Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.105775
Revised: March 12, 2025
Accepted: March 20, 2025
Published online: December 20, 2025
Processing time: 179 Days and 6.4 Hours
Nikethamide, a respiratory stimulant, is used to treat hypoxemia caused by coronavirus disease 2019 (COVID-19), but it carries a risk of convulsions. Mag
To investigate the therapeutic effect of MgSO4 on nikethamide -induced seizures in COVID-19 patients through animal experiments, providing experimental support for the clinical application of MgSO4 in preventing and treating seizures caused by nikethamide.
Forty mice were randomly divided into four groups: (1) Physiological saline; (2) Low-dose MgSO4 (50 mg/kg); (3) Medium-dose MgSO4 (100 mg/kg); and (4) High-dose MgSO4 (200 mg/kg). After 15 minutes of intraperitoneal injection of different doses of MgSO4 or an equal volume of physiological saline, the mice were injected with nikethamide (250 mg/kg).
Compared to the normal saline group, all doses of MgSO4 significantly prolonged the seizure latency and reduced the severity of convulsions. However, they also extended the duration of seizures and correspondingly increased survival time (P < 0.05). The incidence of seizures and mortality rate in the MgSO4-treated groups were significantly lower than those in the normal saline group (P < 0.05).
MgSO4 can prevent and treat seizures caused by nikethamide in mice. This finding has implications for the application of MgSO4 in treating and preventing seizures caused by nikethamide in COVID-19 treatment.
Core Tip: Nikethamide is a respiratory stimulant used for the treatment of coronavirus disease 2019 (COVID-19); however, its use carries the risk of seizure induction. This study demonstrates that magnesium sulfate (MgSO4) can effectively prevent and mitigate nikethamide-induced seizures in a dose-dependent manner. The results indicate that MgSO4 prolongs seizure latency, reduces seizure severity, extends seizure duration, and decreases mortality in the experimental model. These findings provide important insights into the potential clinical application of MgSO4 for managing nikethamide-associated seizure complications in COVID-19 patients.
- Citation: Xu DJ, Zhong Q, Wang GT, Lu X. Preventive and therapeutic effects of magnesium sulfate on nikethamide-induced seizures: Implications for COVID-19 treatment. World J Methodol 2025; 15(4): 105775
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/105775.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.105775
Nikethamide is a respiratory stimulant that primarily acts by stimulating the respiratory center in the brainstem[1,2]. It has historically been used to treat central respiratory and circulatory failure, as well as to counteract central nervous system (CNS) depression caused by sedatives. However, at high doses, nikethamide is known to cause serious side effects, including vomiting, convulsions, and seizure-like symptoms[3]. These convulsive effects are primarily attributed to its ability to induce the prolonged abnormal opening of sodium ions (Na+) channels, leading to excessive neuronal excitation[3,4].
In the context of coronavirus disease 2019 (COVID-19), nikethamide has been explored as a potential treatment for hypoxemia, particularly in critically ill patients[1]. Some studies suggest that nikethamide may improve oxygen saturation, enhance respiratory function, and reduce mortality[5]. However, there remains significant controversy regarding its efficacy in COVID-19 treatment, with conflicting reports on its clinical benefits[6]. Additionally, its use is limited due to the high risk of seizures, making it essential to identify effective strategies for preventing these adverse effects[7,8].
Magnesium sulfate (MgSO4) is a well-established anticonvulsant widely used in conditions such as eclampsia[9]. It exerts its effects by inhibiting N-methyl-D-aspartate receptors (NMDARs) in a voltage-dependent manner[10,11]. Since excessive NMDAR activation is linked to seizure activity, MgSO4 may serve as a potential rescue agent for nikethamide-induced seizures[12-14]. In addition to its anticonvulsant properties, MgSO4 has been suggested to have potential benefits in COVID-19 treatment, including reducing hypoxia and inflammation[15]. However, its role in managing hypoxemia remains unclear, with inconsistent findings in clinical studies[16-18].
This study aimed to evaluate the preventive and therapeutic effects of MgSO4 on nikethamide-induced seizures using an animal model. Specifically, we investigated whether MgSO4 could prolong seizure latency, reduce convulsion severity, extend seizure duration, and improve the survival rate of mice treated with nikethamide. By elucidating its neuroprotective effects, the findings of this study may provide valuable insights for the clinical application of MgSO4 in managing nikethamide-associated seizure complications in COVID-19 patients.
This study was conducted following the ethical guidelines for animal research and was approved by the Ethics Committee of Taizhou University.
Forty pathogen-free Kunming mice, both male and female, weighing between 16 g and 18 g, were obtained from Zhejiang Weitong Lihua Laboratory Animal Technology Co., Ltd. (located in Jiaxing City, Zhejiang Province, China). The company holds the license number SYXK (Zhejiang Province) 2021-0013 and the production license number SCXK (Zhejiang Province) 2019-0001. Three days prior to the experiment, the mice were allowed to acclimate to the laboratory environment and had free access to food and water. To prevent potential behavioral differences among the mice from influencing the research results, all the experiments were conducted at the same time each day.
