Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.107864
Revised: May 22, 2025
Accepted: July 29, 2025
Published online: March 20, 2026
Processing time: 317 Days and 22.5 Hours
Multiple myeloma (MM) is an incurable hematopoietic malignancy defined by the bone marrow's clonal expansion of neoplastic plasma cells. Corticosteroids and monoclonal antibodies (mAbs) have been approved for the treatment of MM over the past 20 years and are now key components of treatment regimens, improving clinical outcomes. Corticosteroids (dexamethasone and prednisone) are frequently used in combination with other agents [proteasome inhibitors and immunomodulatory drugs (IMiDs)], while mAbs (daratumumab, elotuzumab, and isatuximab) have transformed treatment paradigms, particularly for relapsed/refractory cases.
To evaluate the impact of corticosteroids and mAbs on the treatment of MM.
This systematic review integrates results from randomized controlled trials and cohort studies published from 2003 to 2024. This resulted in the identification 26 articles assessing the role of corticosteroids and mAbs in various treatment settings: Newly diagnosed, relapsed, and refractory MM. Seventeen studies were included in the systematic review.
We show that corticosteroid-based combination regimens are critical for achieving rapid tumour regression and increasing overall survival (OS) when combined with proteasome inhibitors (bortezomib or carfilzomib). Mo
While great strides have been made in the treatment, much remains to be learned about long-term safety, efficacy, and potential resistance mechanisms to these treatments.
Core Tip: Corticosteroids and monoclonal antibodies (mAbs) deliver extensive therapeutic value to patients with multiple myeloma (MM) by providing survival improvements, particularly for people with relapsed or refractory disease. The essential medication dexamethasone, alongside other corticosteroids, has long proved beneficial in treatment regimens because it decreases tumor size and works better with other treatments. The development of daratumumab and other mAbs introduced specific tumor cell antigen targeting, producing significant study results for high-risk patients. Modern advancements in cancer therapy have produced promising results, although ongoing research aims to address corticosteroid-related long-term effects, prevent infusion responses in mAbs, and decrease their costly nature.
- Citation: Alam F, Siddiqui H, Nihal A, Andleeb M, Zaman AQU, Khushk MI, Hussain F, Rai R, Fatima FB, Kumar D, Rizvi SAFA, Jabeen S, Jawed I, Qadir U, Zakeri MA. Advancing multiple myeloma therapy: A systematic analysis of corticosteroids and monoclonal antibodies as dual therapeutic agents. World J Methodol 2026; 16(1): 107864
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/107864.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.107864
Multiple myeloma (MM) is a malignant clonal hematologic disease defined as the pathological proliferation of malignant plasma cells in the bone marrow. It is an incurable disease with a multifactorial pathogenesis, including genetic mutations, interactions with the microenvironment of the bone marrow, and failure of the immune system. MM is the second most prevalent hematologic malignancy worldwide that primarily afflicts the geriatric population, with a median age at diagnosis of 69 years[1]. Although treatments for this disease have improved in the past half-century, MM remains a relapsing and refractory disease: MM patients typically go through phases of remission and relapse, with the majority having a poor prognosis. Over the years, conventional treatment strategies for MM included chemotherapy, high-dose chemotherapy with autologous stem cell transplantation, and corticosteroids (e.g., dexamethasone and prednisone). Although these therapies have led to improved rates of disease-free survival, they have been non-curative, as the disease inevitably relapses in most patients[2,3]. As MM remains uncured and often comes back, recent advances in treatment have focused on long-term survival, less risk of relapse, and improved quality of life with the use of new drug mixes.
In the last two decades, the introduction of new agents to the treatment of MM [e.g., proteasome inhibitors, immunomodulatory drugs (IMiDs), and monoclonal antibodies (mAbs)] markedly changed the treatment landscape. These new agents have enhanced survival outcomes, particularly among relapsed or refractory MM, and have evolved into essential components of current treatment approaches. Dexamethasone, a corticosteroid, has stood the test of time as an effective regimen but has fallen short when it comes to the general well-being of the patient with MM[4]. Additionally, corticosteroids are often given as one component of combined treatment, usually alongside established chemotherapeutic agents or modern proteasome inhibitors, to enhance the therapeutic effect at hand[5]. On the other hand, when de
mAbs are one advance in the treatment of MM. They aim at targeted therapies. Any immune system components implicated in MM biology will suffice to engage specific antigens on the malignant plasma cell surface. The human mAb daratumum, directed against CD38, has revolutionized the treatment of MM. It not only prolongs the period free from relapse and development but also decreases times up for recurrence[8,9]. CD38 is a cell surface molecule that, in malignant plasma cells, has been shown to mediate immune-mediated cytotoxicity, the killing of plasma cells by T lymphocytes, and T cell memory, and the ability of daratumumab to inhibit the growth of tumours has been well established in clinical trials[10]. Currently, daratumumab has been established to treat newly diagnosed and relapsed/refractory MM, demonstrating significant efficacy when combined with bortezomib, lenalidomide, and other agents[11,12].
The mAb elotuzumab zaps SLAMF7 (signalling lymphocytic activation molecule family member 7), a plasma cell surface protein, leading to potential benefits when combined with IMiDs like lenalidomide[13]. Elotuzumab also showed considerable benefit in relapsed/refractory MM, including in patients with multiple lines of previous therapy[14]. A second anti-CD38 mAb, siltuximab, has also shown promising activity in relapsed/refractory MM as a single agent or in combination with other agents[15,16].
mAbs' application in MM treatment has contributed to a better survival outcome and improved extra survival for the patients. But, notwithstanding the encouraging efficacy of steroids and mAbs, there are considerable therapeutic hurdles to tackle yet to tailor these drugs for specific patient subtypes such as high-risk MM. High-risk MM is typically regarded with a bad prognosis and is defined by clinically statistically significant genomic alterations (e.g., MYC oncogene translocations), extramedullary disease, and therapy resistance[17]. Further research is needed to optimize therapeutic strategies for these high-risk populations effectively, especially regarding combination regimens incorporating mAbs and corticosteroids[18].
