Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.99785
Revised: March 24, 2025
Accepted: April 8, 2025
Published online: December 20, 2025
Processing time: 370 Days and 5.5 Hours
Irritable bowel syndrome (IBS) is a prevalent functional gastrointestinal (FGITD) disorder, the diagnosis is based on Rome Criteria and other subjective tools. Because IBS overlaps with other FGITD and organic diseases, and the subjective tools do not apply to patients with cognitive decline, objective diagnostic tools are important in this category of patients.
To discuss the role of imaging in IBS diagnosis.
We systematically searched three databases for articles published in the English language with no limitation to a specific period. The literature search was con
Magnetic resonance imaging is superior due to its sensitivity, lack of radiation exposure, and lack of need for bowel preparation. Patients with IBS had smaller colonic and rectal volumes compared to healthy controls and functional consti
Magnetic resonance imaging shows smaller colonic and rectal volumes, and increased activity, thinning, and increased volumes in specific areas of pain modulation. Large trials incorporating all above limitations are needed.
Core Tip: Irritable bowel syndrome (IBS) is a prevalent brain-gut axis disorder, however, the diagnosis is based mainly on Rome Criteria which concentrates on the gastrointestinal tract with ignorance of the brain-gut axis. Furthermore, the Rome criteria are not suitable for patients with cognitive decline. Therefore, tools to address the above diagnostic gaps are highly relevant. In this review, we gave a broader insight into the role of abdominal and brain imaging in diagnosing IBS. The findings, the difference between diarrhea-predominant and constipation-predominant IBS, gender, geography, and cultural background, and the pros and cons of different imaging were discussed.
- Citation: Mirghani HO. Imaging findings of irritable bowel syndrome patients, and the diagnostic value of irritable bowel syndrome: A systematic review. World J Methodol 2025; 15(4): 99785
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/99785.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.99785
Irritable bowel syndrome (IBS) is among the most prevalent disorders of gut-brain cross-talk. The diagnosis of IBS is based largely on the Rome Criteria which are subjective and were developed in the Western countries. Therefore, they cannot be generalized to other parts of the world with different cultures[1].
Due to its chronic nature, its mental and organic impact, and the high prevalence, IBS socioeconomic burden is high, and its effects on the patient's quality of life are significant. In addition, the issue of IBS is dynamic and the understanding of the syndrome is rapidly changing because of the recent advances in relevant clinical research[2]. The prevalence of IBS varies significantly and depends on the methods of diagnosis, Geography, and culture. The Rome Foundation Global Study reported a prevalence of 10.1% and 3.8% when using Rome III, and Rome IV respectively. Importantly, the change in the dynasty of symptoms, the suggestion by the Rome IV Criteria to include the gut-brain interaction, and the overlap with other functional gastrointestinal syndromes have encouraged research in this field[2-4].
ROME 1V Criteria are more restrictive, less likely to diagnose unspecific symptoms, and milder for IBS. Therefore, they are more helpful in tertiary care centers, unlike the ROME III Criteria. Other limitations of the ROME Criteria are overlaps with organic diseases including chronic overlapping pain conditions[5], inflammatory bowel disease[6], and endometriosis, which are concerning[7]. In addition, some nations are less likely to express pain (Asians) bloating/distension. Therefore, IBS could be underestimated[8]. Similarly, the distorted body image and self-criticism created by IBS were thought to mediate the association between IBS, anxiety, and depression[9].
IBS encompasses a wider spectrum of gastrointestinal, extraintestinal, and psychological comorbidities. Because of this, other methods incorporating features other than stool consistency and forms are needed[10].
IBS is a chronic burdensome disorder that is usually misdiagnosed as another condition leading to delayed diagnosis and treatment. The delayed diagnosis of IBS substantially disturbs the quality of life of patients and increases the healthcare cost[11]. Positive diagnostic methods rather than the reliance on exclusion and ROME Criteria are highly needed for a better understanding of the disease and the introduction of multimodal treatment[12].