The reagents utilized in this study included MgSO4 produced by Tianjin Jinyao Pharmaceutical Co., Ltd. (Tianjin, China), with a batch number of H12020994 and a specification of 10 mL/2.5 g. Additionally, the experiment utilized nikethamide injection manufactured by Shanghai Hyundai Hasen Pharmaceutical Co., Ltd. (Shangqiu City, Henan Province, China), with a batch number of H20053727 and a specification of 1.5 mL/0.375 g. Furthermore, sodium chloride injection was provided by Shanghai Baxter Medical Products Co., Ltd. (Shanghai, China), with a batch number of H19983149 and a specification of 1000 mL/9 g.
Using a random number table, the mice were evenly and randomly allocated to four groups, namely, the saline control, low-dose MgSO4 (50 mg/kg), medium-dose MgSO4 (100 mg/kg), and high-dose MgSO4 (200 mg/kg) groups, with 10 mice in each group. The mice were administered different doses of MgSO4 or an equal volume of normal saline via intraperitoneal injection. After 15 minutes, each group of mice received an injection of nikethamide solution (250 mg/kg).
The incubation period of convulsions (in seconds), duration of convulsions (in minutes), number of convulsions, and number of deaths among the mice in each group were observed and recorded. Compared to those prior to administration, convulsions were judged based on increased activity, such as walking, jumping, and irritability. The time from nikethamide injection to the appearance of convulsions was considered the incubation period of convulsions. The duration of convulsions was measured from the onset of convulsions to a state of silence in the mice. The time until death was calculated from the start of nikethamide injection to the moment of death.
The statistical analysis was performed using Statistical Package for the Social Sciences 26.0 (IBM Corp., Armonk, NY, United States), Excel 2019 (Microsoft Corporation, Redmond, WA, United States), and GraphPad Prism 8 (GraphPad Prism v8.0; GraphPad Software). The convulsion latency, convulsion duration, and time until death is expressed as the mean ± SD. Continuous quantitative data were compared among multiple groups using one-way analysis of variance (ANOVA), and Dunnett's t test was used for post hoc testing. Convulsion rates and mortality were expressed as percentages, and differences were compared using the rank sum test. A P value < 0.05 indicated a statistically significant difference.
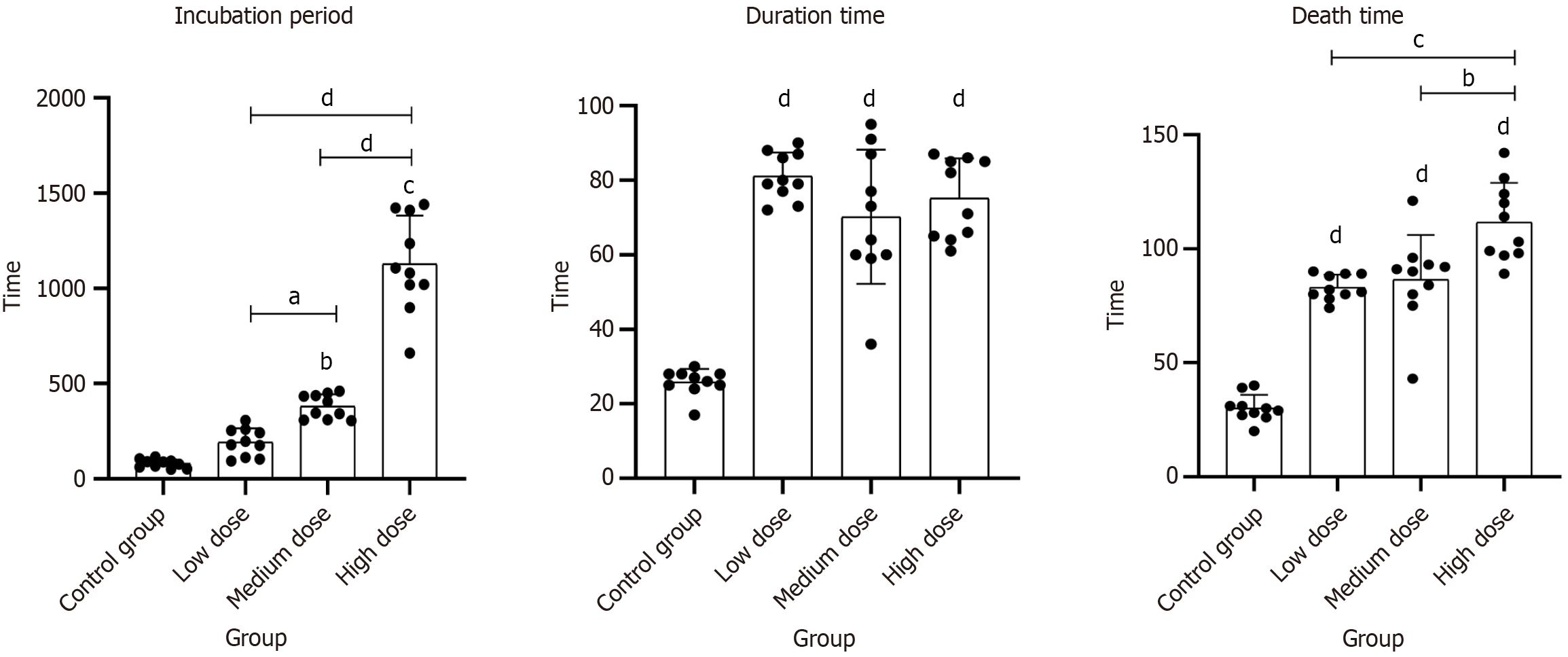
MgSO4 significantly prolonged the incubation period to seizure onset in a dose-dependent manner. The control group exhibited a shortest latency of 80.30 seconds ± 22.32 seconds, whereas magnesium-treated groups showed progressively longer mean latencies: (1) 192.70 seconds ± 73.57 seconds at low dose; (2) 380.20 seconds ± 63.40 seconds at mid dose; and (3) 1129.50 seconds ± 252.51 seconds at high dose. Medium-dose and high-dose MgSO4 groups had a significantly delayed seizure onset compared to control (P < 0.01 for each). Notably, the high-dose MgSO4 group’s latency was about 17 minutes longer than control (P < 0.001), with a mean difference of 1049.2 seconds (95%CI: 900.35–1198.05). An overall one-way ANOVA confirmed a significant effect of treatment on seizure latency (F-statistic = 121.12, P < 0.001). These findings demonstrate a clear dose–response relationship, as higher MgSO4 doses yielded greater delays in seizure initiation, indicating that MgSO4 effectively prolongs seizure onset in this model (Figure 1 and Table 1).