Considerations also had to be utilized regarding the safety profile of cancer treatments relative to their efficacy; cor
This systematic review seeks to summarise the evidence from randomized controlled trials (RCTs) and cohort studies investigating the efficacy of corticosteroids and mAbs in MM treatment over the last 20 years (2003-2024). The review will assess the effectiveness of these therapies as an expert opinion based on their contribution to survival benefit, PFS, or overall response rate (ORR) in newly diagnosed or relapsed/refractory MM patients. This review will also address the limitations of presently existing treatment algorithms, particularly in patients with high-risk MM. It will encourage further exploration of these therapies' safety, durability, and resistance mechanisms.
This systematic aimed to evaluate the effectiveness of corticosteroids and mAbs in treating MM. A systematic literature search was performed to identify eligible RCTs and cohort studies published from 2003 to 2024. The studies were narrow in scope, and the review is highly selective. Systematic data extraction and synthesis were also conducted, and the risk of bias and quality of studies were examined. The following PICO criteria have been applied for the study selection.
Population (P): Patients with MM, including both newly diagnosed and relapsed/refractory MM patients, with a focus on high-risk MM cases.
Intervention (I): Corticosteroids and mAbs used for MM treatment.
Comparison (C): Standard treatment protocols, placebo, or other therapeutic agents used in MM treatment.
Outcome (O): Survival benefit, PFS, ORR, safety, durability, and resistance mechanisms of the therapies.
In this review, studies eligible to be included were RCTs, which are considered the gold standard for evaluating clinical efficacy of interventions. Cohort studies were also used, which collect important facts when RCTs cannot be used. By including this method, we were able to support our findings with both clinical studies and other observations.
The results were expected to provide comparative data on the therapeutic effectiveness of corticosteroids vs mAbs for the treatment of MM.
Patient population: Studies eligible for inclusion included adult patients with a diagnosis of MM in newly diagnosed or relapsed/refractory settings.
Interventions: We only considered studies examining the synergism of corticosteroids (e.g., dexamethasone and prednisone) and mAbs (e.g., daratumumab, elotuzumab, and isatuximab). These therapies are the backbone of the current treatment landscape for MM, and combinations of these agents are linked to improved survival metrics.
Primary outcomes were PFS, OS, and response rates (e.g., complete and partial response and minimal residual disease). These remain key outcomes in assessing the utility of MM therapies. Secondary outcomes such as quality of life, adverse events, and safety profiles were also extracted where they were available.
Timespan: The analysis only included studies published between 2003 and 2024 since this range denotes the treatment landscape evolution for MM, most notably with the emergence of mAbs, such as daratumumab and elotuzumab.
Studies were excluded from the review based on the following criteria:
Non-comparative studies: Studies that evaluated the effects of corticosteroids and mAbs vs other types of treatment regimens or placebos were excluded, as the scope of the review was limited to the relative efficacy of the therapeutic combinations.
Non-peer-reviewed studies/articles: We limited our research only to papers published in peer-reviewed journals. Even though peer review has limitations, it ensured that experts in the field had formally reviewed each study.
Exclusion of studies: We excluded studies that did not report key clinical outcomes, including PFS, OS, or response rates. This included studies that did not measure or report these outcomes sufficiently.
Animal studies and case reports: Animal studies and case reports do not provide generalizable clinical data, so they were excluded.
Studies on pediatric populations: Studies on pediatric patients or patients with primary plasma cell leukaemia were excluded as this review focuses on adult MM cases.
The data extraction was done in a systematic way to maintain both consistency and completeness. Two independent reviewers (with experience in clinical research) extracted data from eligible studies. The following data were abstracted from each study:
Study characteristics: Author(s), year of publication, type of study, and number of participants.
Documented details encompassed: Interventions (corticosteroids and mAbs) used, dosages, treatment regimens, and combinations with other agents.
Outcomes: Primary and secondary outcomes (PFS, OS, response rates defined as complete and partial response), adverse events, and safety profiles.
Data were summarized in tables and analyzed descriptively.
Assessment of risk of bias and quality of studies was key aspects of this systematic review to safeguard the reliability and validity of findings. Potential bias in the included studies was evaluated based on their study design, sample size, randomization process, blinding, and completeness of outcome data. Only RCTs and meta-analyses thereof were included in the analysis as this represents the highest quality evidence; however, cohort studies are also included to provide completeness of the data. Quality of studies was measured using standard tools, particularly the Cochrane Risk of Bias Tool for different domains of bias, including selection, performance, detection, attrition and reporting. Studies were assessed for risk of bias; high risk was defined as flaws in randomization, lack of blinding, or incomplete outcome data. In addition, an assessment of the methodological quality of each study was undertaken, focusing on sample sizes and analyses conducted. Studies that did not meet the minimum quality criteria, such as those with unclear methodological quality or lacking a suitable control group, were excluded from the review. This selection ensured that only sound and well−executed studies were selected, contributing to lower bias and higher quality of the synthesized evidence. This review seeks to elucidate the efficacy of corticosteroids and mAbs in managing MM by critically assessing the risk of bias.
This section will discuss the findings of the systematic review of corticosteroids and the effectiveness of mAbs in MM. These therapies have been clinically evaluated in newly diagnosed and relapsed/refractory MM patients, emphasizing PFS, OS, and response rates.
Corticosteroids, most notably dexamethasone, have formed a part of the backbone of MM therapy for several decades. Their efficacy has been demonstrated in newly diagnosed and relapsing/refractory patients. Rajkumar et al[1] compared high-dose dexamethasone plus lenalidomide vs low-dose dexamethasone alone in newly diagnosed MM patients. They showed a higher ORR for high-dose dexamethasone plus lenalidomide, providing the first evidence for the potency of dexamethasone-enhanced combinations. Similarly, Dimopoulos et al[6] and Facon et al[7] showed that a combination of corticosteroids with other agents significantly benefits patients for PFS and OS, including lenalidomide, bortezomib, and carfilzomib. These findings are consistent with earlier studies showing that using dexamethasone in combination with other myeloma agent’s decreases tumour burden and increases response rates.
