Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.107305
Revised: April 20, 2025
Accepted: June 17, 2025
Published online: December 20, 2025
Processing time: 137 Days and 19.3 Hours
Pancreatic neoplasms present a significant therapeutic challenge due to their complex anatomy and poor prognosis. In recent years, endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) has emerged as a promising mini
Core Tip: Endoscopic ultrasound-guided radiofrequency ablation has emerged as a promising minimally invasive approach for managing pancreatic tumors, providing a valuable alternative for patients who are poor surgical candidates. It effectively achieves significant tumor size reduction, symptom relief, and, in selected cases, prolonged survival, especially in pancreatic neuroendocrine tumors and cystic lesions. Despite a favorable safety profile, including low rates of major complications, standardization of protocols and further technological advancements such as artificial intelligence integration and combination therapies are essential for optimizing treatment outcomes and broadening clinical applicability.
- Citation: Okasha HH, Gadour E, Alyouzbaki AZ, Shaaban HE. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic tumors: Current status and future perspectives. World J Methodol 2025; 15(4): 107305
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/107305.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.107305
Pancreatic cancer is one of the most lethal malignancies worldwide, with the highest incidence rates in developed countries. It is anticipated that the incidence of pancreatic cancer will increase in the coming decades, making it a growing public health concern[1,2]. The majority of patients are diagnosed with advanced disease, and the survival rate for pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer, remains dismal, with a five-year survival rate of less than 10%. This is largely due to the inability to detect the disease early and the absence of effective treatment options once it reaches advanced stages[3].
Pancreatic neuroendocrine tumors (PNETs) and pancreatic cystic lesions (PCLs), while less common, also represent significant clinical challenges and are also associated with high morbidity and mortality. These tumors often present at advanced stages with nonspecific symptoms, which makes them difficult to diagnose early and complicates their treatment options.
Surgical resection remains the gold standard for pancreatic cancer, but many patients are not candidates for surgery due to poor functional status, advanced age, or comorbidities. Moreover, tumors located near critical vasculature or those that are borderline resectable pose additional challenges, highlighting the need for alternative treatment strategies[3,4].
One such emerging strategy is endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA), a minimally invasive procedure that uses thermal energy to destroy tumors. It combines the precision of EUS with the cytotoxic potential of thermal ablation. This technique has shown promise in a variety of pancreatic tumors, including PNETs, PCLs, and PDAC, especially in patients who are not surgical candidates. Recent studies have demonstrated the efficacy of EUS-RFA in achieving tumor shrinkage allowing for potential downstaging, symptom relief, and in some cases, prolonged survival. However, despite these promising outcomes, there remain significant gaps in understanding the optimal indications, procedural standardization, and long-term effectiveness of EUS-RFA[5].
This review aims to evaluate the current evidence surrounding EUS-RFA for pancreatic tumors, discuss its clinical applications, compare it with other therapeutic modalities, and provide a perspective on future directions for this promising technology.
RFA uses high-frequency electrical energy (350-500 kHz) to generate heat in the targeted tissue. The process of ablation involves the conversion of electrical energy into thermal energy, causing the protein molecules within the tissue to denature and leading to coagulative necrosis. The basic principle of RFA is based on the thermal effects that occur when the tissue reaches temperatures of 60-100 °C, which is sufficient to induce irreversible damage to the cellular structure, leading to cell death. The degree of cellular destruction depends on both the peak temperature achieved and the duration of heat exposure. Above 60 °C, irreversible protein denaturation occurs rapidly, while temperatures above 100 °C can result in tissue vaporization and carbonization, which may limit the conduction of further energy[6]. RFA creates a thermal ablation zone characterized by three distinct areas:
Central necrotic zone: Directly affected by the highest temperatures, where immediate tissue coagulation and necrosis occur.
Transitional zone: Experiences lower temperatures that cause partial tissue damage, potentially leading to delayed cellular death via apoptosis.
Peripheral zone: Least affected area that may suffer secondary damage due to heat propagation, influencing tissue integrity and recovery.