| Groups (n = 10) | Variable group | Time (mean ± SD) | 95%CI | P value |
| Normal saline control group | 1 | 80.30 ± 22.32 | 64.33-96.27 | - |
| Low-dose MgSO4 group | 1 | 192.70 ± 73.57 | 140.07-245.33 | 0.174 |
| Medium-dose MgSO4 group | 1 | 380.20 ± 63.40b | 334.85-425.55 | < 0.01 |
| High-dose MgSO4 group | 1 | 1129.50 ± 252.51b | 948.86-1310.14 | < 0.01 |
| Normal saline control group | 2 | 25.80 ± 3.58 | 23.24-28.36 | - |
| Low-dose MgSO4 group | 2 | 81.10 ± 6.33b | 76.57-85.63 | < 0.01 |
| Medium-dose MgSO4 group | 2 | 70.20 ± 18.03b | 57.30-83.10 | < 0.01 |
| High-dose MgSO4 group | 2 | 75.20 ± 10.68b | 67.56-82.84 | < 0.01 |
| Normal saline control group | 3 | 30.10 ± 5.90 | 25.88-34.32 | - |
| Low-dose MgSO4 group | 3 | 83.10 ± 5.53b | 79.15-87.05 | < 0.01 |
| Medium-dose MgSO4 group | 3 | 86.50 ± 19.60b | 72.48-100.52 | < 0.01 |
| High-dose MgSO4 group | 3 | 111.70 ± 17.23b | 99.37-124.03 | < 0.01 |
In contrast to its effect on latency, MgSO4 treatment led to longer seizure durations compared to control animals. The mean duration of seizure activity in the control group was 25.80 minutes ± 3.58 minutes, whereas animals receiving MgSO4 experienced extended seizure episodes (low dose: 81.10 minutes ± 6.33 minutes; mid dose: 70.20 minutes ± 18.03 minutes; high dose: 75.20 minutes ± 10.68 minutes). All magnesium-treated groups had significantly increased seizure durations relative to control. Specifically, the high-dose group had a seizure duration approximately 3 times longer than control (P < 0.01), and even the low-dose group showed a notable increase (P < 0.01 vs control). Statistical analysis (ANOVA with post hoc comparisons) confirmed these differences, with P values < 0.01 for each group (vs control). This result suggests that while MgSO4 delays the onset of seizures, once seizures begin, they persist longer in treated animals. One explanation is that magnesium’s protective effects prevent early mortality or catastrophic seizure termination, thereby allowing seizures to continue for a prolonged period compared to the untreated controls (Figure 1 and Table 1).
MgSO4 markedly improved survival time following seizure induction, with higher doses conferring greater benefit. Control animals succumbed quickly, with a mean survival time of only 30.10 minutes ± 5.90 minutes post-seizure onset. In contrast, MgSO4-treated groups survived substantially longer on average: (1) 83.10 minutes ± 5.53 minutes for the low-dose group; (2) 86.50 minutes ± 19.60 minutes for the mid-dose group; and (3) 111.70 minutes ± 17.23 minutes for the high-dose group. Collectively, the data indicate that MgSO4 treatment improved overall survival in a dose-validated manner. The extended survival times in MgSO4 groups highlight its neuroprotective role, as treated animals were able to withstand seizure activity longer without succumbing. This suggests that higher doses of MgSO4 confer significant protection against lethal seizure consequences, underlining its potential therapeutic value in mitigating seizure-induced mortality (Figure 1 and Table 1).