Because of the established association between IBS, psychological distress, and organic disease, imaging is justifiable in selected patients. Likewise, IBS patients ≥ 50 years old are at an increasing risk of dementia[13], and Rome Criteria might not be applicable among patients with cognitive decline. On the other hand, The United States, and United Kingdom suggested no radiological imaging for IBS diagnosis unless red flags are present. The radiological procedure depends on the most likely alternative diagnosis[14] because evidence shows that colonoscopy, barium enema, and ultrasound yield is low regarding structural changes (diverticulosis, inflammatory bowel disease, and colorectal cancer)[15]. Likewise, psychological stress could affect gut function and brain visceral sensitivity which is the case of IBS[16]. Functional imaging including magnetic resonance imaging (fMRI) and positron emission tomography (PET) significantly advanced our knowledge about gut-brain interaction in particular among patients with IBS. An interesting study used functional near-infrared spectroscopy to assess brain activity among patients with functional dyspepsia, IBS, and healthy control subjects in response to food images. The authors concluded that there was higher prefrontal cortex activity among patients with functional dyspepsia than among their IBS counterparts[17]. Therefore, imaging including abdominal ultrasound, computed tomography (CT), and MRI (abdominal or brain diffusion) could be justifiable in selected patients with cognitive decline and alarm features, in contrast to the subjective Rome Criteria. The current review aimed to discuss the role of imaging in IBS diagnosis.
The current diagnosis of IBS with the ROME Criteria with the reliance on abdominal pain, stool form, and consistency has several limitations because several nations are less likely to express pain but fullness/bloating. In addition, IBS is a wider disease with internal and extra-intestinal symptoms and a great overlap with psychological disorders. Furthermore, ROME Criteria are not working among patients with cognitive decline.
