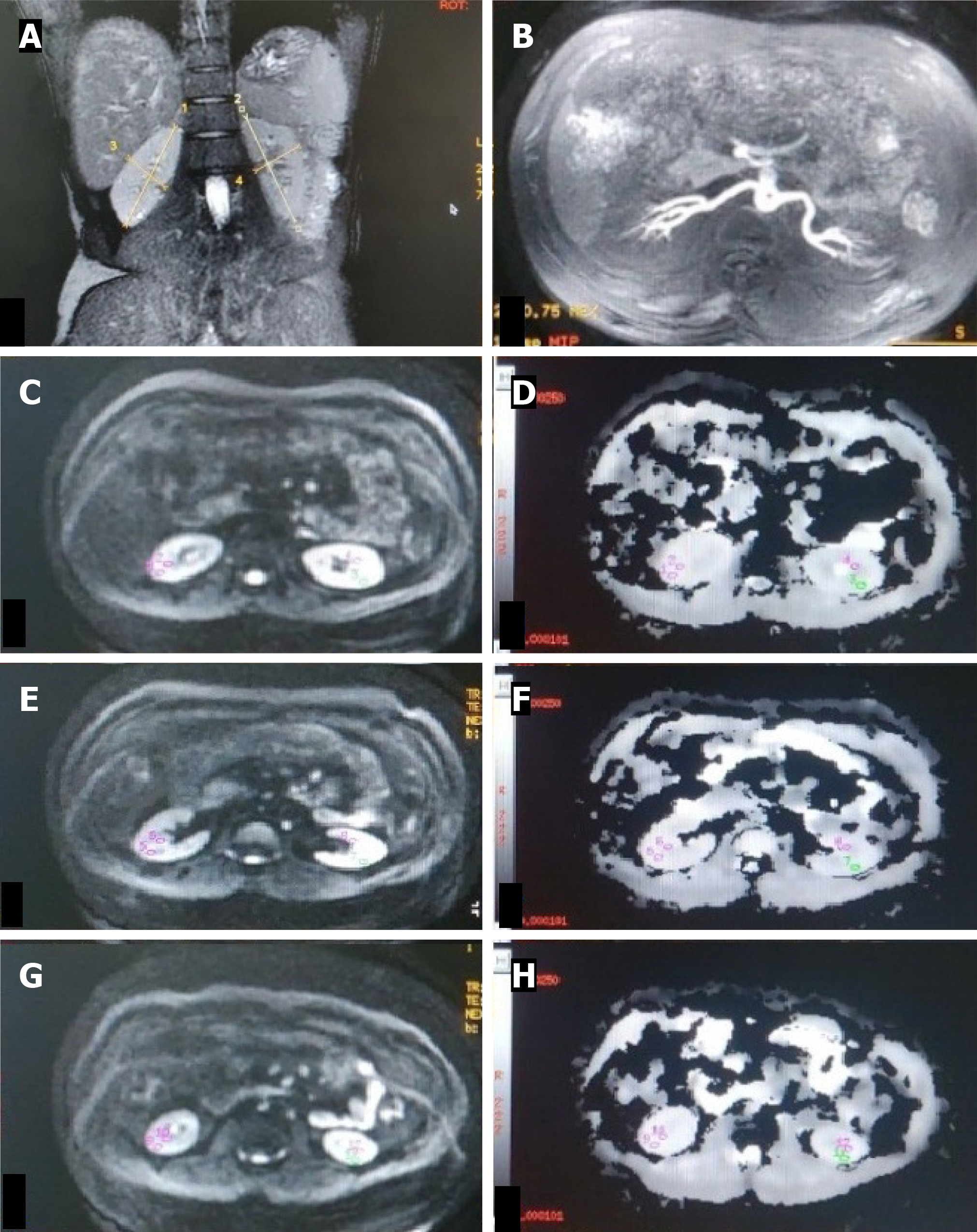
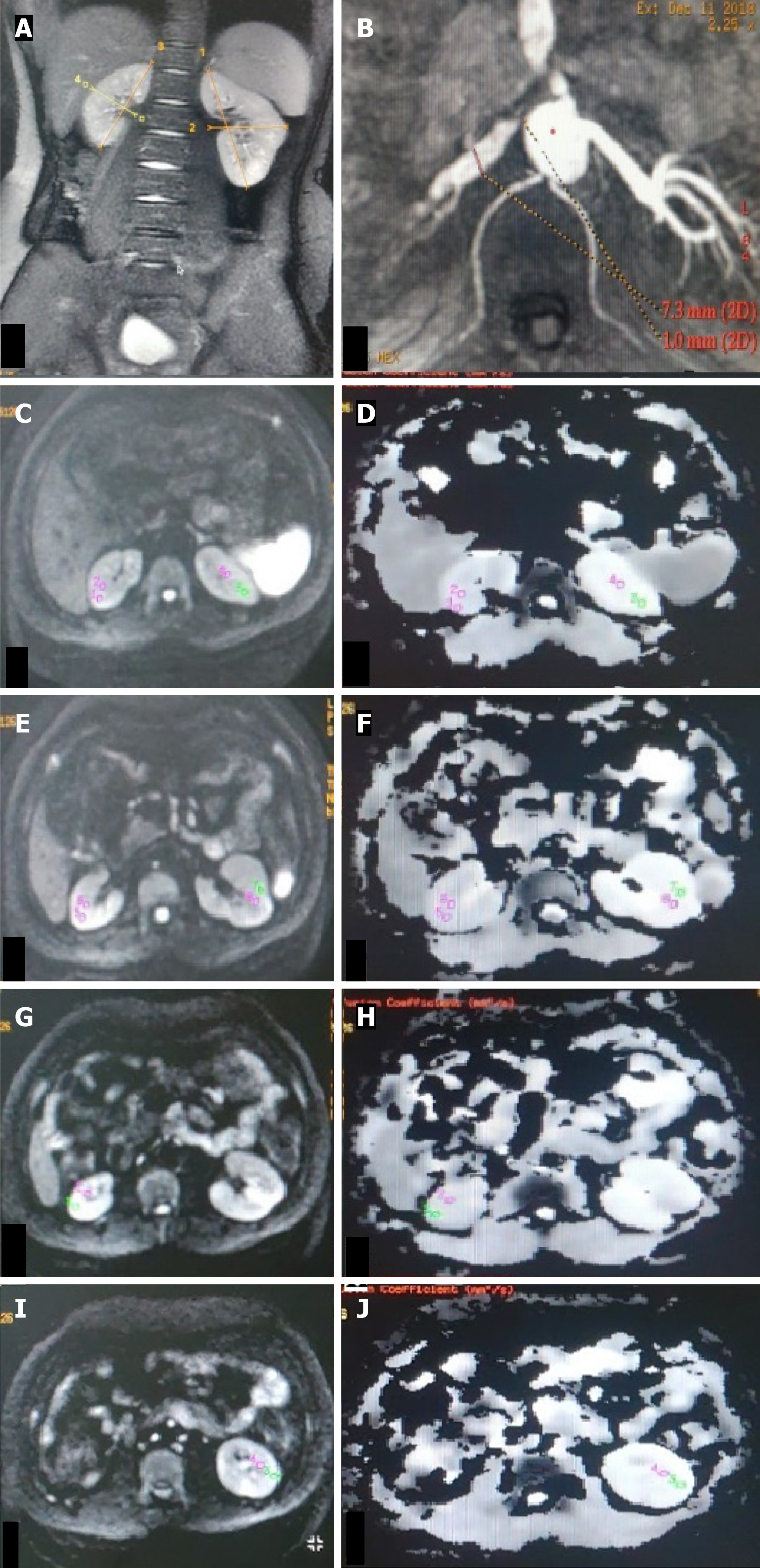
MR imaging was performed with a 3 Tesla MR scanner (Signa Hdxt General Electrics, Milwaukee, United States) using a phased array Torso coil. The magnetic resonance imaging (MRI) protocol was T2W coronal sequence to determine renal size and morphology and NC-MRA for renal arteries followed by DW-MRI.
The NC-MRA sequence was performed using a respiratory-triggered 3D fat-suppressed inflow inversion recovery balanced Steady-state free precession (SSFP) sequence. The sequence was planned in the transverse imaging plane with an imaging range covering both kidneys. The stationary background signal and venous blood signal are suppressed by using the spectral inversion recovery technique. The phase lines were acquired via respiratory triggering. The parallel imaging array spatial sensitivity encoding technique was used in the in-plane phase encode direction with an acceleration factor of 2. MR parameters were as follows: TR 4.1 ms, flip angle 50°, blood suppression inversion time (TI) 1200 ms, receiver bandwidth 125 Hz/pixel, the field of view 360 mm, slice thickness 2 mm, frequency matrix 192, phase matrix 320, phase field of view (FOV) 0.75, and acquisition time 2-3 min.
The DW-MRI sequence was a respiratory-gated 2D Spin-echo Echo-planar imaging. This imaging sequence was planned in the axial plane of the kidney with the MR parameters - TR 10909 ms, TE 74.4 ms, flip angle 75°, matrix size 128 × 128, FOV 380 mm × 380 mm, slice thickness 5 mm and slice gap 1 mm. The images were acquired at b values of 0 and 1000 seconds/mm2 with a Number of Excitations (NEX) of 8.
Two blinded radiologists with > 10 years’ experience in vascular MRI analyzed images on a workstation with access to the source data, maximum intensity projection, and reformatted views. Each renal artery was graded for stenosis on NC-MRA. The percentage of stenosis was calculated using the following formula: [1-(L/R)] × 100, where L and R refer to the diameters of the site of stenosis (L) and the normal site (R). Interobserver agreement was determined (intraclass corre
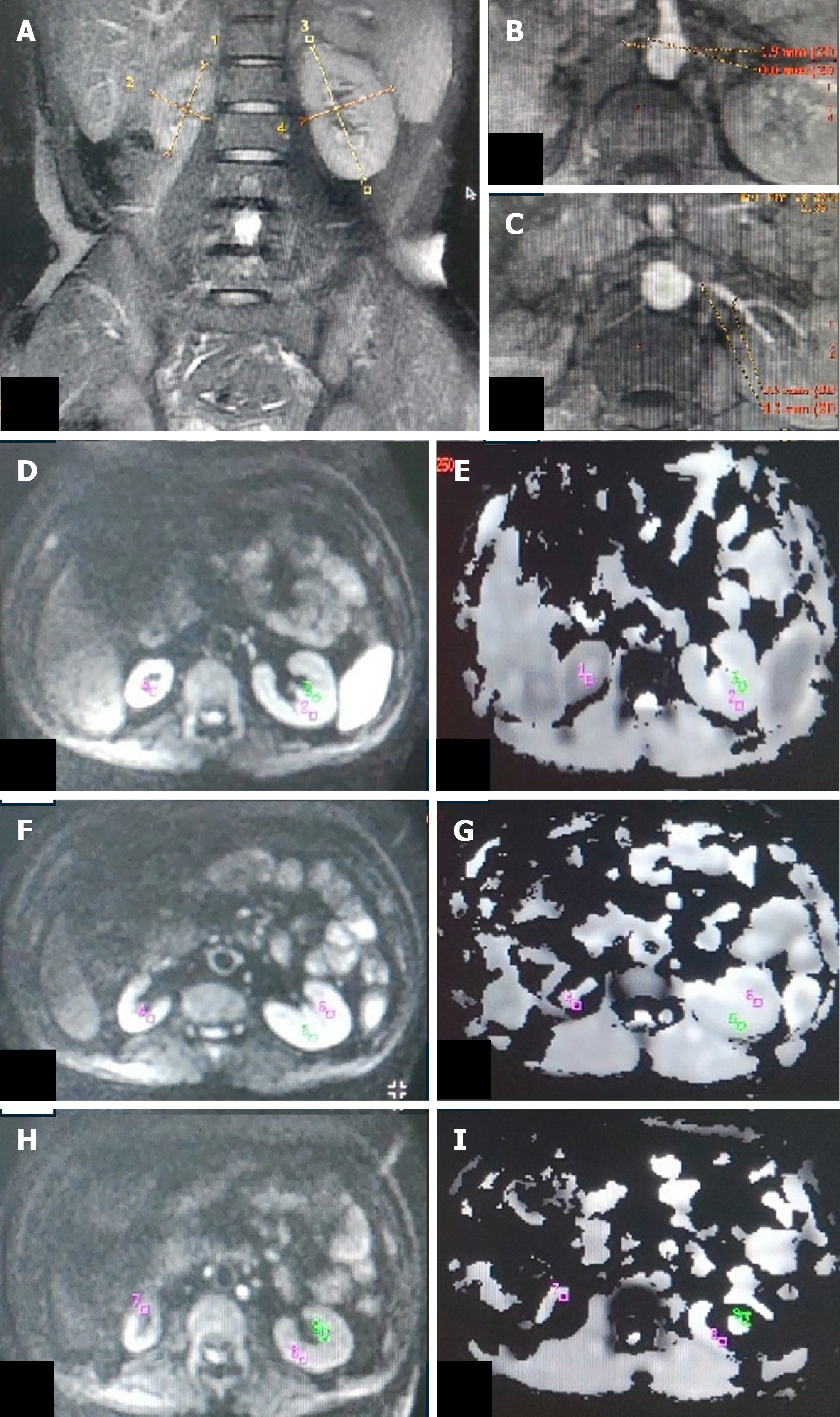
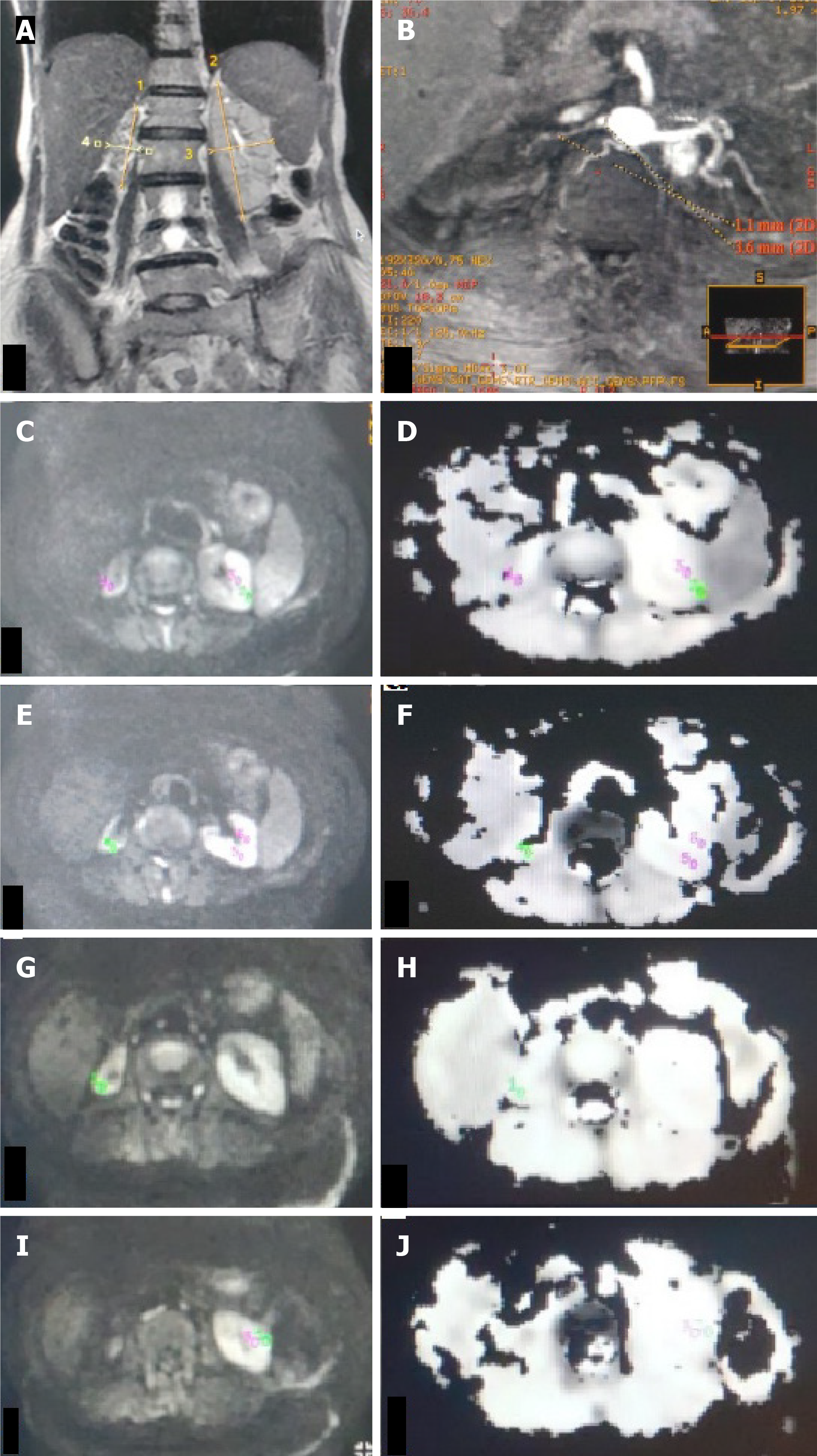
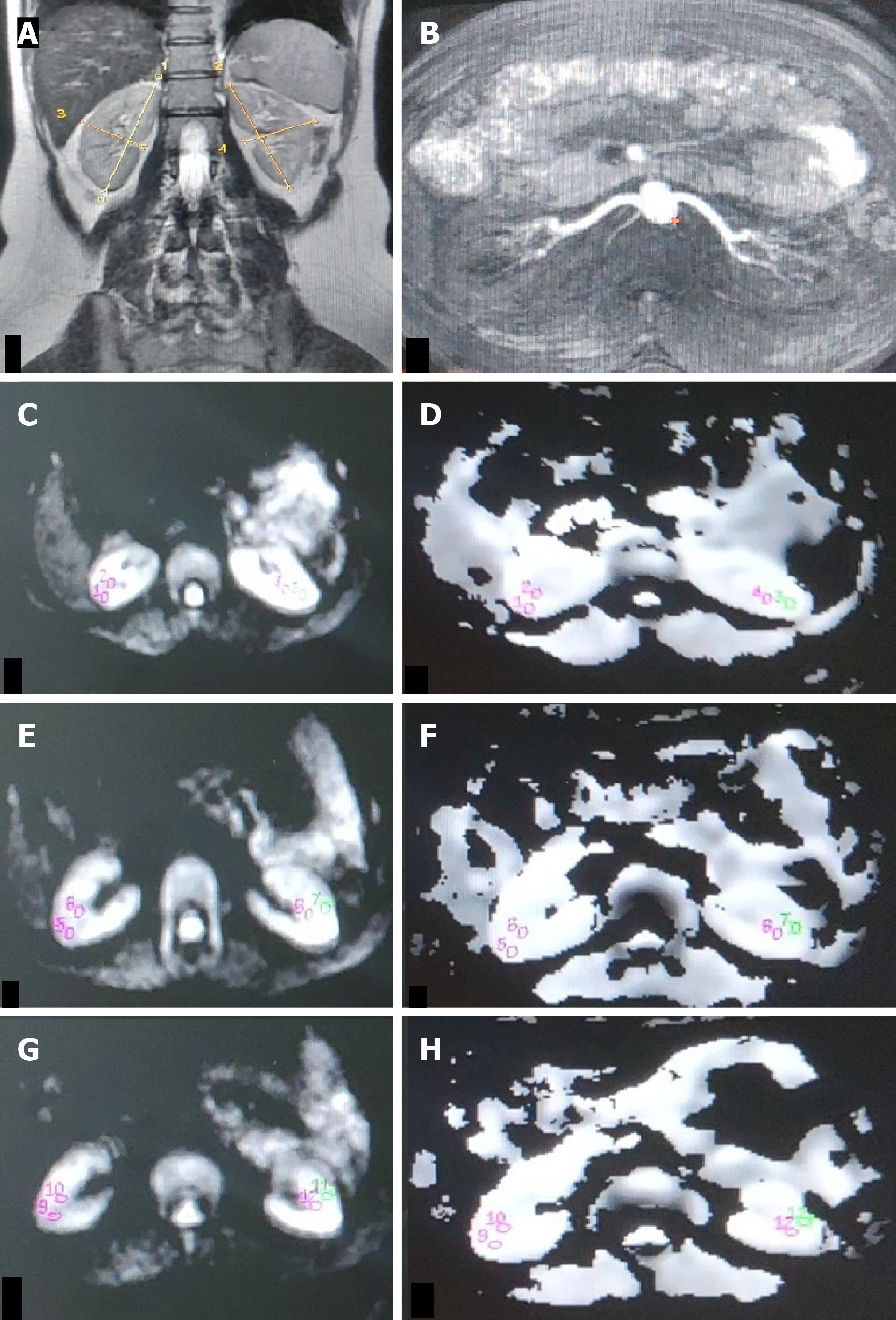
ADC values were calculated from DWI signals using the monoexponential decay equation: ADC = [ln(S0/S1)]/(b1-b0), where S0 and S1 are signal intensities at b-values of 0 and 1000 s/mm², respectively. Transverse ADC maps and ADC values were generated using Functools (GE Healthcare, Wisconsin, United States) in an Advanced workstation. Individual ADC images that yielded optimal cortico-medullary differentiation were selected to define region-of-interest (ROI) (average area - 25 to 55 mm2) manually within the cortex and medulla in the upper, middle, and lower poles of each kidney. In kidneys where the differentiation was not possible, a common cortico-medullary ROI was defined. Average ADC (ADCavg) values for the cortex and medulla were calculated for each kidney and used for statistical analysis.
Renal scintigraphy was performed in 30 kidneys using 99mTc-DTPA. One mCi of Tc99m-DTPA was injected intra
Data were analyzed using SPSS version 23 (IBM Corp.). Continuous variables are reported as mean ± SD or median (interquartile range) based on normality assessed via the Shapiro-Wilk test. Group comparisons (RAS-affected kidneys, contralateral kidneys, essential hypertension kidneys, and healthy controls) were performed using one-way ANOVA with Tukey’s post-hoc test for parametric data (e.g., ADC values) or the Kruskal-Wallis test for non-parametric data (e.g., serum creatinine). Demographic differences (age, sex) between groups were adjusted using analysis of covariance (ANCOVA). Correlations between ADC values and stenosis severity were evaluated using Spearman’s rank-order test, while the relationship between ADC and split GFR was assessed by Pearson’s correlation coefficient. Statistical significance was set at a two-tailed P < 0.05.