Corticosteroids are also a critical component of relapsed/refractory MM treatment. In the OPTIMISM trial, a significant survival benefit was evident in those who received pomalidomide or bortezomib combined with dexamethasone in case of previous lenalidomide exposure[12]. This phenomenon indicates that corticosteroids retain their survival benefit when used even at more severe stages of disease.
While corticosteroids are very effective, they do have some notable side effects. The most significant adverse effects are immune suppression and metabolic disorders such as hyperglycemia and osteoporosis[13]. This is particularly damaging in older patients who are among the most affected populations of MM and may demonstrate a more significant outcome from corticosteroid therapy[14]. Thus, corticosteroids represent integral components of treatment regimens, but their long-term use must be carefully monitored, particularly in at-risk patient groups.
Research suggests that problems associated with corticosteroids are common and significant. The OPTIMISMM trial found that about 30% of patients who received pomalidomide-bortezomib-dexamethasone experienced severe infections, while cases of grade ≥ 3 hyperglycemia were seen in 7%-9%. Reports from studies indicate that corticosteroid use could raise blood sugar levels in up to 12%-18% of elderly MM patients, while rates of infection-related complications can be as high as 25%[21]. It suggests that regular monitoring of metabolism and possible infection is critical during corticosteroid treatments, especially for seniors and those with other health problems.
In recent decades, mAbs targeting antigens present in malignant plasma cells have emerged as an attractive therapeutic modality for the treatment of MM, contributing to considerable clinical outcome improvements, particularly with their use in combination with other therapeutic compounds, including proteasome inhibitors and immunomodulatory drugs. Daratumumab is one such mAb displaying impressive activity in both newly diagnosed as well as relapsed/refractory patients. In the CASTOR trial evaluating daratumumab in combination with bortezomib and dexamethasone, this combination showed a statistically significant PFS benefit compared with bortezomib and dexamethasone alone[5]. This regimen has since become a standard of care for relapsed/refractory MM.
Moreover, the POLLUX study investigating daratumumab in combination with lenalidomide and dexamethasone demonstrated marked and significant PFS improvement over lenalidomide and dexamethasone alone[6]. This study indicated daratumumab's efficacy in improving survival in relapsed/refractory MM patients. Daratumumab's mecha
Apart from daratumumab, elotuzumab has shown promising results when used in combination with lenalidomide and dexamethasone in the ELOQUENT-2 trial. In both trials, the elotuzumab-based regimen was associated with improved PFS compared to lenalidomide and dexamethasone alone in relapsed/refractory MM patients[9]. Elotuzumab targets the SLAMF7 protein on myeloma and natural killer (NK) cells, leading to an increased immune response against MM cells.
Jakubowiak et al[15] conducted a more recent study (2021), which studied the combination of daratumumab with carfilzomib, lenalidomide, and dexamethasone in transplant-eligible, newly diagnosed patients. The four-drug combination achieved notable clinical improvements, including PFS and OS, highlighting the promise of these novel combinations of mAbs with other agents. These results are similar to previous studies that demonstrate the efficacy of daratumumab in combination regimens in patients with newly diagnosed and relapsed MM[5,6,8].
mAbs have much promise, but there are concerns about long-term safety. Infusion-related reactions (notably with daratumumab) and increased risk of infections are the most frequently observed side effects from clinical trials[16]. Careful management is needed for these side effects, as infections in immunosuppressed patients, such as in those receiving mAb therapy with MM, can be life-threatening.
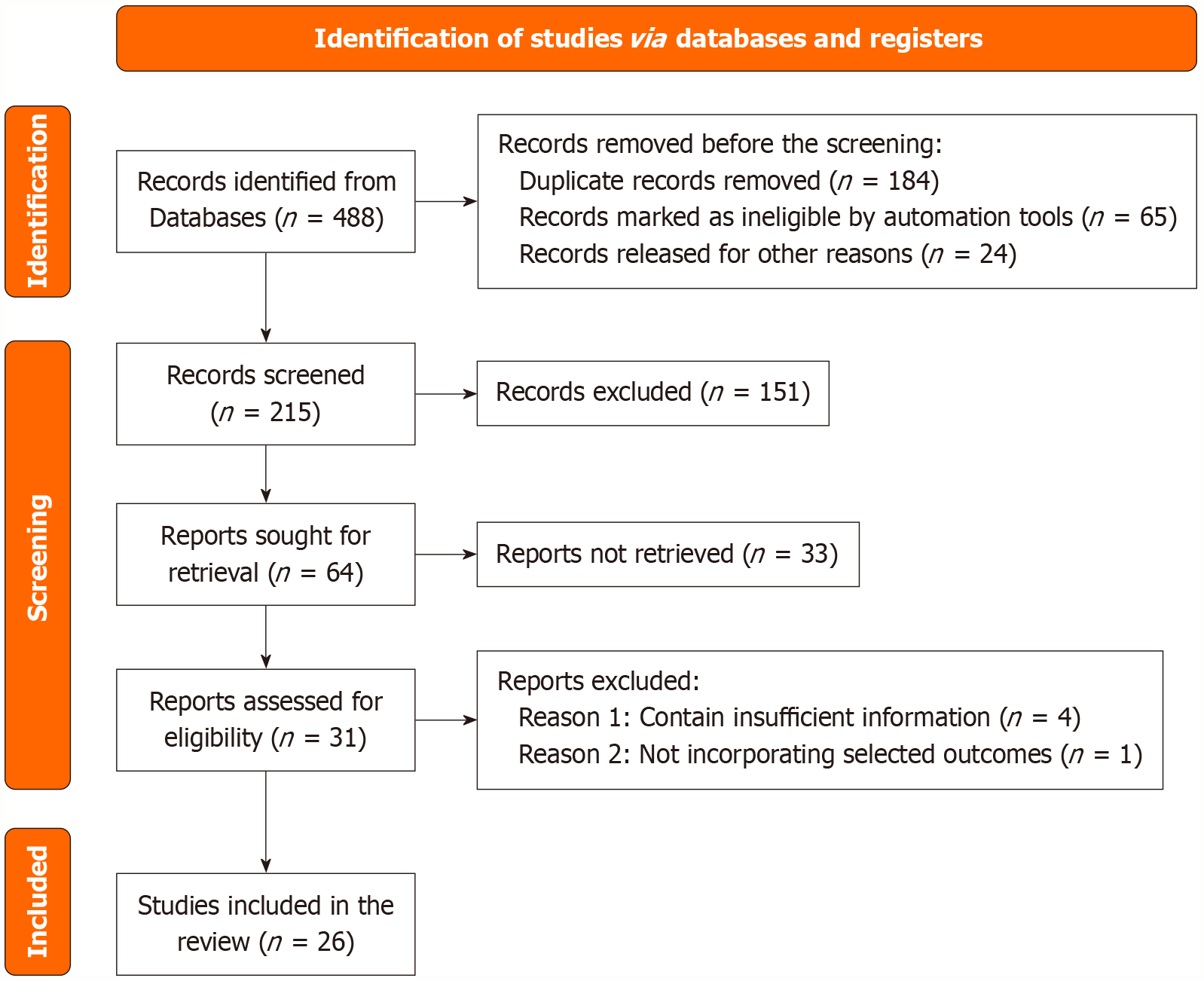
A total of 488 records were identified in the database, of which 273 were rejected due to being duplicates or related to automation, leaving 215 records for screening. After reviewing the title and abstract, 64 studies were reviewed, and 26 studies were found to meet the requirements for the analysis (Table 1). This structured selection process ensures that only relevant and high-quality studies contribute to the final analysis (Figure 1).