MgSO4 exhibited a dose-dependent protective effect by reducing both convulsion incidence and mortality rates (Table 2). The low-dose group showed a convulsion rate of 90% and a mortality rate of 70%, with no significant difference from the control group (P > 0.99). The medium-dose group exhibited a moderate reduction in convulsion rate (60%) and mortality rate (30%), though the differences remained statistically non-significant (P > 0.05). In contrast, the high-dose group demonstrated a significant decrease in both convulsion incidence (20%, P = 0.045) and mortality (10%, P < 0.01), indicating a substantial neuroprotective effect. These results suggest that higher doses of MgSO4 effectively mitigate seizure severity and significantly improve survival outcomes, whereas lower doses may be insufficient to confer full protection. The findings highlight the importance of dose optimization in clinical applications, reinforcing the potential of MgSO4 as a therapeutic strategy for managing pharmacologically induced seizures.
In this study, we demonstrated that MgSO4 significantly protects against nikethamide-induced seizures in mice. All doses of MgSO4 prolonged the seizure latency and increased post-seizure survival time compared to saline, with medium (100 mg/kg) and high (200 mg/kg) doses producing especially strong effects. These doses also significantly reduced the intensity of convulsions and prolonged the duration of seizures. Compared with the control group, the incidence of seizures and mortality rate were significantly lower in the high-dose group. Notably, the anticonvulsant efficacy of MgSO4 was dose-dependent. However, animals receiving the high dose exhibited signs of CNS depression (lethargy and even brief loss of consciousness in some cases), indicating that while seizures were well-controlled, excessive sedation occurred at the highest dose. Only one mouse in the high-dose group succumbed during the experiment, suggesting that 200 mg/kg, though sedating, did not reach a lethal threshold in this model. In summary, MgSO4 dose-dependently prevented or mitigated the convulsive effects of nikethamide—prolonging the seizure-free interval, reducing seizure severity, extending seizure duration, improving survival, and lowering seizure frequency and mortality—albeit with observable sedation at high doses. These findings confirm our hypothesis that MgSO4 can serve as an effective anticonvulsant countermeasure to nikethamide in vivo.
Our results align with and extend the existing literature on respiratory stimulants and anticonvulsant therapies. Nikethamide (also known as coramine) has historically been used to stimulate respiration in acute respiratory failure[3]. However, it is well documented that at high doses or with prolonged use, nikethamide and similar analeptics can provoke adverse effects such as tachypnea, convulsions, and even life-threatening respiratory paralysis[19-21]. The convulsive side effects we observed in control mice given high-dose nikethamide are consistent with these earlier reports. Previous animal studies have begun to elucidate nikethamide’s neural effects: For instance, Qian et al[2] demonstrated that nikethamide’s enhancement of respiratory rhythm in neonatal rat brainstems involves activation of 5-hydroxytryptamine (5-HT) 2A receptors, and additional work implicates gamma-aminobutyric acid(A)_A receptor modulation in nikethamide-induced respiratory changes[4]. These findings support the notion that nikethamide can broadly increase excitatory neurotransmission, which may underlie its seizure-inducing potential.
MgSO4’s efficacy as an anticonvulsant, on the other hand, is well established in certain clinical contexts. It is the treatment of choice for eclamptic seizures in obstetric practice[7,8] and has been reported to exhibit anticonvulsant and neuroprotective properties in various models[5]. Our observation that MgSO4 suppresses seizures is in line with these known anticonvulsant effects. To our knowledge, however, no prior studies have specifically examined MgSO4 for preventing drug-induced convulsions from respiratory stimulants like nikethamide. Thus, our study fills an important gap by demonstrating that magnesium’s protective action can be extended to this unique scenario. In contrast to some reports that magnesium therapy can ameliorate respiratory symptoms in COVID-19 patients (an effect that remains somewhat controversial)[13,14], our work focuses on magnesium’s role in managing a serious side effect of a COVID-19 therapeutic. This distinct perspective underscores the innovative nature of our findings while remaining consistent with the general understanding that MgSO4 is a broad-spectrum anticonvulsant agent.
