Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.106277
Revised: May 6, 2025
Accepted: July 4, 2025
Published online: March 20, 2026
Processing time: 354 Days and 16.8 Hours
The neutrophil-to-lymphocyte ratio (NLR) is a novel inflammatory marker predicting cardiovascular mortality (CVM) and all-cause mortality (ACM).
To investigate the association between the NLR and CVM and ACM in patients with peritoneal dialysis (PD).
We reviewed articles from PubMed, Google Scholar, and Scopus until May 2024 for the association of ACM and CVM in patients with NLR following PD. We used a fixed effects model and I2 statistics to pool hazard ratio (HR) and measure heterogeneity. Leave-one-out sensitivity analysis assessed the robustness of our study. Multivariate meta-regression (MMR) was utilized to identify influencing confounding factors. Joanna Briggs Institute (JBI) tool was used for quality assessment.
Out of 160 articles screened, seven studies from 2011 to 2024 with 4350 patients, a mean age of 49.9 years ± 15 years, and a median follow-up of 4 years were included. We found that higher NLR (> 3) was significantly associated with ACM [adjusted HR (aHR): 1.18, 95%CI: 1.03-1.36, P = 0.016]. However, there was no significant association of NLR (> 3) with CVM (aHR: 1.16, 95%CI: 0.68-1.98, P = 0.59) in patients following PD. Sensitivity analysis showed no variations. JBI tool revealed low bias among the studies. MMR revealed a significant relationship between age and ACM (coefficient: 0.14, P = 0.04).
Our meta-analysis identifies a significant association between NLR (> 3) and ACM outcome, which can help prevent deaths in the elderly and optimize resource use. Caution is needed when predicting mortality in this group as age significantly confounds ACM in patients undergoing PD.
Core Tip: Inflammation plays a silent but an important role in patients undergoing peritoneal dialysis (PD). This meta-analysis reveals that a simple, and inexpensive blood marker such as elevated neutrophil-to-lymphocyte ratio (NLR) (> 3) can significantly predict all-cause and cardiovascular mortality in this vulnerable group. With data from over 4300 patients, our study also identified age as a key confounder, urging clinicians to interpret NLR levels with caution in older adults. These findings position NLR as a powerful, accessible tool for early risk detection and tailored intervention in PD care.
- Citation: Damarlapally N, Vempati R, Mourad D, Vasudevan SS, Mathur G, Khan A, Banda P, Polamarasetty H, Chauhan S, Manjappachar N, Bhatti T, Desai R. Association of neutrophil-lymphocyte ratio with all-cause and cardiovascular mortality in peritoneal dialysis patients: A systematic review and meta-analysis. World J Methodol 2026; 16(1): 106277
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/106277.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.106277
Chronic kidney disease (CKD) is an emerging public health concern due to its progressive nature and association with secondary complications such as cardiovascular disease (CVD) and higher mortality[1]. Individuals with established CKD, particularly stages 3 and 4, have a two-fold to three-fold increased risk for cardiovascular mortality (CVM) over the general population[2]. End-stage renal disease (ESRD) is defined by a glomerular filtration rate (GFR) below 15 mL/minute/1.73 m², necessitating renal replacement therapy[3,4]. According to the United States Renal Data System, the overall prevalence of CKD in the United States is about 14.0% of adults (based on low estimated GFR, albuminuria, or both)[1]. Although the burden of CKD has plateaued over the years, CVD remains the leading cause of morbidity and mortality among these patients.
Peritoneal dialysis (PD) allows for the elimination of waste and fluid balance through the peritoneal membrane. In PD, a dialysis solution (dialysate) is introduced into the abdomen through a surgically implanted tube. The dialysate effectively removes toxins across the peritoneal membrane in patients with renal failure, and helps in correcting imbalances in water, electrolyte, and acid-base levels[3]. The filtration rate is high when the dialysate is clear and fresh but it slows down over time, warranting the process to be repeated 4 times to 6 times a day. The dialyzing fluid contains harmful compounds, including complex end products of glycation produced by glucose degradation products made during the sterilization process[5]. Over time, these toxins accumulate in the body, and reduce renal function in CKD patients further exacerbating the condition.
These toxic accumulations in CKD patients are prone to chronic inflammation, oxidative stress, and metabolic derangements that play a major role in CVD, anemia, malnutrition, CVM and all-cause mortality (ACM). Finding stable and cost-effective biomarkers to identify early complications and predict adverse outcomes remains essential. The classic inflammatory markers like C-reactive protein, interleukin-6, and tumor necrosis factor-alpha are limited by cost, heterogeneity, and episodic availability in the clinical setting[6]. On the other hand, the neutrophil-to-lymphocyte ratio (NLR) has been a promising inflammatory and prognostic marker that can be easily obtained from a standard complete blood count.