| Ref. | Year | Sample size | Study type | Interventions | Outcomes | Key findings |
| Voorhees et al[10] | 2020 | 207 | RCT | Daratumumab + RVd vs RVd | Improved response rates and PFS | sCR rate 424% vs 32.0% |
| Kumar et al[8] | 2020 | 1087 | RCT | Carfilzomib + Rd vs Bortezomib + Rd | No PFS benefit for Carfilzomib over Bortezomib | No significant PFS benefit observed |
| Lonial et al[9] | 2015 | 646 | RCT | Elotuzumab + Rd vs Rd | Improved PFS and response rates with Elotuzumab | PFS improved by approximately 4.5 months (HR 0.70) |
| Bringhen et al[4] | 2018 | 54 | RCT | Weekly Carfilzomib + Cyclophosphamide + Dex | High response rates with Carfilzomib | ORR > 85% |
| Facon et al[7] | 2019 | 737 | RCT | Daratumumab + Rd vs Rd | Significant PFS and ORR improvement with Daratumumab | PFS improved by 13.5 months |
| Dimopoulos et al[6] | 2016 | 569 | RCT | Daratumumab + Rd vs Rd | Improved PFS with Daratumumab | Median PFS: 44.5 vs 18.4 months (HR 0.37) |
| Palumbo et al[5] | 2016 | 498 | RCT | Daratumumab + Bortezomib + Dex vs Bortezomib + Dex | Significantly longer PFS with Daratumumab | Daratumumab group had a 61% risk reduction (HR 0.39) |
| Facon et al[2] | 2007 | 447 | RCT | MPT vs MP vs MEL100 | MPT superior to MP in OS | OS improved with MPT arm (P < 0.05) |
| Richardson et al[3] | 2003 | 202 | RCT | Bortezomib | Bortezomib effective in refractory MM | Significant ORR in heavily pretreated patients |
| Rajkumar et al[1] | 2010 | 445 | RCT | Lenalidomide + Low-dose Dex vs High-dose Dex | Better OS and lower toxicity with low-dose Dex | Low-dose Dex improved OS and had fewer AEs |
| Jakubowiak et al[15] | 2021 | 22 | RCT | Daratumumab + Carfilzomib + Lenalidomide + Dex | Improved outcomes, high response rates | VGPR or better: 100% |
| Richardson et al[12] | 2019 | 559 | RCT | Pomalidomide + Bortezomib + Dex vs Bortezomib + Dex | Improved PFS in the Pomalidomide group | PFS improved by 4.1 months (11.2 vs 7.1 months) |
| Moreau et al[20] | 2021 | 302 | RCT | Isatuximab + Carfilzomib + Dex vs Carfilzomib + Dex | Improved PFS with Isatuximab combination | Median PFS: 35.7 vs 19.2 months |
| Sonneveld et al[18] | 2023 | 709 | RCT | Daratumumab + VRd vs VRd alone | Significant improvement in PFS | PFS: NR vs 62.4 months |
| Bumma et al[13] | 2023 | 503 | Cohort Study | Bortezomib-based vs Lenalidomide maintenance | Different maintenance strategies impact long-term outcomes | Individualized maintenance affects survival |
| Leypoldt et al[19] | 2023 | 125 | RCT | Isatuximab + Carfilzomib + Lenalidomide + Dex | High minimal residual disease negativity rates, long-term PFS benefit | Long-term PFS benefit in high-risk MM |
| Richardson et al[17] | 2021 | 722 | RCT | Ixazomib + Lenalidomide + Dex vs Placebo + Rd | No significant OS benefit, but some subgroup advantages | PFS benefit in some subgroups |
| Takezako et al[16] | 2021 | 40 | RCT | Once-weekly vs twice-weekly carfilzomib | Once-weekly dosing had better ORR and PFS | Improved tolerability with similar efficacy |
| Slade et al[23] | 2023 | 25 | RCT | Elotuzumab + Pomalidomide + Dexamethasone (EPd) | 1-year PFS: 72%; median PFS: 19 months; safe regimen | Median PFS: 19 months |
| Joseph et al[22] | 2024 | 1326 | Cohort Study | Daratumumab + RVd vs RVd alone | Quadruplet therapy improves response and PFS | Improved VGPR and CR rates |
| Wang et al[21] | 2017 | 32 | Retrospective | Low-dose Lenalidomide + Dexamethasone | ORR: 71.9%, median PFS: 13 months, mild side effects | Mild side effects, effective in elderly |
| Bringhen et al[24] | 2014 | 58 | RCT | Carfilzomib + Cyclophosphamide + Dexamethasone | High response rates, PFS: 76%, OS: 87% at 2 years | PFS: 76%, OS: 87% at 2 years |
| Moreau et al[25] | 2021 | 159 | RCT | Teclistamab (BCMA × CD3 bispecific antibody) | ORR: 65%, VGPR: 58%, well tolerated, durable responses | ORR: 65%, VGPR: 58% |
| Dimopoulos et al[11] | 2020 | 466 | RCT | KdD (Carfilzomib, Dexamethasone, Daratumumab) vs Kd | KdD improved PFS (28.6 vs 15.2 months), manageable safety profile | PFS: 28.6 vs 15.2 months |
| Mateos et al[26] | 2022 | 119 | RCT | Lenalidomide + Dexamethasone vs Observation | Median TTP to MM: 9.5 vs 2.1 years, OS benefit with Rd | Median TTP: 9.5 vs 2.1 years |
| Bahlis et al[14] | 2024 | 112 | RCT | DPd (Pomalidomide + Daratumumab + Dexamethasone) | ORR: Favorable OS, median 56.7 months; no new safety signals | Median OS: 56.7 months |
Studies include RCTs and cohort studies, highlighting the variability of available data regarding the therapeutic applicability of corticosteroids and mAbs for managing MM. Nine studies evaluated mAbs in combination with corticosteroids (dexamethasone) in patients receiving other treatments (lenalidomide, bortezomib, and carfilzomib) or other mAbs (daratumumab and elotuzumab). These studies encompassed a diverse set of patient populations, including both newly diagnosed and relapsed/refractory MM patients.