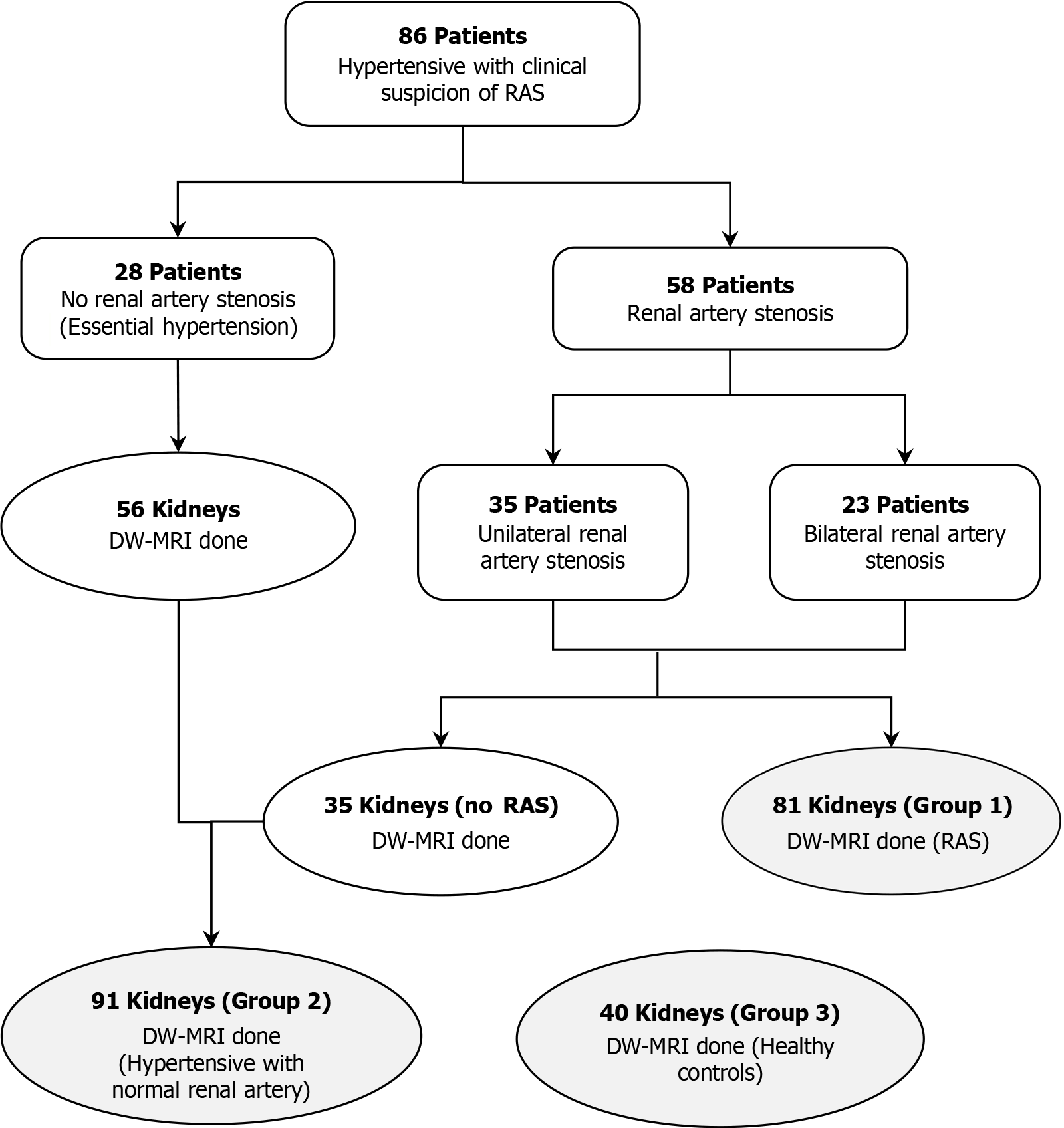
During the study period, 86 hypertensive patients with suspected RAS and 20 healthy control volunteers were enrolled. There were 80 males and 26 females. Among the 86 hypertensive patients, RAS was identified in 58 (67.4%), comprising 50 with atherosclerotic stenosis and 8 with Takayasu arteritis. Of these 58 patients, 23 had bilateral main renal artery stenosis, and 35 had unilateral renal artery stenosis (right artery in 18, left artery in 17).
Overall, there were 212 kidneys from 106 participants, including 58 patients with RAS (81 kidneys, group 1), 28 hypertensive patients with normal renal arteries (56 kidneys, group 2), 20 healthy controls (40 kidneys, group 3), and 35 contralateral kidneys from unilateral RAS patients (Figure 1). Demographic and clinical characteristics are presented in Table 1.
| RAS (n = 58) | Hypertensive group with normal renal arteries | Healthy control volunteers (n = 20) | Total (n = 106) | ||
| Age (years) | 40.9 ± 14.1 | 36.5 ± 13.1 | 28.2 ± 3.9 | ||
| Sex | Male | 37 | 23 | 20 | 80 |
| Female | 21 | 5 | 0 | 26 | |
| Hypertension | 58 | 28 | 0 | 86 | |
| Diabetes mellitus | 10 | 5 | 0 | 15 | |
| Takayasu arteritis | 6 | 2 | 0 | 8 | |
| Chronic kidney disease | 5 | 4 | 0 | 9 | |
| Raised serum creatinine | 39 | 11 | 0 | 50 | |
| Mean serum creatinine (mg/dL) | 2.2 | 1.3 | 0.8 | 1.65 | |
| Mean BUN (mg/dL) | 22.8 | 20.5 | 10.6 | 17.97 | |
| Mean eGFR (mL/min/1.73m2) | 55 | 89.05 | 131 | 91.68 | |
The ADCavg values (1.77 ± 0.3 vs 1.89 ± 0.2, P = 0.044) of kidney cortices were significantly lower in group 1 (Figures 2 and 3) than in group 2 and group 3 (Table 2). Statistically, a significant difference was observed between the ADCavg values (1.89 ± 0.24 vs 1.90 ± 0.11, P = 0.001) of kidneys of group 2 (Figure 4) and group 3, with Group 2 having significantly lower ADCavg values of the cortex as compared to group 3 (Figure 5). Also, ADCavg values (1.77 ± 0.3 vs 1.95 ± 0.2, P = 0.044) of the cortex were significantly lower in kidneys with RAS than that of the contralateral kidney with a normal renal artery in patients with unilateral RAS (Figure 6).