NLR is the equilibrium between innate (neutrophils) and adaptive (lymphocytes) immunity. Increased levels of NLR reflect increased systemic inflammation and impaired immune regulation. Neutrophils are involved in acute inflammation and oxidative injury, whereas lymphocytes are involved in immunosurveillance and regulation. Increased NLR reflects ongoing inflammation accelerating plaque instability and endothelial dysfunction which can lead to CVD and mortality in CKD and PD. The NLR value has also been strongly associated with both inflammation and mortality and can serve as a predictor of mortality in patients undergoing dialysis[7-9]. Researchers are finding its uses in several disorders, including kidney and CVDs, because of its easy acquisition method, low cost, and broad acceptance[10,11]. High NLR values have been linked in studies with poor prognosis in most chronic diseases, such as CVDs, cancers, liver cirrhosis, autoimmune diseases, and sepsis.
Whereas the prognostic utility of NLR has been extensively investigated, its role in predicting ACM and CVM in PD patients is unclear. Thus, this systematic review and meta-analysis seek to assess the link between increased NLR and mortality in patients with PD and its role as a low-cost, early prognostic indicator in this high-risk patient population.
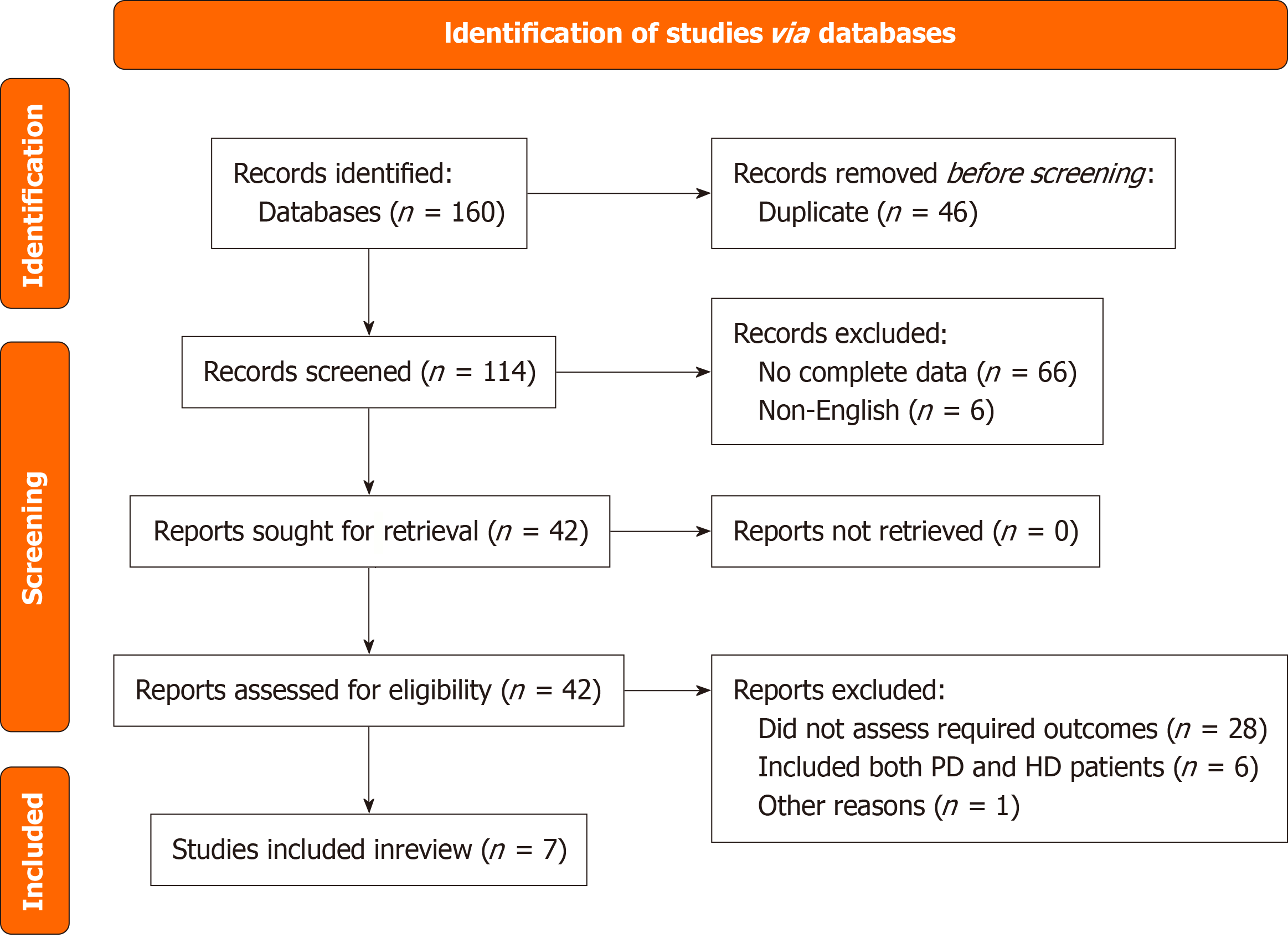
We conducted a comprehensive literature search to examine the association between the NLR with CVM and ACM in patients with ESRD undergoing PD. We only selected articles published between January 1, 2011, and June 30, 2024 using PubMed/MEDLINE, Google Scholar, and Scopus databases. Studies prior to 2011 were not relevant to our topic and lacked the required information on PD. MeSH terms that we have utilized were "peritoneal dialysis", "dialysis", "neutrophil-lymphocyte ratio", "mortality", "cardiovascular death", "prognosis", "risk", "death", "died", "cardiac arrest", and relevant articles and their full texts were collected for further assessment. We have created a Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) chart that depicts the flow of inclusion and exclusion of articles with reasoning (Figure 1). A detailed search criterion has been added in the Supplementary material. We iden
Articles retrieved from the search strategy were imported to the Rayyan, and duplicates were removed: (1) Title and abstract screening was done by two independent reviewers (Damarlapally N and Vempati R); and (2) Any disparity was ruled out by a third reviewer (Mourad D). In our study, we included observational studies, both prospective and retrospective, where the study population included adults with ESRD and undergoing chronic PD and reported NLR along with its association with outcomes in the form of hazard ratio (HR). We have excluded those studies where the study population is of a pediatric age group, not undergoing PD, animal studies, review articles, and those that reported no association with the desired outcomes.