This review was conducted in June and July 2024 with strict adherence to the PRISMA Guidelines to fulfill the objectives which are the role of imaging including ultrasound, CT, and MRI in IBS diagnosis.
The inclusion criteria were Cross-sectional studies, retrospective and prospective studies, randomized trials, and case-control studies conducted on humans were approached. The studies were included if they used abdominal ultrasound, CT, and MRI to diagnose IBS. In addition, brain MRI, structural, and function among patients with IBS were approached.
Case studies, opinions, editorials, and protocols without data were not. Barium studies, radionuclide studies, and colonoscopies were not included. Brain imaging was confined to MRI, therefore, CT brain with its different modalities was also excluded.
The outcome measures were colonic diameters and transit time, and the static and dynamic changes of the brain MRI.
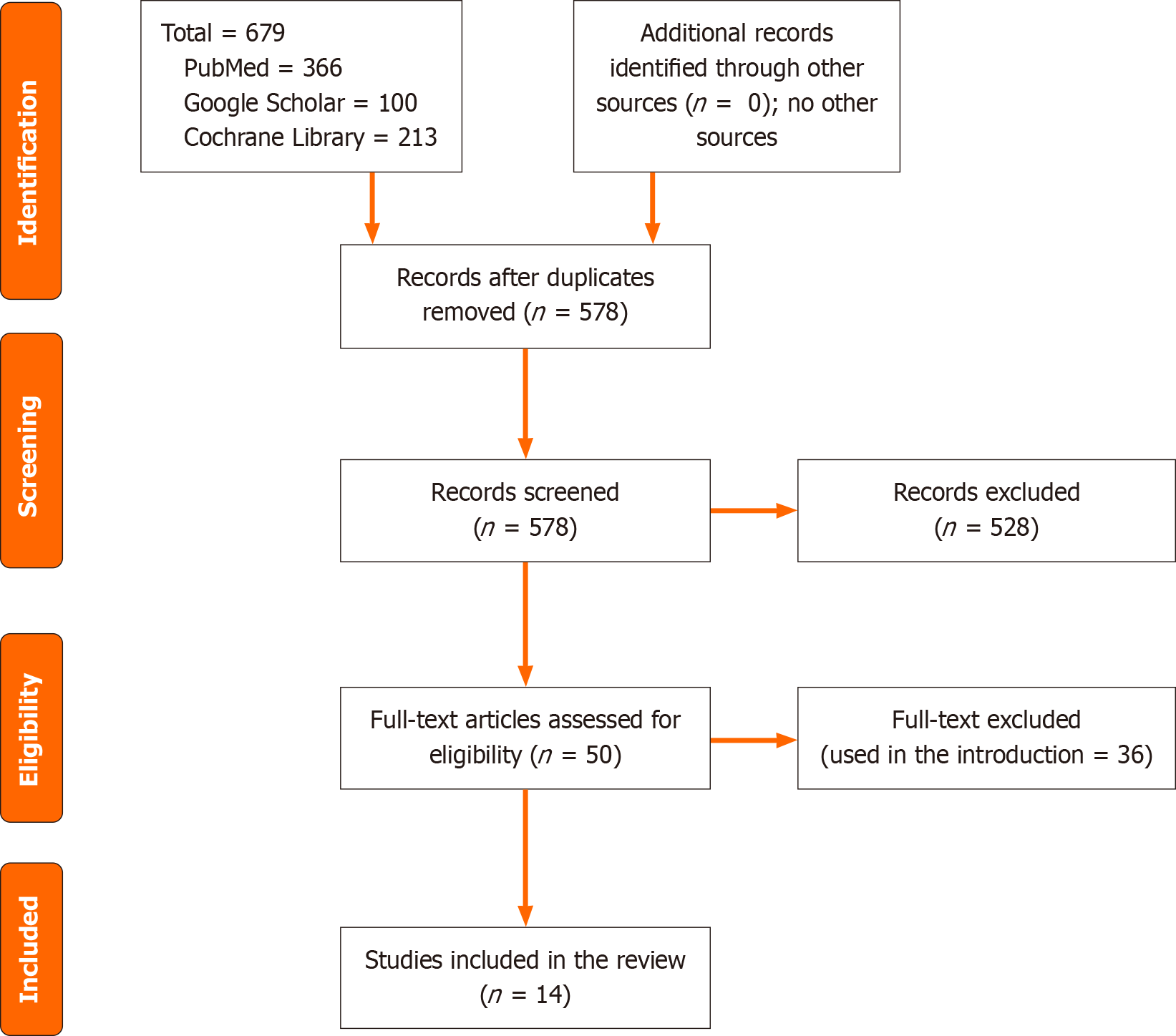
We searched PubMed/MEDLINE, Google Scholar, and Cochrane Library for articles assessing the role of imaging as a diagnostic measure for IBS. The literature search was limited to the articles published in the English language with no limitation to a specific period. The keywords IBS and functional bowel disorders, CT, MRI, functional brain MRI, and static brain MRI were used and linked with the terms "AND" and "OR". The total hits in the above databases were 679 articles, and 578 remained after duplication removal. In the current review, 50 full texts were screened and used in the introduction and article text (Figure 1).
An Excel sheet was used to extract the author's name, year and country of publication, type of study, age, gender, number of participants, and the main results of the imaging procedure (Table 1).