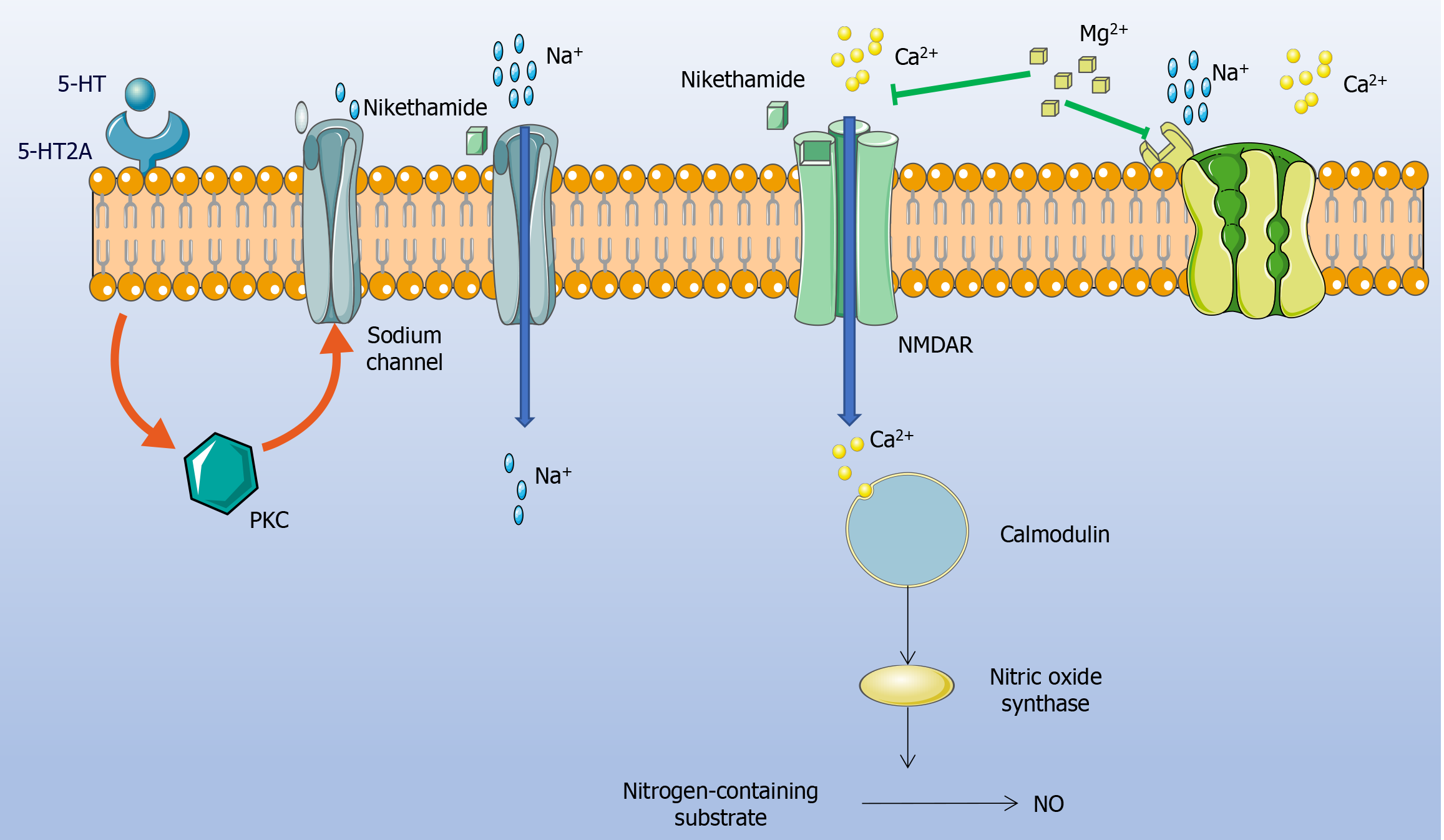
The protective effects of MgSO4 observed in our study can be explained by known neurophysiological mechanisms. Nikethamide exerts its respiratory stimulant action by enhancing CNS excitatory pathways, but this same action can tip into pathological excitation at high doses. Pharmacologically, nikethamide is known to prolong the open state of neuronal sodium channels and to activate NMDARs in the brain. Excessive sodium influx and NMDAR activation lead to a cascade of neuronal hyperexcitation: Increased intracellular Ca2+ entry through NMDAR-associated channels triggers downstream effects such as the overproduction of nitric oxide (a potent vasodilator and neuromodulator), ultimately resulting in aberrant neural firing and convulsions[22-25]. This mechanism explains why an overdose of nikethamide can induce seizures even as it stimulates respiration.