A Microsoft Excel sheet was utilized to extract the data points from the studies, including demographics, study characteristics, and the NLR's predictive validity on outcomes through area under the receiver operating characteristic curve, odds ratio, and HRs on the primary outcomes, such as ACM or CVM. These studies were assessed for quality through the Joanna Briggs Institute (JBI)[12] quality appraisal tool (Table 1)[13-19].
| Criteria | An et al[19] | Liu et al[18] | Zeng et al[17] | Zhang et al[16] | Xu et al[15] | Lau et al[14] | Liu[13] |
| Similar groups from the same population | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Exposures measured similarly | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Exposure is measured validly and reliably | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Confounding factors identified | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Strategies to deal with confounding factors | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Groups are free of outcome at the start | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Outcomes are measured validly and reliably | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Follow-up time reported and sufficient | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Follow up complete or reason for loss described | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Strategies to address incomplete follow-up | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Appropriate statistical analysis | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall appraisal | Include | Include | Include | Include | Include | Include | Include |
We performed all the analysis using Comprehensive Meta-analysis software version 4. Our study pooled the unadjusted and adjusted HR (aHR) by employing a random effects model with a 95%CI, considering assessed heterogeneity greater than 75%. Forest plots were utilized to visualize the pooled HRs. In addition, the I2 statistic was applied to look for the heterogeneity in the outcomes of interest, ACM, and CVM; mild heterogeneity was considered for I2 values between 25%-50%, and moderate heterogeneity for the I2 values between 50%-75%, and severe heterogeneity for the values greater than 75%. Furthermore, a leave-one-out sensitivity analysis was performed to identify each study's influence on the overall effect size estimate. On top of that, we have performed univariate and multivariate meta-regression (MMR) analysis to identify the confounding effect of other variables on ACM and CVM. Egger’s regression and funnel plots were utilized to evaluate publication bias.
Following a keen search through three databases, PubMed, Google Scholar, and Scopus, we found 160 articles related to our topic of interest. After a review of their respective abstracts and titles with the elimination of 46 duplicates, 114 articles were selected for full-text screening. The screening method included a detailed examination according to specified conditions for inclusion and exclusion, including the research population's relevance, the measured outcomes, and enough information required for data analysis. Following a detailed manual screening process, 72 publications were rejected for several reasons, including the fact that they were published in languages other than English, that their conclusions and target audiences did not match the objectives of our study, or that the data was insufficient. Forty-two of the 114 articles were chosen for full-text review. Each article’s methodological diversity and relevance to the outcomes of interest (ACM and CVM) was evaluated to ensure broad representation and minimize selection bias. Seven articles (4 retrospective and 3 prospective studies) from 2011 to 2024 that met our acceptance and rejection criteria were finally chosen for analysis. When aHR were unavailable, we excluded such studies from pooled adjusted analyses and did not perform imputation. These studies were instead included in narrative or sensitivity analyses. These studies covered a broader spectrum of demographics and provided valuable data that increased the article's overall conclusions. The article selection process is shown in the PRISMA diagram (Figure 1).