| Ref. | Country | Study design | Sample size | Age/years | Females, % | Main result |
| Klinge et al[19], 2022 | Denmark | Case-control | 20 | 25-53 | 76% | Low colonic volume and rapid transit time among IBS patients compared to chronic constipation |
| Lam et al[20], 2016 | United Kingdom | Case-control | 48 | 27-53 | 91.3% | Higher ascending colon volume in functional constipation compared to IBS patients |
| Pritchard et al[21], 2014 | United Kingdom | Case-control | 50 | 39 ± 13 | 58.7% | Less ascending colon postprandial volume and high descending colon volume among IBS compared to healthy controls |
| Di Palma et al[28], 2011 | Italy | Case-control | 40 | 31 ± 7 | 66.7% | Rectal volume was smaller among IBS patients |
| Nilsson et al[34], 2015 | Denmark | Cross-sectional | 25 | Not mentioned | All males | No inter-individual and intra-individual variability in colonic volume MRI |
| Grinsvall et al[38], 2021 | Sweden | Case-control | 354 | 27.9 ± 9.9 | All females | Low grey matter volume in the posterior insula and superior frontal gyrus with high grey matter volume and cortical thickness in the somatosensory cortex and subcortical regions |
| Blankstein et al[39], 2010 | Canada | Case-control | 27 | 30.85 ± 8.9 | All females | Increased gray matter in hypothalamus among patients with IBS, and anterior midcingulate cortical thinning |
| Chua et al[40], 2017 | Taiwan | Case-control | 69 | 36.7 ± 7.7 | All females | Supramarginal cortex, middle frontal cortex, and left cuneus showed cortical thinning in IBS patients with a negative correlation with the disease severity |
| Jiang et al[42], 2013 | United States | Case-control | 266 | 34.72 ± 11.12 | 84.6% | No difference in cortical thickness between IBS patients and control subjects. Cortical thickness decreases in the bilateral subgenual anterior cingulate cortex, and increases in the somatosensory and primary motor cortex among women with IBS |
| Mao et al[43], 2020 | China | Cross-sectional | 68 | Not mentioned | Diarrhhea predominant subjects | Enlarged caudate nucleus and thalamus, and altered hemispheric asymmetries among diarrhea-predominant IBS patients |
| Chen et al[44], 2011 | Canada | Cross-sectional | 26 | 20-54 | All females | Mean fractional anisotropy was observed in IBS patients in the fornix and external capsules |
| Zhao et al[45], 2018 | China | Case-control | 30 | 77.5 ± 6.3 | 20% | Gray matter volume is low in occipital and frontal lobes, limbic and hippocampus, and high in angular and temporal gyrus, and insula |
| Icenhour et al[50], 2017 | Sweden | Case-control | 61 | 32-36 | All females | Resting-state functional connectivity is reduced in the amygdala and insula in IBS patients |
| Guleria et al[51], 2017 | India | Case-control | 20 | 30.5 | All males | Homeostatic emotions, autonomic responses to pain, and affective motivation differ in IBS patients compared to controls |
Risk of bias assessment: We used the Newcastle Ottawa Scale to assess the quality of the included studies[18] (Table 2).
| Ref. | Selection bias | Comparability bias | Outcome | Total score |
| Klinge et al[19], 2022 | 3 | 1 | 3 | 7 |
| Lam et al[20], 2016 | 4 | 2 | 3 | 9 |
| Pritchard et al[21], 2014 | 3 | 1 | 3 | 7 |
| Di Palma et al[28], 2011 | 3 | 2 | 2 | 7 |
| Nilsson et al[34], 2015 | 3 | 2 | 2 | 7 |
| Grinsvall et al[38], 2021 | 4 | 2 | 3 | 9 |
| Blankstein et al[39], 2010 | 3 | 2 | 3 | 8 |
| Chua et al[40], 2017 | 3 | 2 | 3 | 8 |
| Jiang et al[42], 2013 | 3 | 2 | 2 | 7 |
| Mao et al[43], 2020 | 3 | 2 | 3 | 8 |
| Chen et al[44], 2011 | 3 | 2 | 2 | 7 |
| Zhao et al[45], 2018 | 3 | 2 | 3 | 8 |
| Icenhour et al[50], 2017 | 3 | 2 | 3 | 8 |
| Guleria et al[51], 2017 | 3 | 2 | 2 | 7 |
The current review results were based on 14 studies, 7 studies were from Europe, 4 from Asia, two were published in Canada, and one from the United States. The studies included 1104 participants, and the majority were females. All the studies were observational studies (11 case-control and 3 were cross-sectional) with good quality (Figure 1, Tables 1 and 2).