| RAS, n = 81 | Hypertension with normal renal arteries, n = 91 | P value (Comparison of patients with and without RAS) | Healthy control volunteers, n = 40 | |
| Age | 40.9 ± 14.1 | 36.5 ± 13.1 | 0.035 | 28.2 ± 3.9 |
| ADCaverage cortex (× 10-3 mm2/s) | 1.77 ± 0.3 | 1.89 ± 0.2 | 0.002 | 1.90 ± 0.1 |
| ADCaverage medulla (× 10-3 mm2/s) | 1.76 ± 0.3 | 1.85 ± 0.2 | 0.021 | 1.84 ± 0.1 |
| Stenosis degree | 64.1 ± 17.56 | - | - |
Similarly, the ADCavg values (1.76 ± 0.3 vs 1.85 ± 0.2, P = 0.013) of the kidney medulla were significantly lower in group 1 than in group 2 and group 3 (Table 2). The ADCavg values (1.76 ± 0.3 vs 1.93 ± 0.2, P = 0.001) of the medulla were also significantly lower in RAS kidneys than those of the contralateral kidney normal renal artery in unilateral RAS affected patients.
The ADCavg values (1.62 ± 0.1 vs 1.78 ± 0.3, P = 0.040) of the cortex showed a statistically significant difference between CKD and non-CKD patients in group 1.
However, the ADCavg values (1.79 ± 0.3 vs 1.74 ± 0.2, P = 0.469) of kidneys did not show any significant change between diabetic and nondiabetic patients in group 1. In addition, even the ADCavg values (1.82 ± 0.2 vs 1.89 ± 0.2, P = 0.777) did not differ significantly between diabetic and nondiabetic patients who had hypertension with normal renal arteries (group 2). The coefficient of variability for ADCavg was 0.14.
The mean DOS in group 1 kidneys was 64.1 ± 17.56. Spearman's correlation analysis revealed that the DOS showed a weak correlation with ADCavg (r = -0.238; P = 0.033). (Table 3).
| Degree of stenosis | ADCavg cortex | ||
| Age | Correlation coefficient | -0.57 | -0.123 |
| Significance (2-tailed) | 0.611 | 0.075 | |
| Degree of stenosis | Correlation coefficient | - | -0.238 |
| Significance (2-tailed) | - | 0.033 |
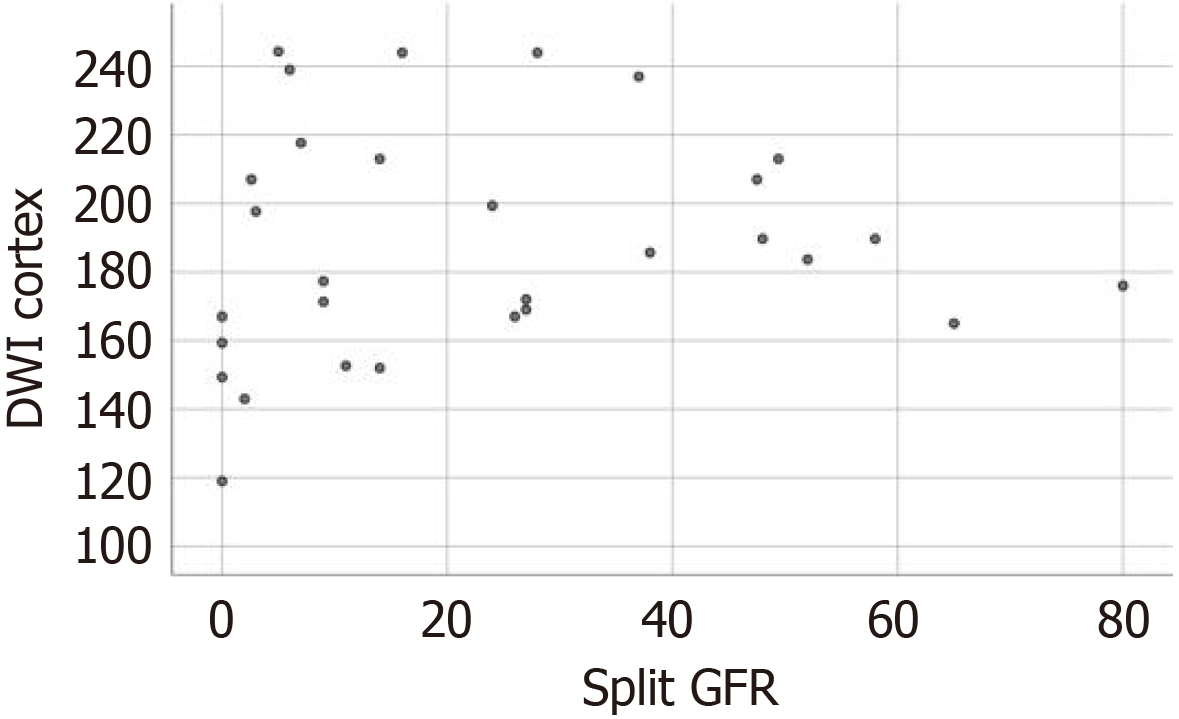
The mean split GFR of the 30 kidneys with RAS in which renal scintigraphy was performed was 23.3 mL/minute (range = 0-80 mL/minute). All 30 kidneys were divided into three groups according to the split GFR measured by renal scintigraphy: Group I - GFR ≥ 40 mL/minute (normal renal function, n = 7); group II - 40 > GFR ≥ 10 mL/minute (mild to moderate renal impairment, n = 11); and group III - GFR < 10 mL/minute (severe renal impairment, n = 12).