The quality of the included studies was evaluated using the Cochrane Risk of Bias tool for RCTs. Table 2 evaluates the risk of bias across RCTs on MM treatments. Most studies demonstrated a low overall risk of bias, with low ratings in randomization, blinding, missing outcome data, and selective reporting. However, studies by Palumbo et al[5], Lonial et al[9], Bahlis et al[14], Takezako et al[16], and Moreau et al[20], had moderate bias in blinding and deviations from intended interventions, indicating potential methodological limitations. Despite these moderate risks, the general trend suggests that the studies were well-conducted and their findings reliable, reinforcing confidence in the reported outcomes for MM therapies (Table 2).
| Ref. | Random bias | Blinding bias | Deviations from intended interventions | Missing outcome data | Outcome measurement bias | Selective reporting bias | Overall risk of bias |
| Voorhees et al[10] | Low | Low | Low | Low | Low | Low | Low |
| Kumar et al[8] | Low | Low | Low | Low | Low | Low | Low |
| Lonial et al[9] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Bringhen et al[4] | Low | Moderate | Low | Low | Low | Low | Low |
| Facon et al[7] | Low | Low | Low | Low | Low | Low | Low |
| Dimopoulos et al[6] | Low | Low | Low | Low | Low | Low | Low |
| Palumbo et al[5] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Facon et al[2] | Low | Moderate | Low | Low | Low | Low | Low |
| Richardson et al[3] | Low | Low | Low | Low | Low | Low | Low |
| Rajkumar et al[1] | Low | Low | Low | Low | Low | Low | Low |
| Jakubowiak et al[15] | Low | Low | Low | Low | Low | Low | Low |
| Richardson et al[12] | Low | Low | Low | Low | Low | Low | Low |
| Moreau et al[20] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Sonneveld et al[18] | Low | Moderate | Low | Low | Low | Low | Low |
| Leypoldt et al[19] | Low | Low | Low | Low | Low | Low | Low |
| Richardson et al[17] | Low | Low | Low | Low | Low | Low | Low |
| Takezako et al[16] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Slade et al[23] | Low | Moderate | Low | Low | Low | Low | Low |
| Bringhen et al[24] | Low | Low | Low | Low | Low | Low | Low |
| Moreau et al[25] | Low | Low | Low | Low | Low | Low | Low |
| Dimopoulos et al[11] | Low | Low | Low | Low | Low | Low | Low |
| Mateos et al[26] | Low | Low | Low | Low | Low | Low | Low |
| Bahlis et al[14] | Low | Moderate | Low | Moderate | Low | Low | Moderate |
The Newcastle-Ottawa Scale was used for cohort studies to assess selection bias, comparability, and outcome assessment. Table 3 presents a quality assessment of three studies, i.e., those conducted by Bumma et al[13], Wang et al[21], and Joseph et al[22], using a total score of 9 based on selection, comparability, and outcome criteria. Each study scored 4 in selection, 2 in comparability, and 3 in outcome, suggesting a high-quality methodology with robust patient selection and outcome measurement (Table 3).
While such potential biases do exist, they have a minimal impact on the overall body of evidence presented, which includes findings supporting the universally represented pooled efficacy of corticosteroids and mAbs upon clinical outcomes in MM patient populations. Sensitivity analyses performed during the review revealed that results were robust when studies at higher risk of bias were included.
mAbs and corticosteroids have shown significant efficacy. Corticosteroids, such as dexamethasone, are still foundational agents and are particularly effective when combined with other agents such as lenalidomide and bortezomib. mAbs, notably daratumumab and elotuzumab, have demonstrated significant improvements in PFS and OS in patients with newly diagnosed and relapsed/refractory MM. Although these therapies prove effective, the long-term safety profile of these infusions (particularly about the risks of infection and infusion-related reactions) remains an essential subject of current research (Table 1).
Many studies have reported key results in evaluating corticosteroids and mAbs for MM. Most trials focused on PFS, OS, and ORR as the main measures. Sometimes, researchers included rates for complete response, very good partial response, and minimal residual disease negativity in later studies[10,19]. Moreover, continuous elotuzumab + pomalidomide + dexamethasone maintenance after a second autologous transplant achieved broad responses with manageable toxicity, though progression was common on longer follow-up[23].
In several trials, daratumumab was used, and standard treatments performed better. For example, in the POLLUX trial, patients who got daratumumab plus lenalidomide/dexamethasone experienced a progression rate decline from 18.4 to 44.5 months. The CASTOR trial[5] found that the risk of progression dropped by 61% (hazard ratio = 0.39) in patients treated with daratumumab plus bortezomib/dexamethasone instead of the control.