Colonic volume and transit time: MRI showed low colonic volume and rapid transit time among patients with IBS compared to chronic constipation and healthy control subjects[19] supporting a previous study conducted by Lam et al[20] who found higher ascending colon volume in functional constipation. More studies confirmed the above findings and attributed the findings to increased sensitivity to colon distention in patients with IBS because both constipation and diarrheal IBS had increased rectal sensitivity and less call for stool[21-24]. Importantly, there is substantial intra and inter-individual variation that should be controlled for before reaching a firm conclusion[25]. In addition, the colonic volume changes are not uniform through IBS spectra, Pritchard et al[21] observed a rise in ascending colon volume and a decrease in the descending colon following a meal in constipation-predominant IBS and healthy subjects suggesting a retrograde movement from the left colon[26], the reverse happen later. By contrast, the retrograde movement is not observed in diarrheal-predominant and mixed IBS[27]. Similarly, MRI showed smaller rectal volume among patients with IBS suggesting an increased tone in the resting state[28].
The current evidence of low colonic volume among patients with IBS is promising for the classification of colonic disorders. Future research to investigate whether patients with larger colonic diameters respond better to prokinetics or laxatives. MRI studies could help further the classification of patients and treatment guidance[27,28]. In addition, the separation of the colonic content (gas, fluid, or solid) using postprocessing of MRI could be helpful for the individualization of therapy (drug/diet)[29]. However, the current evidence was based on observational studies with female predominance which are more prone to bias. In addition, the MRI is not cost-effective and is not available in the primary care setting.
Regarding the colonic transit time, less transit time was reported among IBS compared to functional constipation, however, the study was performed after laxative stimulation and not under normal conditions[20]. A previous study[30] used radiopaque markers and found low transit time among patients with IBS compared to patients with diarrhea and constipation. Radiopaque markers and colonic scintigraphy although useful, radiation exposure is a big limitation[31].
Other attractive imaging are ultrasound and CT which can provide structural evidence. Ultrasound studies on IBS patients showed increased gallbladder and colon contractility with impaired pyloric and stomach contraction and transit, hyperechoic rectum with acoustic shadow is characteristic[32]. However, ultrasound is operator–dependent and is not sensitive to colonic volume evaluation, and CT radiation exposure is a major limitation[33]. Therefore, MRI is more attractive in the diagnosis of IBS and functional constipation, because it is sensitive, no need for bowel preparation, and no radiation exposure (a big advantage since most IBS patients are females of reproductive age)[34].
The gut-brain interaction: The pathophysiology of IBS is complex where gastrointestinal tract (GIT) symptoms are induced by any combination of visceral hypersensitivity, motility disorders, disturbance in mucosa and immunity, and altered gut microbiota diversity and function[35]. Interoceptive signals originate from the GIT and memories for them are extensively modulated by cognition, emotion (depression and anxiety), and motivational factors. Therefore, the pathophysiology is wide and involves the GIT (gut connectome), wide areas of the brain (brain connectome), and input from the autonomic nervous system, and neuroendocrine[36]. The multifactorial nature of IBS makes the diagnosis challenging, there is a need for objective measures to address the brain static and dynamic change to assist the diagnosis, suggest future research, and guide IBS therapy.
Rectal nociception and MRI brain abnormalities: The gut-brain connections are not only important in food digestion, but also related to mood disorders, stress, altered bowel motility, and abdominal pain, because of that rectal inflation among patients with IBS activates the emotion arousal, attention, and visceral sensation areas of the brain[37].
Higher cortical thickness and grey matter volume in the somatosensory cortex and subcortical regions were found among women with IBS, the reverse was observed in the superior frontal gyrus and posterior insula[38]. Blankstein et al[39] used functional MRI and found high grey matter volume in the hypothalamus with thinning in the anterior midcingulate might be related to continuous stress and activation of the hypothalamic-pituitary-adrenal axis. Chronic abnormal input from the GIT or pre-existing brain abnormalities that predisposed to IBS could explain the findings. Importantly, Chua et al[40] confirmed the cortical thinning, but with a negative correlation with the duration and severity of abdominal pain. The contradiction in the above results could be explained by cultural and gender disparities in pain perception[41], people from the East are more tolerant of pain compared to their counterparts from the Western region and females tolerate pain more[42].