A major factor influencing the efficiency of RFA is the heat sink effect, which occurs when blood vessels nearby dissipate heat, reducing the temperature of the tissue and potentially impairing the efficacy of the treatment by reducing the ablation zone. This effect can be especially problematic in highly vascularized tumors, such as those in the pancreas, where careful consideration of the device settings and procedural technique is required to achieve optimal results[7].
The success of EUS-RFA is largely dependent on the technology used, with several devices currently available for clinical practice. The two main types of devices used are monopolar and bipolar probes, each offering distinct advantages. Devices designed with internal cooling or modified electrode geometry aim to mitigate this. Moreover, real-time temperature monitoring during EUS-RFA is an evolving area aimed at enhancing safety and control of the ablation process. Recent preclinical models have demonstrated that combining RFA with adjunctive agents or varying waveform energy delivery may optimize lesion creation.
The Habib™ EUS-RFA probe is one of the most widely used monopolar probes. It consists of a thin needle electrode that can be inserted into the tumor through a 19-G endoscopic needle, offering precision and minimal tissue damage during insertion.
The EUSRA probe is another widely used device that enables controlled ablation of pancreatic tumors with adjustable electrode exposure lengths, allowing for fine control of the ablation zone.
The Hybrid Cryotherm Probe combines RFA with cryotherapy, a technique that uses cooling and heating to enhance the ablation effect. This hybrid approach is particularly useful in treating large or centrally located tumors.
Ablation settings typically range from 10 to 60 W, with single or multiple 10-20 second cycles depending on tumor size and location. The choice of probe, power setting, and ablation duration depends on the tumor's characteristics and location[6].
Monopolar systems like the Habib™ EUS-RFA and the EUSRA Probe are favored for their precision and minimal tissue disruption during insertion. This device allows for precise control of the ablation area, suitable for targeting small to medium tumors with clear boundaries.
Bipolar systems, such as the Hybrid Cryotherm Probe, offer advantages in managing larger or vascular-rich tumors by combining RFA with cryotherapy. This method reduces the heat sink effect, which is crucial in highly vascularized areas like the pancreas[6,7].
Clinical applications: (1) Monopolar probes: Ideal for precise, small-scale ablations where the risk of damaging surrounding tissues is minimal. Commonly used in well-demarcated lesions away from major blood vessels; and (2) Bipolar probes: Better suited for complex cases with larger or irregularly shaped tumors or those located near critical structures, as they allow for a more controlled and uniform thermal spread[7]. EUS-RFA is frequently compared to other local ablation modalities, as summarized in Table 1.
| Technique | Mechanism of action | Advantages | Disadvantages (complications) |
| Endoscopic ultrasound-guided radiofrequency ablation | Thermal ablation via high-frequency current | Minimally invasive, precise targeting, low morbidity | Risk of pancreatitis, limited penetration depth |
| Microwave ablation | Electromagnetic energy creates heat | Faster ablation, deeper penetration | Less precise targeting, potential for heat sink effect |
| Cryoablation | Freezing-induced necrosis | Lower pain, preservation of tissue integrity | Requires multiple sessions, risk of adjacent organ injury |
| Irreversible electroporation | Non-thermal, disrupts cell membranes | No heat sink effect, preserves vascular structures | Expensive, limited availability |
While EUS-RFA has shown considerable promise, direct comparisons with other local ablation modalities remain limited. Microwave ablation offers deeper tissue penetration and faster energy delivery, which may be advantageous in larger lesions, but it often lacks the precise targeting that EUS guidance provides[8]. Cryoablation offers a favorable safety profile due to controlled tissue freezing, yet may require multiple sessions for efficacy and carries the risk of cryoshock[9]. Irreversible electroporation is a non-thermal ablation method that preserves vascular structures and avoids the heat sink effect, making it suitable for tumors near critical blood vessels. However, its high cost and limited availability can be barriers to widespread use[10]. Future head-to-head trials are needed to guide modality selection based on tumor characteristics, location, and patient-specific factors.