For our meta-analysis, we included seven studies[13-15] containing 4350 patients receiving PD[16-19]. The characteristics of populations in these studies differed: Mean ages ranged from 47.4 years ± 15.6 years to 59 years ± 12.3 years. The variation in age distributions reflects the different populations of patients under study and correlates with various stages of chronic renal disease and its related complications. The studies also differed in sample size, with the most extensive having 1778 individuals and the least having 138. Despite the fact that the sample sizes differed, each study made an important addition to the examination of the connection between mortality outcomes and NLR. The studies accurately represented the patient populations by incorporating a wider range of demographic and clinical details (Tables 2 and 3)[13-19].
| Ref. | Study type | Country | Duration in years (n) | Follow up in years (n) | Sample size (n) | Age in years (mean ± SD) | Males | Body mass index (kg/m2) (mean ± SD) |
| An et al[19] | P | China | 2 | 4 | 138 | 53 ± 17 | 81 (58.7) | 21.9 ± 2.7 |
| Liu et al[18] | R | China | 7 | 9 | 1778 | 47.4 ± 15.6 | 1058 (59.5) | 21.5 ± 3.0 |
| Zeng et al[17] | R | China | 7.5 | 3 | 1502 | 51 ± 15.5a | 852 (56.7)a | 22.1 ± 3.04 |
| Zhang et al[16] | P | China | 5.4 | 2.4 | 140 | 54.7 ± 15.7a | 84 (57.1) | 23.47 ± 3.7 |
| Xu et al[15] | P | China | 15 | N/A | 378 | N/A | N/A | N/A |
| Lau et al[14] | R | China | 2 | 1 | 225 | 59 ± 12.3 | 129 (57.3) | N/A |
| Liu[13] | R | China | 1 | N/A | 189 | N/A | N/A | N/A |
| Ref. | Serum albumin (g/dL) (mean ± SD) | Absolute neutrophil (109/L) (mean ± SD) | Absolute lymphocytes (109/L) (mean ± SD) | Leukocytes (109/L) (mean ± SD) | Neutrophil-to-lymphocyte ratio (mean ± SD) | Hemoglobin (g/dL) (mean ± SD) | C-reactive protein (mg/dL) (mean ± SD) | Cholesterol (mg/dL) (mean ± SD) | Diabetes mellitus |
| An et al[19] | 3.9 ± 0.5 | 5.0 ± 1.7a | 1.5 ± 0.5a | N/A | 3.33 ± 3.4 | 100.2 ± 20.3 | 1.3 ± 0.9a | 216.6 ± 58 | 33 (23.9) |
| Liu et al[18] | 3.7 ± 0.5 | N/A | N/A | 6.6 ± 0.52 | 2.87 ± 0.3 | 101 ± 21 | N/A | 189 ± 11 | 449 (25.3) |
| Zeng et al[17] | 3.5 ± 0.5a | 4.1 ± 1.6a | 1.2 ± 0.52a | 6.1 ± 2.0a | 3.3 ± 1.5a | 8.7 ± 1.9a | N/A | 162.4 ± 42.9 | 342 (22.8) |
| Zhang et al[16] | 3.5 ± 5 | 4.4 ± 2.1a | 1.4 ± 0.6a | 6.5 ± 2.4a | 2.87 ± 0.4 | 8.8 ± 2.2 | 3.15 ± 1.7 | 162.02 ± 44.9 | 31 (22) |
| Xu et al[15] | 3 ± 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lau et al[14] | 3.5 ± 0.4 | 4.6 ± 2.0a | 1.1 ± 0.4a | 6.6 ± 2.2a | 3.9 ± 3.06a | 8.9 ± 1.3 | 0.41 ± 0.3 | N/A | 115 (51.1) |
| Liu[13] | 3 ± 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Through the JBI Academic Analysis Tool, we measured multiple components of study design, actions, and reports, and the quality of the included studies (Table 1)[13-19]. Most of the studies were of excellent quality, with few chances of bias in areas like result reporting, data collecting, and patient selection. Some studies showed possible low biases in data selection, addressing the missing data, and adjusting for possible errors in confounding factors. All seven studies were incorporated into the meta-analysis irrespective of those concerns because the overall risk of inclusion was considered to be acceptable.