Enlarged caudate nucleus and thalamus were observed in diarrhea-predominant IBS with thalamic rightward asymmetries and leftward asymmetries in the caudate nucleus in contrast to the finding in the healthy controls[43]. Chen et al[44] conducted a study among IBS females and showed higher mean fractional anisotropy in the insula and fornix while matter compared to their counterparts without the syndrome. Zhao et al[45] conducted a study among elderly IBS patients, structural MRI, diffusion tensor imaging analysis, and Voxel-based morphometry studies supported by Blankstein et al[39] and Chen et al[44]. An important finding with clinical implication is the association between depression and similar cortical thinning observed in IBS, additionally, depression is prevalent among IBS patients[46,47]. Hence, some tricyclic antidepressants might be an effective therapy for IBS[48].
Brain resting state activity in IBS: The resting state brain activity among patients with IBS differs significantly from healthy volunteers, high activity resting state was observed in wide areas including the postcentral gyrus, frontal, and temporal lobe. In contrast, the cingulate and paracingulate gyrus showed decreased resting state activity. Importantly, resting-state functional connectivity is related to visceral sensitivity and symptom severity[49,50]. Interestingly, the brain activation is different in constipation and diarrheal-predominant IBS[51].
Only English literature should be included, and various screening criteria resulted in a small number of articles being included.
Patients with IBS had smaller colonic and rectal volumes compared to healthy controls and functional constipation. Dynamic and static MRI of the brain showed increased activity, thinning, and increased volumes in specific areas of pain modulation. High colonic diameter and decreased transit time detected by MRI are promising for the diagnosis of IBS due to its sensitivity, lack of radiation exposure, and lack of need for bowel preparation. Imaging could be beneficial for the diagnosis of IBS among patients with alarm symptoms and cognitive dysfunction because Rome Criteria are subjective and need patients' responses. Imaging results could direct response to specific IBS treatment (diet/medications). The above abnormalities are not uniform and vary significantly according to the type of IBS, duration, intensity of symptoms, gender, and culture. Because of that, high patient selection is needed. Large trials incorporating all IBS subtypes, both women and men and controlling for sociodemographic and cultural factors are needed.
We want to acknowledge the Saudi Digital Library for accessing the databases.
| 1. | Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 2. | Huang KY, Wang FY, Lv M, Ma XX, Tang XD, Lv L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J Gastroenterol. 2023;29:4120-4135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (18)] |
| 3. | Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99-114.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1381] [Article Influence: 276.2] [Reference Citation Analysis (0)] |
| 4. | Ray G, Ghoshal UC. Epidemiology of Disorders of the Gut-Brain Interaction: An Appraisal of the Rome IV Criteria and Beyond. Gut Liver. 2024;18:578-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Slade GD, Greenspan JD, Fillingim RB, Maixner W, Sharma S, Ohrbach R. Overlap of Five Chronic Pain Conditions: Temporomandibular Disorders, Headache, Back Pain, Irritable Bowel Syndrome, and Fibromyalgia. J Oral Facial Pain Headache. 2020;34:s15-s28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | DiVasta AD, Zimmerman LA, Vitonis AF, Fadayomi AB, Missmer SA. Overlap Between Irritable Bowel Syndrome Diagnosis and Endometriosis in Adolescents. Clin Gastroenterol Hepatol. 2021;19:528-537.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Fang XC, Fan WJ, Drossman DD, Han SM, Ke MY. Are bowel symptoms and psychosocial features different in irritable bowel syndrome patients with abdominal discomfort compared to abdominal pain? World J Gastroenterol. 2022;28:4861-4874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 9. | Geller S, Levy S, Avitsur R. Psychological distress in individuals with irritable bowel syndrome: the roles of body image and self-criticism. Health Psychol Behav Med. 2024;12:2334466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Black CJ, Yiannakou Y, Guthrie EA, West R, Houghton LA, Ford AC. A Novel Method to Classify and Subgroup Patients With IBS Based on Gastrointestinal Symptoms and Psychological Profiles. Am J Gastroenterol. 