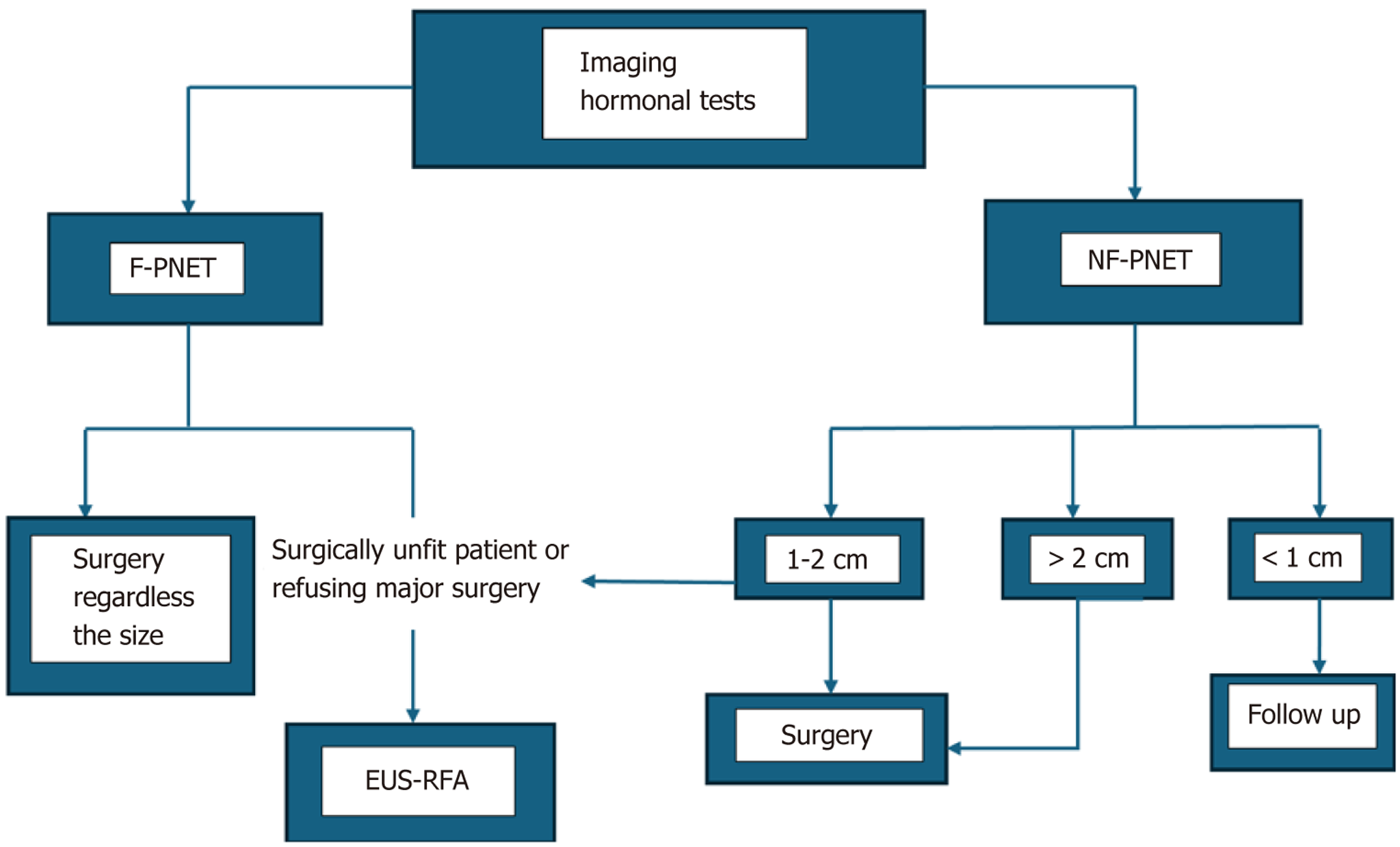
PNETs are one of the most common indications for EUS-RFA (Figure 1). Surgical resection remains the standard of care for PNETs, but EUS-RFA offers an alternative approach for patients with smaller lesions, especially functional tumors like insulinomas. It is also a good option for patients who are poor surgical candidates due to age or comorbidities, or patients refusing major surgery. The success rates for insulinoma ablation with EUS-RFA are impressive, with studies showing 85%-95% resolution of symptoms[4].