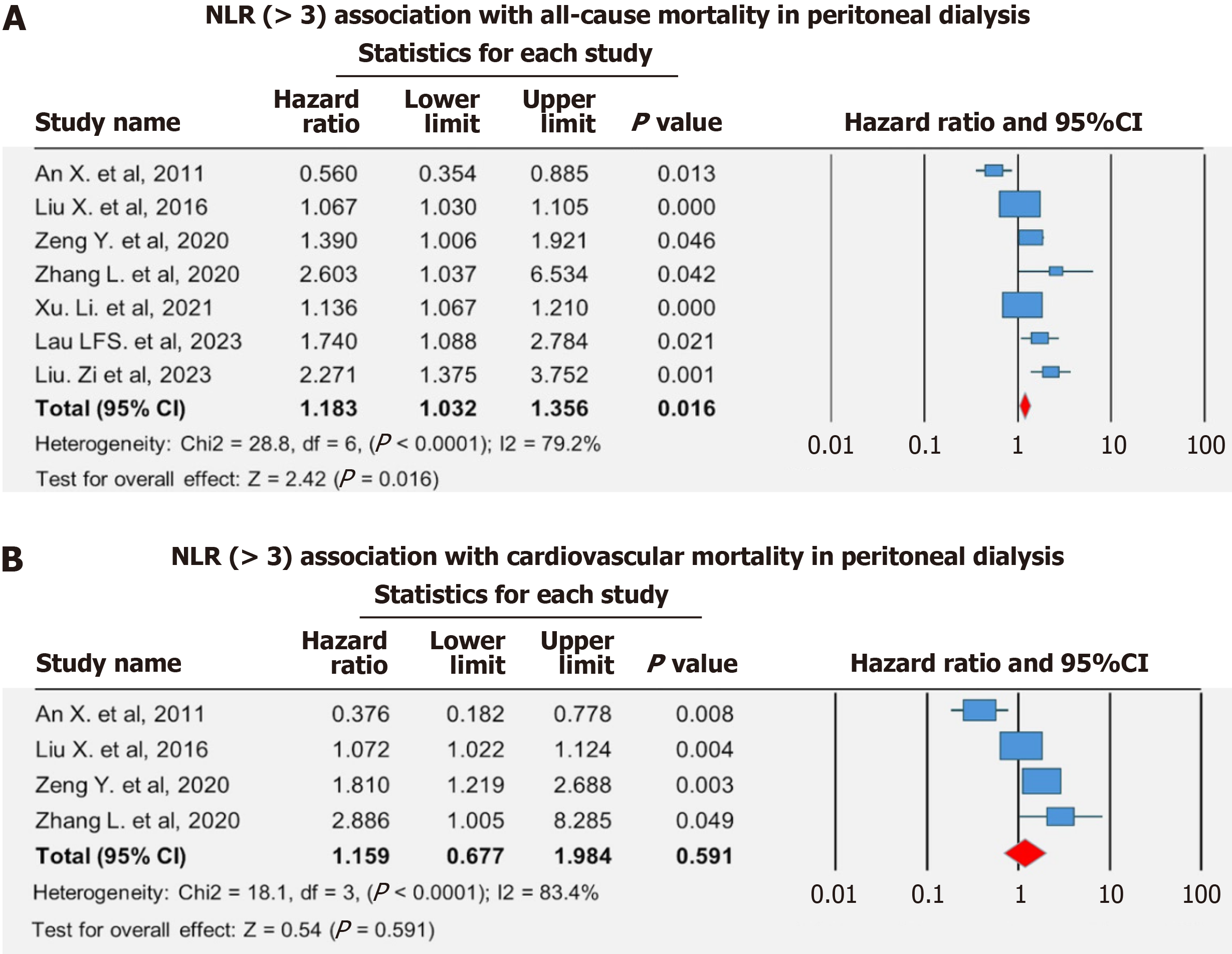
Our study provides substantial proof that a high NLR is a strong and independent marker of ACM and CVM in individuals undergoing PD. Meanwhile, the specific aHR and confidence intervals were not uniformly available among the individual studies due to reporting differences, making it challenging to determine the exact strength of the association. Apart from variations in the specific data collection and analyses across various individual studies, a statistically significant relationship was found across ACM outcomes (Figure 2)[13-19].
ACM: One of the primary focuses of our research was to know the association between NLR and ACM. Higher NLR levels were associated with a higher risk of ACM. Our analysis reported a pooled adjusted hazards ratio of 1.18 (95%CI: 1.03-1.36, P = 0.016) for the association between NLR > 3 and ACM outcome. This result was consistent across multiple patient populations, showing that NLR may be a useful prognostic biomarker for PD patients. Our subgroup analysis revealed that retrospective studies, those with sample sizes ≥ 350, studies with male proportion ≥ 59%, diabetes prevalence ≥ 32%, and serum albumin levels < 3.5 were significantly associated with an increased risk of ACM (Table 4).
| Variables | Number of studies | Number of patients | Adjusted hazard ratio (95%CI) | P value | I2 |
| Study design | |||||
| Prospective | 3 | 656 | 1.06 (0.58-1.97) | 0.84 | 83.6 |
| Retrospective | 3 | 2129 | 1.10 (1.00-1.21) | 0.047 | 77 |
| Sample size | |||||
| < 350 | 4 | 692 | 1.27 (0.74-2.15) | 0.38 | 84.8 |
| ≥ 350 | 3 | 3658 | 1.11 (1.03-1.19) | 0.004 | 61.3 |
| Males | |||||
| < 59 | 2 | 1640 | 0.90 (0.37-2.18) | 0.81 | 90.1 |
| ≥ 59 | 3 | 2143 | 1.08 (1.02-1.14) | 0.01 | 45.8 |
| Diabetes | |||||
| < 32 | 3 | 1780 | 1.18 (0.55-2.56) | 0.67 | 85.4 |
| ≥ 32 | 2 | 2003 | 1.07 (1.04-1.10) | < 0.0001 | < 0.0001 |
| Neutrophil-to-lymphocyte ratio (109/L) | |||||
| 2.87-3.2 | 2 | 1918 | 1.47 (0.64-3.41) | 0.37 | 72.2 |
| 3.3-3.89 | 3 | 1865 | 0.99 (0.69-1.42) | 0.96 | 80.4 |
| Serum albumin (g/dL) | |||||
| < 3.5 | 3 | 2069 | 1.42 (1.01-1.99) | 0.04 | 76.4 |
| ≥ 3.5 | 4 | 2281 | 1.05 (0.95-1.17) | 0.32 | 73.6 |
CVM: Our other primary outcome included the association of CVM with NLR in the same group of PD patients. An increased risk of cardiovascular death was also significantly associated with greater NLR levels, much like ACM. Our study revealed that NLR > 3 was not associated with a greater risk of developing CVM than those with the lower levels, with a pooled aHR of 1.16 (95%CI: 0.68-1.98, P = 0.59) in patients following PD (Figure 2). NLR may serve as a useful adjunctive biomarker for cardiovascular risk stratification in patients undergoing PD.