When combined with different drugs, corticosteroids showed different levels of effectiveness. Taking low-dose dexamethasone together with lenalidomide was found to increase OS, but pomalidomide-bortezomib-dexamethasone increased PFS by 4.1 months relative to bortezomib-dexamethasone ( lenalidomide-exposed patients)[12].
In various studies, including recent ones, treatments with corticosteroids and mAbs have demonstrated very good outcomes. For people with high-risk disease, researchers found that daratumumab-based combinations had better outcomes compared to others[18,19].
Many studies analyzing time-to-event outcomes found that a backbone treatment of both a corticosteroid and mAbs often performed better than the separate use of either treatment.
This paper discusses the combination of the two and highlights the benefits of corticosteroids and mAbs as an essential improvement in MM—especially in recent years[1]. These agents demonstrated promising efficacy in improving PFS and OS in a broad range of patient populations, including newly diagnosed, relapsed, and refractory patients. As anti-inflammatory and immune-modulating drugs, corticosteroids like dexamethasone and prednisone have been a cornerstone of MM therapy for decades. However, despite their considerable therapeutic benefits, the side effects, especially after prolonged use, remain a significant problem. mAbs for the treatment of MM are very promising as they have revolutionized the treatment, but some concerns have been raised regarding their cost, accessibility, and long-term safety.
Corticosteroids, such as dexamethasone, have been a mainstay of MM treatment for decades; they are typically used in conjunction with a variety of other agents, including IMiDs and proteasome inhibitors. Several studies have also shown that they decrease intra-tumour burden whilst potentiating the activity of other chemotherapeutics. Rajkumar et al[1] demonstrated that when dexamethasone and lenalidomide (Revlimid) were combined in newly diagnosed patients, the response rates were higher than thosewith lenalidomide alone, highlighting the importance of corticosteroids. Similarly, the results reported by Dimopoulos et al[6] and Facon et al[7] showed that corticosteroid-based regimens with lena
While results from clinical trials are available, newer studies show that corticosteroids and mAbs work together to treat MM. Dexamethasone decreases inflammation by reducing the activity of certain cytokines, triggering the removal of malignant plasma cells, and reducing overall tumor-related inflammation. These actions could help daratumumab, which targets CD38, perform better in fighting against myeloma cells. The antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis processes are activated by daratumumab. Dexamethasone is believed to improve the action of NK cells by lowering the number of T cells and myeloid-derived suppressor cells. Corticosteroids can potentiate daratumumab's therapeutic effects, causing a stronger and longer response. Dexamethasone may also improve tolerability by reducing reactions linked to the infusion of mAbs, mainly through cytokine suppression[11,20].
Corticosteroids have proven therapeutic efficacy; however, they can cause numerous side effects that negatively impact the quality of life in MM patients. Persistent intake of corticosteroids results in overall immune suppression, which is particularly consequential in MM patients who exhibit inherent immune compromise due to their underlying disease pathology. This is an essential issue in connection with the required steroid immunotherapy. Additionally, corticosteroids cause metabolic derangements (e.g., hyperglycemia, weight gain, and increased appetite) that negatively influence existing comorbidities in MM patients. Another significant side effect is osteoporosis, which increases the risk of fractures, particularly in older people who are disproportionately affected by MM. These side effects can be especially concerning in the elderly population, who are often the predominant group of patients with MM. The difficulty in managing chronic side effects of corticosteroid therapy has led to investigating corticosteroid-sparing regimens and designing newer, less toxic agents, including mAbs and targeted therapies. Reducing corticosteroid doses or optimizing their use in combination regimens is attempting to minimize these toxicities without compromising their therapeutic effectiveness.
The most exciting approach for MM treatment is mAbs, targeted therapy that effectively retrieves molecular me
The POLLUX trial also provided evidence for the combination of daratumumab with lenalidomide and dexamethasone[6]. Note that this study demonstrated that adding daratumumab significantly improved PFS and response rates compared to lenalidomide and dexamethasone alone. However, as mAbs were associated with enhanced efficacy in improving patient outcomes, their use should be considered a mainstay in the frontline treatment regimens, particularly in patients with high-risk MM. Moreover, mAbs have been shown to overcome the mechanisms of drug resistance developed in relapsed and refractory MM, particularly when conventional therapies (proteasome inhibitors and im
A second anti-SLAMF7 mAb, elotuzumab, has shown activity when combined with lenalidomide and dexamethasone[9]. In patients with relapsed/refractory MM, elotuzumab-based combinations have been linked with significantly improved PFS compared to lenalidomide plus dexamethasone alone. This mechanism seems to increase the activity of NK cells involved in the immune response against myeloma cells. Moreover, this provides a new immunologic approach for the treatment of MM that is distinct from standard chemotherapies that rely on direct cytotoxicity. mAb therapy for the treatment of MM has been extended with the incorporation of elotuzumab and daratumumab, allowing patients to have more options to consider in the first line and the relapsed setting.
mAbs are effective, but they pose their challenges. One of the most significant changes is the affordability of such therapies. Daratumumab and elotuzumab are mAbs with high price tags, creating access concerns, especially in low-income and middle-income countries. mAb treatment imposes substantial economic burdens on healthcare systems in high-income settings and has economic consequences for patients with financial distress at treatment initiation with these therapies. This highlights a challenge concerning the cost of mAbs and a quandary concerning treatment affordability. This challenge will probably improve with biosimilars, that is, low-cost alternatives to branded mAbs. If successful, these biosimilars, like many for daratumumab, will represent an affordable solution for MM patients in terms of cost-effectiveness.
Another consideration is the safety of mAbs, particularly infusion-related reactions or infections, which need to be evaluated in the context of risk for active disease. Infusion reactions such as fever, chills, and hypotension are common when administering mAbs and can delay the continuation of therapy. Although these reactions are mild, they can affect treatment time and the patient's comfort. Due to their immunosuppressive capacity, mAbs can result in infusion-related responses and an increased infection risk, especially when combined with other immunosuppressive drugs. In clinical practice, though, careful monitoring of the patients for infections and adequate prophylactic strategies are crucial concerning mAbs.