2021;116:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Chey WD, Hashash JG, Manning L, Chang L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology. 2022;162:1737-1745.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 12. | Carter KA. Irritable bowel syndrome: Clinical practice update. JAAPA. 2024;37:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Chen CH, Lin CL, Kao CH. Irritable Bowel Syndrome Is Associated with an Increased Risk of Dementia: A Nationwide Population-Based Study. PLoS One. 2016;11:e0144589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Kavanagh RG, O'Grady J, Carey BW, O'Connor OJ, Maher MM. Review of the role of abdominal imaging in irritable bowel syndrome. World J Radiol. 2018;10:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 15. | O'Connor OJ, McSweeney SE, McWilliams S, O'Neill S, Shanahan F, Quigley EM, Maher MM. Role of radiologic imaging in irritable bowel syndrome: evidence-based review. Radiology. 2012;262:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Katsumata R, Hosokawa T, Manabe N, Mori H, Wani K, Ishii K, Tanikawa T, Urata N, Ayaki M, Nishino K, Murao T, Suehiro M, Fujita M, Kawanaka M, Haruma K, Kawamoto H, Takao T, Kamada T. Brain activity in response to food images in patients with irritable bowel syndrome and functional dyspepsia. J Gastroenterol. 2023;58:1178-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Skrobisz K, Piotrowicz G, Drozdowska A, Markiet K, Sabisz A, Naumczyk P, Rydzewska G, Szurowska E. Use of functional magnetic resonance imaging in patients with irritable bowel syndrome and functional dyspepsia. Prz Gastroenterol. 2019;14:163-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-iix, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1728] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 19. | Klinge MW, Krogh K, Mark EB, Drewes AM, Brix L, Isaksen C, Dedelaite M, Frøkjaer JB, Fynne LV. Colonic volume in patients with functional constipation or irritable bowel syndrome determined by magnetic resonance imaging. Neurogastroenterol Motil. 2022;34:e14374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Lam C, Chaddock G, Marciani L, Costigan C, Paul J, Cox E, Hoad C, Menys A, Pritchard S, Garsed K, Taylor S, Atkinson D, Gowland P, Spiller R. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol Motil. 2016;28:861-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Pritchard SE, Marciani L, Garsed KC, Hoad CL, Thongborisute W, Roberts E, Gowland PA, Spiller RC. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol Motil. 2014;26:124-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, Gowland P, Spiller R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152:124-133.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Carrington EV, Scott SM, Bharucha A, Mion F, Remes-Troche JM, Malcolm A, Heinrich H, Fox M, Rao SS; International Anorectal Physiology Working Group and the International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol. 2018;15:309-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Sharif H, Hoad CL, Abrehart N, Gowland PA, Spiller RC, Kirkham S, Loganathan S, Papadopoulos M, Benninga MA, Devadason D, Marciani L. Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics (Basel). 2021;11:974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Moreno-Osset E, Bazzocchi G, Lo S, Trombley B, Ristow E, Reddy SN, Villanueva-Meyer J, Fain JW, Jing J, Mena I. Association between postprandial changes in colonic intraluminal pressure and transit. Gastroenterology. 1989;96:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy SN, Mena I, Snape WJ Jr. Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology. 1991;101:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Di Palma L, Coletta M, Tomba C, Forzenigo LV, Biondetti P, Basilisco G. Magnetic resonance imaging of rectal volume in patients with irritable bowel syndrome. Dig Liver Dis. 2011;43:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Mark EB, Bødker MB, Grønlund D, Østergaard LR, Frøkjaer JB, Drewes AM. MRI analysis of fecal volume and dryness: Validation study using an experimental oxycodone-induced constipation model. J Magn Reson Imaging. 