Leave-one-out sensitivity analyses were performed to test the strength of the meta-analysis findings. This analysis includes estimating the change in the overall results by removing each study, one at a time. Since we found no significant effect on the overall findings, the conclusions are stable and not dependent on one study for both ACM and CVM. There was no further statistically significant association of higher CVM risk with NLR > 3 even on one-study removal method as analyzed on the primary analysis. This outcome supports the meta-analysis's conclusions, although the included studies were heterogeneous (Supplementary Figure 1)[13-19].
Univariate meta-regression did not yield a significant association with age (P = 0.41) or male gender (P = 0.94) with ACM. However, MMR analysis showed a significant association between age and ACM outcomes (coefficient: 0.14, 95%CI: 0.001-0.2905, P = 0.04) which signifies that increasing age can be linked to increased risk of developing ACM and caution must be employed while analyzing NLR in the elderly (Supplementary Figure 2).
There was absence of publication bias on studies with NLR > 3 outcomes in ACM and CVM as illustrated in Supple
As this systematic review was based on previously published studies, no ethical approval was required. All included studies were assumed to have obtained appropriate ethical approval.
NLR has become a novel marker with substantial predictive value in recent years[8]. In this meta-analysis, we combined data from seven studies to evaluate the significance of NLR in patients undergoing PD. The results in our study and the reported HR across the included studies provide evidence of a significant increase in ACM and CVM risk for patients with elevated NLR.
The results of this study align with those from recent research. Previous studies have reported similar associations between NLR and outcomes[20,21]. Zeng et al[17] reported a significant association between NLR and CVM, particularly in the younger PD population aged < 60 years. Aging is linked to a decline in immune function, leading to reduced responsiveness of immune cells to cytokine signals. Studies indicate this diminished signaling occurs regardless of the stimulus dose and is consistently seen in older individuals which possibly explains the greater correlation in younger PD population[17]. The possible explanations behind these associations, NLR and ACM, and NLR and CVM, could be that CKD patients have an ongoing inflammatory state[22] and immune dysregulation[23], leading to a higher NLR. An elevated NLR may indicate an imbalance that weakens the body’s ability to fight infections, contributing to higher ACM[7]. Additionally, chronic inflammation is known to increase the risks of heart disease and can lead to CVM. According to Valdivielso et al[24], chronic inflammation, as indicated by a high NLR, accelerates the development of atherosclerosis and contributes to plaque instability and makes patients more prone to cardiovascular events, such as heart attacks and strokes, which are leading causes of death in PD patients[25].
Following MMR analysis, we found a significant relationship between age, an independent variable, and ACM out
NLR can be considered an impactful prognostic tool for PD patients. It is readily accessible from the peripheral blood and easily calculated as a ratio between neutrophils and lymphocytes. It engages two aspects of the immune system: (1) The innate system, represented by neutrophils; and (2) The adaptive system, represented by lymphocytes[27]. A higher neutrophil and lower lymphocyte count reflect a higher NLR value.
Neutrophils are the first line of defense of the human body and exert their function through the formation of pha
NLR is a straightforward and cost-effective test that has proven to be of important predictive and prognostic value in many chronic diseases, including CVD, tumors, liver diseases, autoimmune diseases, and vascular diseases[7]. In clinical practice, NLR could serve as a low-cost, easily accessible biomarker to identify high-risk PD patients who may benefit from closer monitoring or early intervention. Until standardized cut-offs are defined, clinicians might consider using relative increases in NLR (e.g., > 3-4 based on current evidence) as a flag for further evaluation of inflammatory status and potential cardiovascular risk management.
This is the first meta-analysis evaluating NLR as a prognostic value in PD patients. This review included diverse patient demographics, making the results generalizable to a wide array of PD patients. The quantitative analysis was also done as per guidelines, with the quality and sensitivity analysis testament to the quality and robustness of this review’s findings.