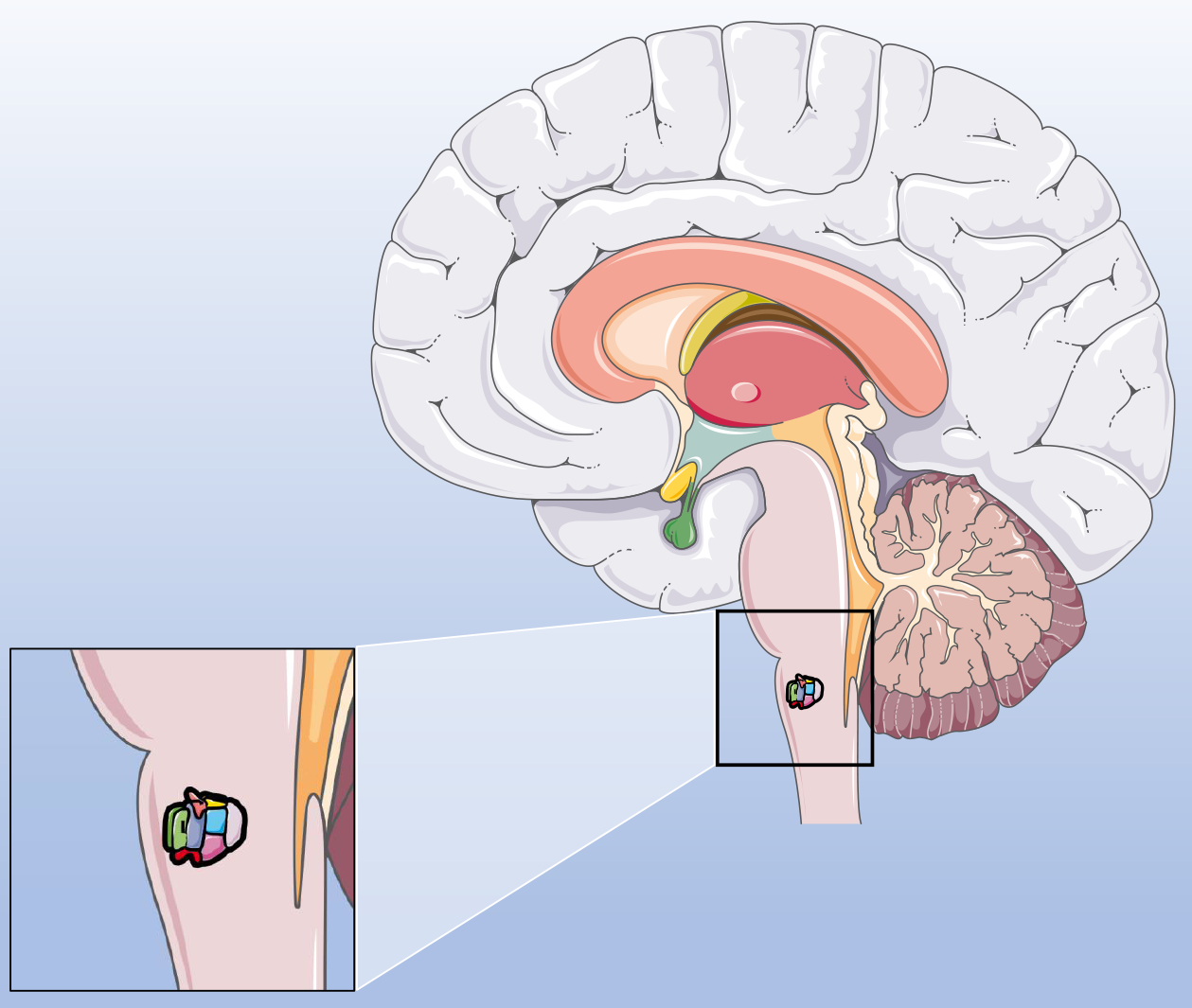
The respiratory rhythm control center in the pre-Bötzinger complex (Figure 2) is modulated by multiple neurotransmitters, with 5-HT being the most important neurotransmitter[26,27]. Endogenous activation of 5-HT2A[28] receptors is necessary for the production of respiratory rhythm in vitro. Protein kinase C can antagonize the blockade of the endogenous activation network of 5-HT2A receptors. Endogenously activated 5-HT2A receptors regulate Na+ conduction through the protein kinase C pathway to maintain this process[22,23]. Nikethamide alters the conformation of sodium ion channels and keeps them open for a long time, thereby altering the sodium ion current and affecting sodium ion distribution, leading to the induction of epilepsy and convulsions[24,25].
Magnesium ions is a natural noncompetitive antagonist of NMDARs that mainly blocks calcium ions (Ca2+) influx through voltage-gated channels (Figure 3). By regulating the activity of Na+-Ca2+ channels in neurons, MgSO4 injection has a good antagonistic effect on seizures induced by nikethamide[12-14]. By limiting calcium entry and stabilizing neuronal membranes, magnesium can prevent the excitotoxic escalation that leads to seizures. Additionally, Mg2+ has a general inhibitory influence on neurotransmission—for example, it can dampen excessive release of excitatory transmitters and interfere with the sodium-calcium exchange cycle that sustains high-frequency neuronal firing. In our experiment, these properties of magnesium almost certainly underlie the prolonged seizure latency, reduced seizure severity, and extended seizure duration observed in the treated mice. Essentially, MgSO4 raises the seizure threshold by inhibiting the neuronal hyperexcitation induced by nikethamide. This mechanistic interpretation is supported by the observed dose-response: Higher doses of magnesium produced deeper CNS depression (e.g. sedation and decreased locomotor activity), indicating a stronger blockade of excitatory neural activity[29,30]. However, this also reflects the drug’s narrow therapeutic window–the same mechanisms that quell seizures can, in excess, suppress normal neural function (leading to respiratory and circulatory depression at very high levels of magnesium). Overall, the interplay between nikethamide and magnesium in our study highlights a classic excitatory-inhibitory counterbalance in neuropharmacology: Nikethamide drives neural activity up, while magnesium pushes it down, and at appropriate dosing, magnesium successfully restrains nikethamide’s overstimulation of the CNS.