2019;50:733-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Horikawa Y, Mieno H, Inoue M, Kajiyama G. Gastrointestinal motility in patients with irritable bowel syndrome studied by using radiopaque markers. Scand J Gastroenterol. 1999;34:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Kim ER, Rhee PL. How to interpret a functional or motility test - colon transit study. J Neurogastroenterol Motil. 2012;18:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Klinge MW, Borghammer P, Lund S, Fedorova T, Knudsen K, Haase AM, Christiansen JJ, Krogh K. Enteric cholinergic neuropathy in patients with diabetes: Non-invasive assessment with positron emission tomography. Neurogastroenterol Motil. 2020;32:e13731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Okawa Y. Can irritable bowel syndrome be detected by ultrasound? Drug Discov Ther. 2020;14:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Nilsson M, Sandberg TH, Poulsen JL, Gram M, Frøkjaer JB, Østergaard LR, Krogh K, Brock C, Drewes AM. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol Motil. 2015;27:1755-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Mukhtar K, Nawaz H, Abid S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J Gastroenterol. 2019;25:552-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (3)] |
| 36. | Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, Schweinhardt P, Van Oudenhove L, Borsook D. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut. 2019;68:1701-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 38. | Grinsvall C, Ryu HJ, Van Oudenhove L, Labus JS, Gupta A, Ljungberg M, Törnblom H, Mayer EA, Simrén M. Association between pain sensitivity and gray matter properties in the sensorimotor network in women with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Chua CS, Bai CH, Shiao CY, Hsu CY, Cheng CW, Yang KC, Chiu HW, Hsu JL. Negative correlation of cortical thickness with the severity and duration of abdominal pain in Asian women with irritable bowel syndrome. PLoS One. 2017;12:e0183960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Belfer I. Nature and nurture of human pain. Scientifica (Cairo). 2013;2013:415279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One. 2013;8:e73932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Mao CP, Chen FR, Sun HH, Shi MJ, Yang HJ, Li XH, Ding D. Larger regional volume of the thalamus in diarrhea-predominant irritable bowel syndrome: a cross-sectional study. Brain Imaging Behav. 2020;14:2302-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Zhao L, Wang Y, Zhang Y. Microstructural changes in the brain in elderly patients with irritable bowel syndrome. Aging Med (Milton). 2018;1:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, Averill C, Jia-Wei Yang G, Wong E, Tang CY, Krystal JH, Iosifescu DV, Charney DS. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp. 2016;37:3214-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bülow R, Selonke M, Völzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy-Duchesne B, Rentería ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya-Maldonado R, Gruber O, Krämer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx BWJH, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD, Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 910] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 48. | Xie C, Tang Y, Wang Y, Yu T, Wang Y, Jiang L, Lin L. Efficacy and Safety of Antidepressants for the Treatment of Irritable Bowel Syndrome: A Meta-Analysis. PLoS One. 2015;10:e0127815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Su C, Liu W, Wang Q, Qiu S, Li M, Lv Y, Yu Y, Jia X, Li H. Abnormal resting-state local spontaneous functional activity in irritable bowel syndrome patients: A meta-analysis. J Affect Disord. 2022;302:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Icenhour A, Witt ST, Elsenbruch S, Lowén M, Engström M, Tillisch K, Mayer EA, Walter S. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin. 2017;15:449-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Guleria A, Karyampudi A, Singh R, Khetrapal CL, Verma A, Ghoshal UC, Kumar D. Mapping of Brain Activations to Rectal Balloon Distension Stimuli in Male Patients with Irritable Bowel Syndrome Using Functional Magnetic Resonance Imaging. J Neurogastroenterol Motil. 2017;23:415-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/