For nonfunctional PNETs, especially those < 2 cm, EUS-RFA offers an excellent palliative option, achieving significant tumor reduction and potentially prolonging patient survival. In cases where surgical resection is not feasible, EUS-RFA can provide a less invasive means of controlling tumor growth and relieving symptoms related pain and compression of surrounding structures[5,11].
PCLs, particularly intraductal papillary mucinous neoplasms and mucinous cystic neoplasms, represent a growing area of focus for EUS-RFA. These lesions can be difficult to manage, especially in patients with high-risk features such as mural nodules or growing lesions. Traditionally, surgical resection has been considered the gold standard for these cysts; however, the increasing evidence supporting the use of EUS-RFA shows that it can be a less invasive option for patients with small, symptomatic cysts, especially those located in the head region.
Studies show that RFA of PCLs can result in up to 70% cyst resolution, significantly reducing the need for repeat surgical interventions. When mural nodules or high-risk features are present, EUS-RFA may offer a valuable bridge to surgery, shrinking the lesion and making it more resectable[12].
EUS-RFA has been increasingly considered for PDAC, particularly in cases where tumors are classified as locally advanced or borderline resectable. The strategic use of EUS-RFA offers several clinical benefits:
Locally advanced tumors: For tumors that cannot be surgically removed without significant risk of morbidity or incomplete resection, EUS-RFA can be utilized to enhance the efficacy of chemotherapy. By reducing the tumor mass and disrupting the stromal barrier, EUS-RFA may improve chemotherapeutic drug delivery and absorption. Moreover, this technique has been associated with significant pain reduction, directly impacting patient quality of life by targeting nerves involved in pain transmission within or adjacent to the tumor.
Borderline resectable PDAC: In borderline resectable cases, EUS-RFA can play a crucial role in downstaging the tumor, potentially converting an inoperable tumor into a surgically manageable condition. By reducing tumor size and involvement with critical vascular structures, EUS-RFA increases the likelihood of a successful surgical resection with negative margins, which is a key determinant of long-term survival[13]. However further prospective studied are needed to verify the efficacy of RFA in downstaging of PDAC.
While the application of EUS-RFA in primary pancreatic tumors is well-documented, its role in managing pancreatic metastases from other primary cancers like renal cell carcinoma (RCC) and colorectal cancer (CRC) is emerging as a promising therapeutic option. Initial studies and clinical reports suggest that EUS-RFA can effectively target and ablate metastatic lesions within the pancreas, offering a minimally invasive alternative to systemic chemotherapy or major surgery, which can be particularly advantageous in patients burdened with significant comorbidities or those who have previously undergone multiple treatments.
Metastatic RCC: RCC metastases to the pancreas are often resistant to chemotherapy, making local control strategies like EUS-RFA valuable. EUS-RFA can provide localized tumor control without the systemic side effects associated with conventional treatments[14].
Metastatic CRC: For CRC metastases, which often present as multiple small lesions within the pancreas, EUS-RFA can be used to ablate lesions that are not amenable to resection or when patient preference dictates a less invasive approach[15].
The success of EUS-RFA in clinical practice is evidenced by a growing body of research, particularly in PNETs and PCLs, where significant reductions in tumor size, symptom relief, and improved quality of life have been reported.
PNETs: Complete ablation has been reported in up to 87% of cases, with some studies indicating a near 90% recurrence-free survival at 12 months. These statistics suggest a robust potential for EUS-RFA not only as a palliative measure but also as a curative approach in selected cases. Detailed longitudinal studies have shown that symptom relief, particularly in insulinomas, correlates strongly with reduced hospitalizations and improved patient-reported outcome measures[4,16].