Our meta-analysis was based on a small number of studies due to the small number of eligible articles. Additionally, the nature of the studies included had inherent limitations. The studies lacked identification of a standard cutoff value for NLR to facilitate its usage and interpretation accurately, necessitating implementing more extensive multi-centric studies in PD patients. We found considerable heterogeneity in our study owing to the association of different populations, varying data collection methods, and comorbidities such as diabetes, hypertension, and atherosclerosis among the study sample. The chemical nature of dialysate fluids, catheter-related infections, varying duration of PD, and PD-related complications could also have influenced the heterogeneity. A major limitation is the variability in NLR cut-off values used across studies, ranging widely depending on population characteristics and study design. This inconsistency hinders the clinical applicability of NLR as a prognostic marker. Future research must aim to identify and validate a standardized threshold specific to PD populations to enhance practical utility. More extensive multi-center studies should be done focusing on the standardized cut-off values to achieve uniform results. Given the observational nature of the included studies, our findings should be interpreted as associations rather than causal relationships. Confounding factors cannot be fully excluded. Sources of heterogeneity likely include variations in dialysis duration, comorbidity burden, and geographic differences in healthcare practices. Although subgroup (especially in studies related to CVM) and sensitivity analyses were limited by available data, future meta-analyses should incorporate these factors to better elucidate sources of variability.
NLR, which depicts the imbalance between neutrophils and lymphocyte counts, can indicate inflammation and poor immune function, both associated with cardiovascular risk and mortality. Conditions such as diabetes, malnutrition, dyslipidemia, hypertension, and chronic systemic inflammation, which influence atherosclerosis pathogenesis, are closely associated with NLR in patients undergoing PD containing pro-inflammatory dialysate solution. A high NLR ratio can be used as a prognostic indicator to detect these cardiovascular-risk patients, enable more effective strategies, and prevent disease progression. Further research and clinical trials are needed to substantiate its use in clinical practice. NLR can be a cost-effective and non-invasive biomarker to assess prognosis and is particularly helpful in resource-limited settings.
Our meta-analysis found that a higher NLR was significantly associated with an increased risk of ACM and CVM with PD. These results suggest that NLR can be used as a prognostic tool to prevent early morbidity and mortality in this subset of patients. Additional research is required to confirm its role as an early indicator to prevent the progression of CKD to ESRD. Due to the limitations encountered in this review, we recommend that NLR values be used by employing a standardized methodology. Randomized controlled trials, preferably with large sample sizes and multi-centered, are highly recommended, as they can significantly reduce the effect of confounding factors on the relationship between NLR and ACM and establish further relationship with estimating CVM risks.
| 1. | United States Renal Data System. USRDS Annual Data Report: Epidemiology of kidney disease in the United States [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases. 2023. [cited 23 June 2025]. Available from: https://usrds-adr.niddk.nih.gov/2023/chronic-kidney-disease/1-ckd-in-the-general-population. |
| 2. | Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3261] [Cited by in RCA: 3197] [Article Influence: 199.8] [Reference Citation Analysis (0)] |
| 3. | Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, Koch V, McCulloch M, Numanoglu A, Nourse P, Pecoits-Filho R, Ponce D, Warady B, Yeates K, Finkelstein FO. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34:494-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 743] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 5. | Morelle J, Sow A, Fustin CA, Fillée C, Garcia-Lopez E, Lindholm B, Goffin E, Vandemaele F, Rippe B, Öberg CM, Devuyst O. Mechanisms of Crystalloid versus Colloid Osmosis across the Peritoneal Membrane. J Am Soc Nephrol. 2018;29:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Faraj SS, Jalal PJ. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: case-control study. Ann Med Surg (Lond). 2023;85:2291-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 7. | Yang N, Yang K, Pan S, He Q, Jin J. Progress in the application of the neutrophil-to-lymphocyte ratio in dialysis-related complications. Ren Fail. 2023;45:2259996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 9. | Chandra A, Raj G, Awasthi NP, Rao N, Srivastava D. Evaluation of the relationship between blood cell parameters and vascular calcification in dialysis-dependent end-stage renal disease patients. Saudi J Kidney Dis Transpl. 2020;31:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 11. | Xie T, Xin Q, Chen R, Zhang X, Zhang F, Ren H, Liu C, Zhang J. Clinical Value of Prognostic Nutritional Index and Neutrophil-to-Lymphocyte Ratio in Prediction of the Development of Sepsis-Induced Kidney Injury. Dis Markers. 2022;2022:1449758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Joanna Briggs Institute. Critical appraisal tools [Internet]. 2020. [cited 23 June 2025]. Available from: https://jbi.global/critical-appraisal-tools. |