Our findings have direct implications for improving the safety of COVID-19 treatments involving respiratory stimulants. Severe COVID-19 pneumonia[15-18] often leads to acute hypoxemic respiratory failure, partly due to dysregulated pulmonary vascular function and inflammation in the lungs (e.g. impaired hypoxic pulmonary vasoconstriction and widespread alveolar damage)[31-33]. In such cases, respiratory stimulants like nikethamide have been employed to drive ventilation in an attempt to counter profound hypoxia[3,34]. Nikethamide may offer transient benefits when respiratory muscle function remains partly intact[34,35], but its utility is severely limited by the risk of precipitating convulsions at higher doses or prolonged use. Our study suggests a solution to this dilemma: Co-administering MgSO4 could prevent or attenuate nikethamide-induced seizures, thereby enabling the therapeutic use of nikethamide in critical COVID-19 patients who might otherwise be prone to drug-provoked convulsions. In practical terms, MgSO4 could be considered as a prophylactic adjunct in COVID-19 cases where nikethamide or similar respiratory analeptics are indicated for refractory hypoxemia.
Several factors make MgSO4 an attractive option in this context. It is inexpensive, widely available, and familiar to clinicians due to its long-standing use in conditions like preeclampsia, where it is valued for both efficacy and safety when used within the proper dose range[6,36]. Importantly, magnesium’s mode of action (sedation and seizure prevention) addresses exactly the side effect that limits nikethamide’s application. By mitigating convulsions, magnesium might expand the viable dosing range of nikethamide, potentially improving oxygenation in COVID-19 patients without inducing neurological complications. However, clinicians must also be mindful of MgSO4’s narrow therapeutic window[37]. The sedative effects we observed at high doses in mice parallel known risks in humans–excessive magnesium can cause respiratory depression, loss of deep tendon reflexes, and cardiac arrhythmias or arrest if levels become toxic[7]. Thus, any clinical protocol emerging from this research would need to balance efficacy with careful monitoring (e.g. frequent reflex checks and magnesium level measurements) to avoid iatrogenic harm. In summary, incorporating MgSO4 as a countermeasure could substantially enhance the safety profile of nikethamide therapy for COVID-19-related respiratory failure, but it would require judicious dosing and monitoring in the clinical setting.
While promising, these results should be interpreted in light of several limitations. First, our study was conducted in a mouse model, and the extent to which the findings translate to humans remains uncertain. Mice were administered acute doses of nikethamide and MgSO4; this controlled scenario may not capture the full complexity of COVID-19 patients who could have co-morbidities, ongoing inflammation, and concurrent medications. The number of animals per group was limited, which, despite yielding statistically significant outcomes, suggests that further studies with larger sample sizes are warranted to confirm the robustness of the protective effect. Additionally, we focused on short-term outcomes (seizure latency, duration, and survival in the acute phase after nikethamide exposure). We did not assess longer-term neurological status or potential cumulative toxicity, which could be relevant if MgSO4 were to be used repeatedly or continuously in a clinical setting.
Mechanistically, we inferred magnesium’s site of action (NMDAR blockade and general CNS depression) from existing knowledge, but we did not directly measure neural activity markers in our experiments. Future research could include electrophysiological studies or neurochemical assays (for example, measuring glutamate release, calcium influx in neurons, or electroencephalogram monitoring during seizures) to directly validate the proposed mechanism of seizure suppression by magnesium in the context of nikethamide. It would also be valuable to explore optimal dosing strategies–for instance, whether a moderate dose of MgSO4 might suffice to prevent seizures without causing undue sedation, or if fractionated dosing could maintain protection with lower peak magnesium levels.
Another important direction is to evaluate MgSO4 in more clinically relevant models. This could include studies in larger animals or in rodent models that mimic COVID-19 pneumonia (e.g. using viral infection or inflammatory agents to induce lung injury and hypoxemia) to see if magnesium retains its protective effect against nikethamide-induced seizures under those conditions. Ultimately, a carefully controlled clinical trial in COVID-19 patients receiving nikethamide (or analogous respiratory stimulants) could be conducted to determine safety, optimal dosing, and efficacy of magnesium co-therapy. Such trials should closely monitor not only respiratory parameters and seizure occurrence but also potential side effects of magnesium, ensuring patient safety.
In conclusion, our work provides a proof-of-concept for using MgSO4 to broaden the therapeutic utility of nikethamide by mitigating its most serious side effect. Further research and development are needed to transition these findings from bench to bedside, but the therapeutic implications are significant: If successful, this strategy could enhance treatment options for severe COVID-19 and improve outcomes by safely addressing refractory hypoxemia without added risk of seizures. The balance of efficacy and safety will be the guiding principle in these future investigations.
All the authors express their gratitude to the ethics committee of the medical college of Taizhou University for their help. We also express our gratitude to our colleague for the data support provided for this experiment.