PDAC: The efficacy of EUS-RFA for PDAC is more limited and more challenging due to the aggressive nature of the tumor and its dense vascular environment. However, early studies have shown promising results in terms of tumor necrosis and pain management which is valuable for improving patient quality of life. Combined treatment strategies such as EUS-RFA + chemotherapy showed improved progression-free survival[17] (Table 2).
| Tumor type | Technical success (%) | Complete ablation (%) | Symptom relief (%) | Major complication rate (%) |
| Neuroendocrine tumors | 98 | 87 | 95 | 5 |
| Pancreatic cystic lesions | 95 | 70 | 60 | 7 |
| Pancreatic ductal adenocarcinoma | 90 | 30 | 50 | 10 |
| Metastases | 85 | 50 | 65 | 8 |
Standardized surveillance protocols are crucial for monitoring post-ablation outcomes and early detection of recurrence. Follow-up typically includes cross-sectional imaging such as contrast-enhanced CT or MRI at 1, 3, and 6 months post-procedure, followed by annual scans if stable[18]. For PNETs, periodic hormone panels and chromogranin A levels are useful adjuncts. In PCLs, EUS may be repeated to assess cyst resolution or residual septations[19]. Developing evidence-based surveillance pathways tailored to tumor type, size, and ablation extent will improve consistency in post-RFA care.
The most common short-term complication associated with EUS-RFA is post-ablation pancreatitis, which occurs in approximately 7%-10% of cases. This complication is typically mild and resolves with conservative management, such as fasting and intravenous fluids. However, in some cases, it can lead to significant morbidity, requiring more intensive management. The risk of pancreatitis is higher in tumors located near the pancreatic duct or those with larger volumes of treated tissue. Preventive strategies, including the use of preprocedure intake of NSAID or prophylactic pancreatic duct stenting, have been shown to reduce the risk of pancreatitis in high-risk patients[12,16].
Other short-term complications include abdominal pain, bleeding, and infection. These are generally transient and managed conservatively. Bowel perforation and gastric ulcers are rarer but more serious complications that can occur, especially in tumors located near the duodenum or gastric wall. The incidence of major complications is typically low, with studies showing rates of around 1% or less[16].
Long-term complications of EUS-RFA are less well documented due to the relative novelty of the technique. However, chronic pain or recurrence of treated tumors can occur in certain cases particularly in patients with repeated or high-intensity ablations. Recurrence is more common in tumors that are not completely ablated, in larger tumors where the energy used during the procedure might not have been sufficient to destroy the entire tumor mass or in aggressive tumor subtypes. For PNETs, the recurrence rate is generally low (around 10% at 12 months), with higher recurrence seen in histopathologic stage G2 tumors, larger tumors or those located near major blood vessels[12].
Pancreatic fistula formation is another potential long-term complication, particularly in cases where there is significant pancreatic duct injury during the procedure. This can result in prolonged drainage or the need for surgical intervention in rare cases. Advances in imaging and the use of real-time monitoring during the procedure have significantly reduced this risk[20].
In addition to clinical risks, the economic burden associated with EUS-RFA is an important consideration. Although EUS-RFA may reduce hospitalization and surgery costs in the long term, the upfront expense of the procedure, specialized equipment, and operator expertise can be substantial. Cost-effectiveness studies are still limited, but early data suggest that EUS-RFA may be more economical in selected patient groups, particularly those unfit for surgery or with localized, small-volume disease. Further analysis comparing direct and indirect healthcare costs across treatment modalities is warranted to better understand the overall financial impact on patients and healthcare systems[21].
One of the key limitations of EUS-RFA is the lack of standardization in procedural techniques, including variations in electrode selection, energy settings, and ablation duration. This lack of uniformity makes it challenging to compare outcomes across studies. Standardized guidelines for optimal energy settings, ablation zones, and procedural steps are needed to improve consistency and maximize the success rate of the procedure. Ongoing multicenter trials and expert consensus meetings are likely to address these gaps and provide a framework for practice.
The development of protocols for patient selection, particularly focusing on tumor size, location, and vascular involvement, will also be critical. Further research into biomarkers that predict response to RFA, such as tumor-specific antigens or genetic markers, will help guide patient selection and improve treatment outcomes.
There have been significant advances in ablation technology, with newer devices offering enhanced precision and minimized collateral damage. The development of multi-needle probes and cooled-tip RFA devices is particularly noteworthy. These devices allow for more controlled and uniform energy delivery, reducing the risk of complications such as thermal injury to adjacent structures[13].