| 13. | Liu ZC, Xu K, Bu HX, Dou XF. [Predictive value of prognostic nutritional index combined with NLR and PLR in all-cause mortality of patients with peritoneal dialysis]. Tianjjin Yiyao. 2023;5:290-294. [DOI] [Full Text] |
| 14. | Lau LFS, Ng JKC, Fung WWS, Chan GCK, Cheng PM, Chow KM, Leung CB, Li PK, Szeto CC. Relationship between Serial Serum Neutrophil-Lymphocyte Ratio, Cardiovascular Mortality, and All-Cause Mortality in Chinese Peritoneal Dialysis Patients. Kidney Blood Press Res. 2023;48:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Xu LC, Zhou FF, Li M, Dai ZW, Cai KD, Zhu BX, Luo Q. Predictive Value of Peripheral Blood Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio on Patient Survival with Peritoneal Dialysis. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Nie Y, Guo M, Wang L, Shi Y, Jiang X, Ding X, Xu X, Ji J. Neutrophil to Lymphocyte Ratio as a Predictor of Long-Term Outcome in Peritoneal Dialysis Patients: A 5-Year Cohort Study. Blood Purif. 2021;50:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Zeng Y, Chen Z, Chen Q, Zhan X, Long H, Peng F, Zhang F, Feng X, Zhou Q, Liu L, Peng X; Evergreen Tree Nephrology Association; Guo G, Zhang Y, Wang Z, Wen Y, Li J, Liang J. Neutrophil to Lymphocyte Ratio Predicts Adverse Cardiovascular Outcome in Peritoneal Dialysis Patients Younger than 60 Years Old. Mediators Inflamm. 2020;2020:4634736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Liu X, Huang R, Wu H, Wu J, Wang J, Yu X, Yang X. Patient characteristics and risk factors of early and late death in incident peritoneal dialysis patients. Sci Rep. 2016;6:32359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | An X, Mao HP, Wei X, Chen JH, Yang X, Li ZB, Yu XQ, Li ZJ. Elevated neutrophil to lymphocyte ratio predicts overall and cardiovascular mortality in maintenance peritoneal dialysis patients. Int Urol Nephrol. 2012;44:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Zhao WM, Tao SM, Liu GL. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2020;42:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Ao G, Wang Y, Qi X, Wang F, Wen H. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: a meta-analysis. Clin Exp Nephrol. 2021;25:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, Warady BA. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int Rep. 2021;6:1775-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 23. | Lee TH, Chen JJ, Wu CY, Lin TY, Hung SC, Yang HY. Immunosenescence, gut dysbiosis, and chronic kidney disease: Interplay and implications for clinical management. Biomed J. 2024;47:100638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Valdivielso JM, Rodríguez-Puyol D, Pascual J, Barrios C, Bermúdez-López M, Sánchez-Niño MD, Pérez-Fernández M, Ortiz A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler Thromb Vasc Biol. 2019;39:1938-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1312] [Article Influence: 262.4] [Reference Citation Analysis (2)] |
| 26. | Cheng L, Hu N, Song D, Chen Y. Mortality of Peritoneal Dialysis versus Hemodialysis in Older Adults: An Updated Systematic Review and Meta-Analysis. Gerontology. 2024;70:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int J Mol Sci. 2022;23:3636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 621] [Article Influence: 155.3] [Reference Citation Analysis (1)] |
| 28. | Usman NUB, Winson T, Basu Roy P, Tejani VN, Dhillon SS, Damarlapally N, Panjiyar BK. The Impact of Statin Therapy on Cardiovascular Outcomes in Patients With Diabetes: A Systematic Review. Cureus. 2023;15:e47294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Sampath S, Damarlapally N, Vasudevan SS, Bareja S, Mehta K, Jayachandran Y, Ashfaque M, Arote DV, Nawab S, Srivastava A, Desai R. Association of adverse cardiovascular outcomes with high sensitivity cardiac troponins in stable coronary artery disease patients. J Am Coll Cardiol. 2024;83:904. [DOI] [Full Text] |
| 30. | Berliner N. Leukocytosis and Leukopenia. In: Goldman L, Schafer AI, editors. Goldman's Cecil Medicine. Netherlands: Elsevier, 2012. |
| 31. | Romejko K, Markowska M, Niemczyk S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int J Mol Sci. 2023;24:10470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 32. | Guo M, Wang Z, Yang R, Liu K, Zeng J, An T. High neutrophil/lymphocyte ratio and low lymphocyte percentage are independent risk factors for new-onset CKD. Clin Immunol Commun. 2022;2:165-171. [DOI] [Full Text] |
| 33. | Xiong J, Qiao Y, Yu Z, Huang Y, Yang K, He T, Zhao J. T-Lymphocyte Subsets Alteration, Infection and Renal Outcome in Advanced Chronic Kidney Disease. Front Med (Lausanne). 2021;8:742419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Hartzell S, Bin S, Cantarelli C, Haverly M, Manrique J, Angeletti A, Manna G, Murphy B, Zhang W, Levitsky J, Gallon L, Yu SM, Cravedi P. Kidney Failure Associates With T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front Immunol. 2020;11:583702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/