The mean renal ADCs of the three groups I, II, and III were 1.89 +/-0.17, 1.94 +/-0.36, and 1.83 +/-0.39 × 10-3 mm2/s, respectively. The statistical analysis revealed a significant difference in the renal ADCs of the kidneys with normal (group 1) and impaired function (group 2 and 3) (F = 6.938, P = 0.014). However, the difference in the renal ADCs of the kidneys with mild to moderate (group 2) and severe functional impairment (group 3) was not statistically significant (P = 0.817).
Statistical analysis revealed a very weak positive correlation between ADC values and split GFR (r = 0.111, P = 0.559). A scatter plot of ADC values vs split GFR in 30 examined kidneys is shown in Figure 7.
We studied the ADC values of kidneys in patients with RAS, hypertension, and normal controls and correlated ADC value with DOS. We found that diffusivity decreases in kidneys with RAS, which was observed as decreases in the ADC values of the cortex and medulla on DWI pulse sequences. However, the decline in diffusivity showed a weak association with the DOS of the main renal artery. We also correlated split GFR with ADC values in 30 RAS affected kidneys and found a very weak positive association.
In the present study, the mean ADC values of the cortex and medulla in RAS affected kidneys were lower than that of contralateral kidneys in patients with unilateral RAS with a statistically significant difference (P < 0.05) at b 1000. This finding could be due to low blood perfusion in RAS affected kidneys, especially in the cortex, confirmed by our results. ADC reduction in RAS likely reflects interstitial fibrosis and tubular atrophy owing to chronic hypoxia. Hypertension-associated ADC decline may involve arteriolar hyalinization impairing microvascular flow. Furthermore, the mean ADC values of RAS affected kidneys were also lower than that of the kidneys of essential hypertensive and normal healthy controls, and were statistically significantly different (P < 0.05). These findings were consistent with a previously published study by Yildirim et al[11] which included a smaller sample size of 20 patients; however, only the cortex ADC values were evaluated in that study (Table 4). The study by Namimoto et al[20] was conducted at lower b values, although it should not be compared with our study, they also observed significantly lower ADC values in RAS patients compared to normal patients, similar to our study (Table 5).
| Ref. | Groups | Cortex mean ADC | Medulla mean ADC |
| Yildrim et al[11], 2007 | RAS affected kidneys | 1.8 ± 0.2 | |
| Contralateral and EH kidneys | 2.0 ± 0.1 | ||
| Our study | RAS affected kidneys | 1.77 ± 0.3 | 1.76 ± 0.3 |
| Contralateral kidneys | 1.95 ± 0.2 | 1.93 ± 0.2 | |
| EH kidneys | 1.89 ± 0.3 | 1.85 ± 0.2 | |
| Normal kidneys | 1.90 ± 0.1 | 1.84 ± 0.1 | |
We observed a very weak correlation between the main renal artery’s DOS and ADCavg values in 81 RAS affected kidneys. Yildirim et al[11] correlated DOS with ADC values in only 13 RAS-affected kidneys and found a strong correlation (r = -0.754; P = 0.003) between the two. A large number of RAS-affected kidneys in our study may have been responsible for this weak correlation. Our results should also be interpreted in light of the fact that factors such as chronicity of the disease, development of collateral circulation, length, and site of stenosis also determines the extent of damage within the kidney apart from just the DOS[22]. Renal revascularization procedures are performed in patients with hemodynamically significant stenosis of at least 50% as there is a close interrelationship between severity of stenosis and kidney atrophy due to ischemic nephropathy[23,24]. Additionally, improvement of renal function is also an essential parameter for renal revascularization. Although conventional renal angiography is the gold standard for diagnosing RAS it is an invasive procedure. NC-MRA is a non-invasive technique and provides a fast and viable means of detecting RAS[2]. Although NC-MRA can overestimate stenosis, we found that our patients with stenosis greater than 50% (n = 64) had ADCavg values lower than 1.80 × 10-3 mm2/s. However, in the study by Yildrim et al[11], the ADC values were lower than 2.0 × 10-3 mm2/s in all the patients (n = 5) with RAS greater than 50%.
The effect of hypertension on the ADC values of kidneys has not yet been studied. We found that the ADC values of the cortex of kidneys in the essential hypertensive group and normal contralateral kidneys of unilateral RAS affected patients were lower than the ADC values of normal healthy control volunteers with a statistically significant difference. This lower value could be due to hypertension-induced alteration of microvasculature due to end-organ damage. However, RAS affects the ADC values more severely than hypertension. ADC alone lacks diagnostic utility but may stratify severe RAS (e.g., ADCavg < 1.80 × 10-3 mm2/s in > 50% stenosis) when combined with NC-MRA.
The GFR is considered the best index of overall renal function. Currently, the reference standard for split GFR uses Tc 99m DTPA as a filtration marker, but this method uses ionizing radiation and has low spatial resolution[25]. Hence, if we can estimate split GFR using ADC values, it can evaluate individual renal function in a radiation-free manner without intravenous contrast agents. Our study of 15 patients with RAS (30 kidneys) established a very weak correlation between renal ADCs and split GFR. However, it is interesting to note that there was a significant difference in the renal ADCs among the groups with normal and impaired renal function, but the difference between mild to moderate and severe renal impairment groups was not statistically significant. This insignificant difference could be due to the small sample size. Xu et al[13] in 2007 found a moderate correlation between ADC values and split GFR function in 110 kidneys; however, only global ADC values were measured in that study. Sulkowska et al[26] conducted a study in a healthy population considering global GFR and showed no correlation between ADC values and GFR. The weak ADC-GFR correlation (r = 0.111) suggests DWI primarily reflects microstructural rather than functional changes in RAS. Clinically, this implies: (1) ADC may identify structural damage before GFR decline occurs; (2) Revascularization decisions should not rely solely on ADC values; (3) Combined anatomical-functional assessment (NC-MRA + DWI + GFR) provides the most comprehensive evaluation.