Another exciting area is the integration of robotic systems and artificial intelligence (AI) into EUS-RFA. AI is increasingly being integrated into endoscopic systems to enhance image interpretation, tumor detection, and procedural planning. AI algorithms can assist operators in accurately identifying ablation zones, predicting the optimal trajectory for probe insertion, and dynamically monitoring the treatment response in real time[22,23]. Robotic-assisted EUS may also offer improved precision and control, minimizing operator-dependent variability and enhancing procedural reproducibility, particularly in difficult anatomical locations[24].
Combination therapies are expected to play a significant role in the future of EUS-RFA for pancreatic cancer. Combining EUS-RFA with chemotherapy or immunotherapy has shown potential in preclinical studies and early-phase clinical trials. For example, combining gemcitabine or FOLFIRINOX with EUS-RFA could not only shrink the tumor but also increase the efficacy of chemotherapy by improving tumor vascularity and drug delivery to the tumor site[17].
In addition, the combination of EUS-RFA and immune checkpoint inhibitors is an exciting area of investigation. Tumor necrosis induced by RFA can release tumor antigens, which, when combined with immune checkpoint inhibitors, could trigger an immune response against the tumor. This synergy between local and systemic therapies could be a game-changer in the management of locally advanced pancreatic cancer[25].
EUS-RFA represents a significant advancement in the minimally invasive treatment of pancreatic tumors. It offers a viable alternative for patients who are poor candidates for surgery or refusing major surgery, with growing evidence supporting its safety and efficacy in various pancreatic neoplasms, particularly in the management of PNETs and PCLs. Recent developments in device technology, procedural techniques, and adjunctive therapies have significantly broadened its clinical application. To optimize outcomes, future efforts should focus on procedural standardization, robust long-term clinical trials, integration with systemic therapies, and enhanced imaging guidance through AI. Moreover, real-world cost-benefit analyses and patient-centered outcomes are needed to refine its role in practice. As the field evolves, EUS-RFA may become a cornerstone in the multidisciplinary management of pancreatic tumors.
| 1. | Ilic I, Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J Gastroenterol. 2022;28:4698-4715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (3)] |
| 2. | Shou S, Liu R, He J, Jiang X, Liu F, Li Y, Zhang X, En G, Pu Z, Hua B, Pang B, Zhang X. Current and projected incidence rates of pancreatic cancer in 43 countries: an analysis of the Cancer Incidence in Five Continents database. BMJ Open Gastroenterol. 2025;12:e001544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18:1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 4. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Khoury T, Sbeit W, Fusaroli P, Campana D, Brighi N, Napoleon B, Lisotti A. Safety and efficacy of endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine neoplasms: Systematic review and meta-analysis. Dig Endosc. 2024;36:395-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 708] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | Strand NH, Hagedorn JM, Dunn T, Johnson B, Abd-Elsayed A, Covington S, Freeman J, Dawodu A, Maloney J. Advances in radiofrequency ablation: mechanism of action and technology. Ann Palliat Med. 2024;13:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, Cuffari S, Dionigi G, Rotondo A, Fugazzola C. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 9. | Saccomandi P, Lapergola A, Longo F, Schena E, Quero G. Thermal ablation of pancreatic cancer: A systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35:398-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Narayanan G, Daye D, Wilson NM, Noman R, Mahendra AM, Doshi MH. Ablation in Pancreatic Cancer: Past, Present and Future. Cancers (Basel). 