Given their compelling effectiveness with other critical agents like proteasome inhibitors and IMiDs, an examination of mAbs as frontline therapies for high-risk MM patients is warranted. Multiple studies have shown that adding mAbs to frontline regimens significantly improves clinical outcomes. For instance, the combination of daratumumab with carfilzomib, lenalidomide, and dexamethasone has shown improved results in newly diagnosed, transplant-eligible patients[15]. The addition of mAbs to both standard double and triple drug first-line therapies holds the potential of improving survival, especially in patients at a higher risk for progression, as demonstrated by a QUADRUPLET regimen that provides significantly greater response rates and PFS compared to that in doublet regimens. However, the ideal timing and patient selection for mAb therapy remain under study due to cost and the potential for long-term side effects.
The paper introduces a fresh method to manage high-risk MM cases through simultaneous corticosteroid and mAb administration, and evaluates the combined effectiveness of corticosteroids and mAbs in extending survival rates, alongside discussing their associated safety risks and persistent adverse effects. This paper also examines how biosimilars might increase accessibility to medication, especially for people living in low-income communities. Through examination of high-risk patients combined with systematic review methodology, the authors create new possibilities to enhance therapeutic approaches for MM treatment.
The article contributes an innovative perspective to non-innovative treatment combinations of corticosteroids with mAbs in MM by focusing on synergistic effects in high-risk disease populations. The lengthy systematic review and analysis of research over the past two decades and the detailed evaluation of biosimilar safety and resistance properties and financial implications have brought a new direction to past studies. Due to the extensive knowledge gap and insufficient available research, the current paper delivers a comprehensive, unique evaluation integrating multiple MM therapies with high-risk patient management strategies and expense estimates.
To summarize the nature of the existing data, the use of corticosteroids and mAbs has changed the treatment landscape of MM, with corticosteroids being an essential contributor to tumour reduction and mAbs as agents that have a significant impact on PFS or OS. However, corticosteroids have adverse effects, especially with long-term use, and the costs of mAbs and associated safety considerations will need to be considered in clinical practice. Continued development of alternative therapies, including biosimilars and corticosteroid-sparing regimens, and characterization of the safety and efficacy of mAbs will be essential in improving treatment success and ensuring that effective therapies are widely available to all patients with MM.
The review demonstrates how corticosteroids and mAbs, particularly daratumumab and elotuzumab, are important advancements in MM treatment. Corticosteroid and mAb treatment approaches are vital to PFS and OS measurements, particularly for high-risk patient groups. Long-term corticosteroid treatments create health problems because they weaken the immune system and result in metabolic dysfunctions along with osteoporosis risks, which become particularly critical for senior patients. The therapeutic advantages of mAbs in cancer treatment come with financial burdens and infusion-related adverse effects that affect their availability, particularly in underserved healthcare settings. Future medical investigations need to assess mAbs' long-term effects and performance while studying how corticosteroids can be reduced in combination treatments and developing patient-specific indicators for therapeutic approaches. Further research can be explored by researching new treatment combinations that pair mAbs with bispecific antibodies or CAR T-cell approaches, as this could have a positive impact on patients’ outcomes since treatment with both has a potential synergy. Making mAbs affordable and accessible through biosimilars is key to enhancing MM treatment access to more patients. Research developments through the upcoming 5-10 years will modify MM treatment guidelines to create individualized therapy options that offer enhanced effectiveness and accessibility and aim to improve results for all persons receiving treatment.
| 1. | Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR; Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, Dib M, Voillat L, Maisonneuve H, Troncy J, Dorvaux V, Monconduit M, Martin C, Casassus P, Jaubert J, Jardel H, Doyen C, Kolb B, Anglaret B, Grosbois B, Yakoub-Agha I, Mathiot C, Avet-Loiseau H; Intergroupe Francophone du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 3. | Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2009] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 4. | Bringhen S, D'Agostino M, De Paoli L, Montefusco V, Liberati AM, Galieni P, Grammatico S, Muccio VE, Esma F, De Angelis C, Musto P, Ballanti S, Offidani M, Petrucci MT, Gaidano G, Corradini P, Palumbo A, Sonneveld P, Boccadoro M. Phase 1/2 study of weekly carfilzomib, cyclophosphamide, dexamethasone in newly diagnosed transplant-ineligible myeloma. Leukemia. 2018;32:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:754-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1203] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 6. | Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O'Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1167] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 7. | Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, Goldschmidt H, O'Dwyer M, Perrot A, Venner CP, Weisel K, Mace JR, Raje N, Attal M, Tiab M, Macro M, Frenzel L, Leleu X, Ahmadi T, Chiu C, Wang J, Van Rampelbergh R, Uhlar CM, Kobos R, Qi M, Usmani SZ; MAIA Trial Investigators. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380:2104-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 786] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 8. | Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, Parker TL, Menter A, Yang X, Parsons B, Kumar P, Kapoor P, Rosenberg A, Zonder JA, Faber E Jr, Lonial S, Anderson KC, Richardson PG, Orlowski RZ, Wagner LI, Rajkumar SV. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:1317-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 9. | Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H, Belch A, Reece D, Beksac M, Spencer A, Oakervee H, Orlowski RZ, Taniwaki M, Röllig C, Einsele H, Wu KL, Singhal A, San-Miguel J, Matsumoto M, Katz J, Bleickardt E, Poulart V, Anderson KC, Richardson P; ELOQUENT-2 Investigators. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1074] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 10. | Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, Chari A, Silbermann R, Costa LJ, Anderson LD Jr, Nathwani N, Shah N, Efebera YA, Holstein SA, Costello C, Jakubowiak A, Wildes TM, Orlowski RZ, Shain KH, Cowan AJ, Murphy S, Lutska Y, Pei H, Ukropec J, Vermeulen J, de Boer C, Hoehn D, Lin TS, Richardson PG. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 11. | Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H, Klippel Z, Zahlten-Kumeli A, Usmani SZ. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 12. | Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, Lindsay J, Weisel K, White D, Facon T, San Miguel J, Sunami K, O'Gorman P, Sonneveld P, Robak P, Semochkin S, Schey S, Yu X, Doerr T, Bensmaine A, Biyukov T, Peluso T, Zaki M, Anderson K, Dimopoulos M; OPTIMISMM trial investigators. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:781-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 13. | Bumma N, Dhakal B, Fraser R, Estrada-Merly N, Anderson K, Freytes CO, Hildebrandt GC, Holmberg L, Krem MM, Lee C, Lekakis L, Lazarus HM, Mian H, Murthy HS, Nathan S, Nishihori T, Parrondo R, Patel SS, Solh M, Strouse C, Vesole DH, Kumar S, Qazilbash MH, Shah N, D'Souza A, Sidana S. Impact of bortezomib-based versus lenalidomide maintenance therapy on outcomes of patients with high-risk multiple myeloma. Cancer. 2023;129:2179-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Bahlis NJ, Samaras C, Reece D, Sebag M, Matous J, Berdeja JG, Shustik J, Schiller GJ, Ganguly S, Song K, Seet CS, Acosta-Rivera M, Bar M, Quick D, Fonseca G, Liu H, Gentili C, Singh P, Siegel D. Pomalidomide/Daratumumab/Dexamethasone in Relapsed or Refractory Multiple Myeloma: Final Overall Survival From MM-014. Clin Lymphoma Myeloma Leuk. 2024;24:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Jakubowiak A, Usmani SZ, Krishnan A, Lonial S, Comenzo RL, Wang J, de Boer C, Deraedt W, Weiss BM, Schecter JM, Chari A. Daratumumab Plus Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Newly Diagnosed Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2021;21:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Takezako N, Shibayama H, Handa H, Hagiwara S, Ozaki S, Suzuki K, Kosugi H, Ri M, Sugiura I, Choi I, Miyamoto T, Iida S. Once-weekly vs. twice-weekly carfilzomib dosing in a subgroup of Japanese relapsed and refractory multiple myeloma patients from a randomized phase 3 trial (A.R.R.O.W.) and comparison with ENDEAVOR. Int J Hematol. 2021;113:219-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Richardson PG, Kumar SK, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, Stoppa AM, Gimsing P, Garderet L, Touzeau C, Buadi FK, Laubach JP, Cavo M, Darif M, Labotka R, Berg D, Moreau P. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J Clin Oncol. 2021;39:2430-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, Hulin C, Antonioli E, Leleu X, Mangiacavalli S, Perrot A, Cavo M, Belotti A, Broijl A, Gay F, Mina R, Nijhof IS, van de Donk NWCJ, Katodritou E, Schjesvold F, Sureda Balari A, Rosiñol L, Delforge M, Roeloffzen W, Silzle T, Vangsted A, Einsele H, Spencer A, Hajek R, Jurczyszyn A, Lonergan S, Ahmadi T, Liu Y, Wang J, Vieyra D, van Brummelen EMJ, Vanquickelberghe V, Sitthi-Amorn A, de Boer CJ, Carson R, Rodriguez-Otero P, Bladé J, Moreau P; PERSEUS Trial Investigators. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024;390:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 350] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 19. | Leypoldt LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, Munder M, Reinhardt HC, Nogai A, Görner M, Ko YD, de Wit M, Salwender H, Scheid C, Graeven U, Peceny R, Staib P, Dieing A, Einsele H, Jauch A, Hundemer M, Zago M, Požek E, Benner A, Bokemeyer C, Goldschmidt H, Weisel KC. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2024;42:26-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 107] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 20. | Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Špička I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y, Suzuki K, Risse ML, Asset G, Macé S, Martin T; IKEMA study group. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 21. | Wang C, He Z, Shi Y, Zhang L, Chen Y, Chen Z, Yu L. Low-dose lenalidomide and dexamethasone combination treatment in elderly patients with relapsed and refractory multiple myeloma. Hematology. 2017;22:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Joseph NS, Kaufman JL, Gupta VA, Hofmeister CC, Dhodapkar MV, Boise LH, DiCamillo SM, Roberts D, Nooka AK, Lonial S. Quadruplet therapy for newly diagnosed myeloma: comparative analysis of sequential cohorts with triplet therapy lenalidomide, bortezomib and dexamethasone (RVd) versus daratumamab with RVD (DRVd) in transplant-eligible patients. Blood Cancer J. 2024;14:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 23. | Slade M, Fiala MA, Kirchmeyer M, King J, Gao F, Schroeder MA, Stewart AK, Stockerl-Goldstein K, Chen C, Vij R. Continuous Elotuzumab, Pomalidomide, and Dexamethasone Maintenance Following Second Autologous Transplantation for Multiple Myeloma: Results of a Prospective Phase 2 Multicenter Trial. Transplant Cell Ther. 2023;29:764.e1-764.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, Musto P, Boccadifuoco L, Offidani M, Omedé P, Gentilini F, Ciccone G, Benevolo G, Genuardi M, Montefusco V, Oliva S, Caravita T, Tacchetti P, Boccadoro M, Sonneveld P, Palumbo A. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Popat R, Usmani S, Garfall A, van de Donk N, Nahi H, San-miguel J, Oriol A, Nooka A, Martin T, Rosinol L, Chari A, Karlin L, Benboubker L, Mateos M, Bahlis N, Moreau P, Besemer B, Martínez-lópez J, Sidana S, Pei L, Trancucci D, Verona R, Girgis S, Olyslager Y, Jaffe M, Uhlar C, Stephenson T, Van Rampelbergh R, Banerjee A, Goldberg J, Kobos R, Krishnan A. P06: UPDATED RESULTS FROM THE PHASE 1/2 Majestec-1 study of teclistamab, a B-cell maturation antigen X CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Hema Sphere. 2022;6:14-14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Mateos MV, Hernández MT, Salvador C, Rubia J, de Arriba F, López-Corral L, Rosiñol L, Paiva B, Palomera L, Bargay J, Oriol A, Prosper F, López J, Arguiñano JM, Bladé J, Lahuerta JJ, San-Miguel J. Lenalidomide-dexamethasone versus observation in high-risk smoldering myeloma after 12 years of median follow-up time: A randomized, open-label study. Eur J Cancer. 2022;174:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/