This aligns with growing recognition that fibrosis (detected by ADC) and filtration (measured by GFR) represent distinct pathological axes in renal disease progression.
Our study’s limitation is the significant difference in the age and sex distribution in patients with RAS, essential hypertension, and normal controls. We did not evaluate the effect of age and sex on the ADC values of kidneys. All the patients with RAS were diagnosed solely with NC-MRA. While NC-MRA offers advantages for CKD patients, its use as the sole diagnostic modality without conventional angiographic confirmation represents a key limitation. The lack of hemodynamic assessment (e.g., pressure gradients) further limits functional correlation with ADC values. Age/sex disparities were adjusted but may still confound the results. The weak ADC-GFR correlation (r = 0.111) may stem from DWI’s insensitivity to glomerular function or confounding factors like collateral circulation. The small sample size (n = 30) may limit power. Lastly, we did not measure ADC value post-intervention. Despite these limitations, 212 kidneys were evaluated; ours is one of the largest cohorts comparing the ADC values of kidneys with RAS. Furthermore, this study is among the first to correlate ADC with NC-MRA-derived DOS. Hence, it also includes RAS patients with CKD in whom CE-MRA could not be performed due to the risk of NSF.
We conclude that a significantly lower ADC value in the kidney cortex on DW-MRI was observed in RAS and hypertensive patients. However, ADC value had a weak correlation with DOS and measured split GFR of the affected kidney. DW-MRI could be useful in estimating the kidney's functional status in a non-invasive manner without gadolinium, particularly in CKD patients and could be used as a supportive technique to NC-MRA to triage patients planned for interventional procedures.
| 1. | Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 638] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Leung DA, Hagspiel KD, Angle JF, Spinosa DJ, Matsumoto AH, Butty S. MR angiography of the renal arteries. Radiol Clin North Am. 2002;40:847-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Lal H, Singh RKR, Yadav P, Yadav A, Bhadauria D, Singh A. Non-contrast MR angiography versus contrast enhanced MR angiography for detection of renal artery stenosis: a comparative analysis in 400 renal arteries. Abdom Radiol (NY). 2021;46:2064-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Li JC, Jiang YX, Zhang SY, Wang L, Ouyang YS, Qi ZH. Evaluation of renal artery stenosis with hemodynamic parameters of Doppler sonography. J Vasc Surg. 2008;48:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Ebrahimi B, Rihal N, Woollard JR, Krier JD, Eirin A, Lerman LO. Assessment of renal artery stenosis using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging analysis. Invest Radiol. 2014;49:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | François CJ, Lum DP, Johnson KM, Landgraf BR, Bley TA, Reeder SB, Schiebler ML, Grist TM, Wieben O. Renal arteries: isotropic, high-spatial-resolution, unenhanced MR angiography with three-dimensional radial phase contrast. Radiology. 2011;258:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Mannelli L, Maki JH, Osman SF, Chandarana H, Lomas DJ, Shuman WP, Linnau KF, Green DE, Laffi G, Moshiri M. Noncontrast functional MRI of the kidneys. Curr Urol Rep. 2012;13:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Hueper K, Rong S, Gutberlet M, Hartung D, Mengel M, Lu X, Haller H, Wacker F, Meier M, Gueler F. T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol. 2013;48:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Perazella MA, Rodby RA. Gadolinium-induced nephrogenic systemic fibrosis in patients with kidney disease. Am J Med. 2007;120:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Yildirim E, Kirbas I, Teksam M, Karadeli E, Gullu H, Ozer I. Diffusion-weighted MR imaging of kidneys in renal artery stenosis. Eur J Radiol. 2008;65:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Yang D, Ye Q, Williams DS, Hitchens TK, Ho C. Normal and transplanted rat kidneys: diffusion MR imaging at 7 T. Radiology. 2004;231:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging. 2007;26:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M. Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology. 2010;255:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Müller MF, Prasad PV, Bimmler D, Kaiser A, Edelman RR. Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology. 1994;193:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Siegel CL, Aisen AM, Ellis JH, Londy F, Chenevert TL. Feasibility of MR diffusion studies in the kidney. J Magn Reson Imaging. 1995;5:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology. 2005;235:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Li XM, Yang L, Reng J, Xu GH, Zhou P. Non-invasive evaluation of renal structure and function of healthy individuals with multiparametric MRI: Effects of sex and age. Sci Rep. 2019;9:10661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier PH, Taouli B, Lee VS. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010;254:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832-837. [PubMed] [DOI] [Full Text] |
| 21. | Fukuda Y, Ohashi I, Hanafusa K, Nakagawa T, Ohtani S, An-naka Y, Hayashi T, Shibuya H. Anisotropic diffusion in kidney: apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging. 2000;11:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | De Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J, Bartunek J, Vanderheyden M, Heyndrickx GR. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Hany TF, Debatin JF, Leung DA, Pfammatter T. Evaluation of the aortoiliac and renal arteries: comparison of breath-hold, contrast-enhanced, three-dimensional MR angiography with conventional catheter angiography. Radiology. 1997;204:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 188] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Caps MT, Zierler RE, Polissar NL, Bergelin RO, Beach KW, Cantwell-Gab K, Casadei A, Davidson RC, Strandness DE Jr. Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 1998;53:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Cousins C, Mohammadtaghi S, Mubashar M, Strong R, Gunasekera RD, Myers MJ, Peters AM. Clearance kinetics of solutes used to measure glomerular filtration rate. Nucl Med Commun. 1999;20:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Sulkowska K, Palczewski P, Duda-Zysk A, Szeszkowski W, Wojcik D, Kownacka-Piotrowska D, Gołebiowski M. Diffusion-weighted MRI of kidneys in healthy volunteers and living kidney donors. Clin Radiol. 2015;70:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/