2021;13:2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Imperatore N, de Nucci G, Mandelli ED, de Leone A, Zito FP, Lombardi G, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a systematic review of the literature. Endosc Int Open. 2020;8:E1759-E1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Barthet M, Giovannini M, Gasmi M, Lesavre N, Boustière C, Napoleon B, LaQuiere A, Koch S, Vanbiervliet G, Gonzalez JM. Long-term outcome after EUS-guided radiofrequency ablation: Prospective results in pancreatic neuroendocrine tumors and pancreatic cystic neoplasms. Endosc Int Open. 2021;9:E1178-E1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Testoni SGG, Petrone MC, Reni M, Rossi G, Barbera M, Nicoletti V, Gusmini S, Balzano G, Linzenbold W, Enderle M, Della-Torre E, De Cobelli F, Doglioni C, Falconi M, Capurso G, Arcidiacono PG. Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers (Basel). 2021;13:4512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Chanez B, Caillol F, Ratone JP, Pesenti C, Rochigneux P, Pignot G, Thomassin J, Brunelle S, Walz J, Salem N, Giovannini M, Gravis G. Endoscopic Ultrasound-Guided Radiofrequency Ablation as an Future Alternative to Pancreatectomy for Pancreatic Metastases from Renal Cell Carcinoma: A Prospective Study. Cancers (Basel). 2021;13:5267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Figueiredo Ferreira M, Garces-Duran R, Eisendrath P, Devière J, Deprez P, Monino L, Van Laethem JL, Borbath I. EUS-guided radiofrequency ablation of pancreatic/peripancreatic tumors and oligometastatic disease: an observational prospective multicenter study. Endosc Int Open. 2022;10:E1380-E1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Napoléon B, Lisotti A, Caillol F, Gasmi M, Ah-Soune P, Belle A, Charachon A, Cholet F, Eyraud PY, Grandval P, Gonzalez JM, Habersetzer F, Koch S, Le Rhun M, Mangialavori L, Musquer N, Palazzo M, Poincloux L, Privat J, Sportes A, Stouvenot M, Subtil C, Thomassin L, Vanbiervliet G, Vidal G, Vuitton L, Giovannini M, Barthet M. Risk factors for EUS-guided radiofrequency ablation adverse events in patients with pancreatic neoplasms: a large national French study (RAFPAN study). Gastrointest Endosc. 2023;98:392-399.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Oh D, Seo DW, Song TJ, Park DH, Lee SK, Kim MH. Clinical outcomes of EUS-guided radiofrequency ablation for unresectable pancreatic cancer: A prospective observational study. Endosc Ultrasound. 2022;11:68-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Moyer MT, Canakis A. Endoscopic Ultrasound-Guided Ablation of Pancreatic Mucinous Cysts. Gastrointest Endosc Clin N Am. 2024;34:537-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Vargas A, Dutta P, Carpenter ES, Machicado JD. Endoscopic Ultrasound-Guided Ablation of Premalignant Pancreatic Cysts and Pancreatic Cancer. Diagnostics (Basel). 2024;14:564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Scopelliti F, Pea A, Conigliaro R, Butturini G, Frigerio I, Regi P, Giardino A, Bertani H, Paini M, Pederzoli P, Girelli R. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Kumar S, Saumoy M, Oh A, Schneider Y, Brand RE, Chak A, Ginsberg GG, Kochman ML, Canto MI, Goggins MG, Hur C, Kastrinos F, Katona BW, Rustgi AK. Threshold Analysis of the Cost-effectiveness of Endoscopic Ultrasound in Patients at High Risk for Pancreatic Ductal Adenocarcinoma. Pancreas. 2021;50:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Zhang D, Wu C, Yang Z, Yin H, Liu Y, Li W, Huang H, Jin Z. The application of artificial intelligence in EUS. Endosc Ultrasound. 2024;13:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Khalaf K, Terrin M, Jovani M, Rizkala T, Spadaccini M, Pawlak KM, Colombo M, Andreozzi M, Fugazza A, Facciorusso A, Grizzi F, Hassan C, Repici A, Carrara S. A Comprehensive Guide to Artificial Intelligence in Endoscopic Ultrasound. J Clin Med. 2023;12:3757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 24. | Kaan HL, Ho KY. Robot-Assisted Endoscopic Resection: Current Status and Future Directions. Gut Liver. 2020;14:150-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Moond V, Maniyar B, Harne PS, Bailey-Lundberg JM, Thosani NC. Harnessing endoscopic ultrasound-guided radiofrequency ablation to reshape the pancreatic ductal adenocarcinoma microenvironment and elicit systemic immunomodulation. Explor Target Antitumor Ther. 2024;5:1056-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/