| 1. | Tejera E, Munteanu CR, López-Cortés A, Cabrera-Andrade A, Pérez-Castillo Y. Drugs Repurposing Using QSAR, Docking and Molecular Dynamics for Possible Inhibitors of the SARS-CoV-2 M(pro) Protease. Molecules. 2020;25:5172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Qian ZB, Wu ZH. [Role of 5-HT(2A) receptor in increase in respiratory-related rhythmic discharge activity by nikethamide in neonatal rat transverse medullary slices]. Sheng Li Xue Bao. 2008;60:216-220. [PubMed] |
| 3. | Sedoul P. Use Of Sedatives, Relaxants, And Respiratory Stimulants In Respiratory Failure. Ann N Y Acad Sci. 1965;121:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Qian ZB, Qi Y, Wu ZH. [GABA A receptor participates in respiratory enhancement induced by nikethamide in neonatal rats]. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:301-304. [PubMed] |
| 5. | Kong N, Gao C, Xu MS, Xie YL, Zhou CY. Spontaneous pneumomediastinum in an elderly COVID-19 patient: A case report. World J Clin Cases. 2020;8:3573-3577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Mishra M. Prone positioning. Clin Med (Lond). 2020;20:e280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Peppin JF, Pergolizzi JV Jr, Fudin J, Meyer TA, Raffa RB. History of Respiratory Stimulants. J Pain Res. 2021;14:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Anthwal T, Nain S. 1,3,4-Thiadiazole Scaffold: As Anti-Epileptic Agents. Front Chem. 2021;9:671212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Vossler DG, Bainbridge JL, Boggs JG, Novotny EJ, Loddenkemper T, Faught E, Amengual-Gual M, Fischer SN, Gloss DS, Olson DM, Towne AR, Naritoku D, Welty TE. Treatment of Refractory Convulsive Status Epilepticus: A Comprehensive Review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020;20:245-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol. 2022;226:S1237-S1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Brookfield KF, Mbata O. Magnesium Sulfate Use in Pregnancy for Preeclampsia Prophylaxis and Fetal Neuroprotection: Regimens in High-Income and Low/Middle-Income Countries. Obstet Gynecol Clin North Am. 2023;50:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 12. | Basha PM, Nayeemunnisa. Effect of methyl parathion on Na(+)-K+ and Mg2+ adenosine triphosphatase activity in developing central nervous system in rats. Indian J Exp Biol. 1993;31:785-787. [PubMed] |
| 13. | Kaur S, Dhawan J, Gupta R, Chawla S. Comparison of Magnesium Sulfate and Ketamine with Ropivacaine in Supraclavicular Brachial Plexus Block: A Randomized Controlled Trial. Anesth Essays Res. 2020;14:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Mehmel M, Jovanović N, Spitz U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients. 2020;12:1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 15. | Miyamoto T, Stein L, Thomas R, Djukic B, Taneja P, Knox J, Vossel K, Mucke L. Phosphorylation of tau at Y18, but not tau-fyn binding, is required for tau to modulate NMDA receptor-dependent excitotoxicity in primary neuronal culture. Mol Neurodegener. 2017;12:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Tang CF, Ding H, Jiao RQ, Wu XX, Kong LD. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Pourdowlat G, Mousavinasab SR, Farzanegan B, Kashefizadeh A, Meybodi ZA, Jafarzadeh M, Baniasadi S. Evaluation of the efficacy and safety of inhaled magnesium sulphate in combination with standard treatment in patients with moderate or severe COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Shaikh N, Khatib MY, Wraidat MAA, Mohamed AS, Al-Assaf AA, Tharayil AGM, Abujaber AA, Nashwan AJ. Nebulized fentanyl for respiratory symptoms in patients with COVID-19 (ventanyl trial). Medicine (Baltimore). 2022;101:e28637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Morales-Quinteros L, Camprubí-Rimblas M, Bringué J, Bos LD, Schultz MJ, Artigas A. The role of hypercapnia in acute respiratory failure. Intensive Care Med Exp. 2019;7:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Palmer BF, Clegg DJ. Respiratory Acidosis and Respiratory Alkalosis: Core Curriculum 2023. Am J Kidney Dis. 2023;82:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Chen CF, Fang HS. Effect of ephedrine and coramine (nikethamide) on hypoxic tolerance in mice. Taiwan Yi Xue Hui Za Zhi. 1974;73:401-403. [PubMed] |
| 22. | Jia Z, Yazdani M, Zhang G, Cui J, Chen J. Hydrophobic gating in BK channels. Nat Commun. 2018;9:3408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Carr R, Weinstock IA, Sivaprasadarao A, Müller A, Aksimentiev A. Synthetic ion channels via self-assembly: a route for embedding porous polyoxometalate nanocapsules in lipid bilayer membranes. Nano Lett. 2008;8:3916-3921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Radouco-Thomas C, Frommel E, Burgermeister-Guex G, Ducommun M. [The point of peripheral impact of coramine, cardiazol, picrotoxin and scilliroside and their relation to cholinergic action. I]. Schweiz Med Wochenschr. 1953;83:511-513. [PubMed] |
| 25. | Wu XY, Zhao JL, Zhang M, Li F, Zhao T, Yang LQ. Sedative, hypnotic and anticonvulsant activities of the ethanol fraction from Rhizoma Pinelliae Praeparatum. J Ethnopharmacol. 2011;135:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Picardo MCD, Sugimura YK, Dorst KE, Kallurkar PS, Akins VT, Ma X, Teruyama R, Guinamard R, Kam K, Saha MS, Del Negro CA. Trpm4 ion channels in pre-Bötzinger complex interneurons are essential for breathing motor pattern but not rhythm. PLoS Biol. 2019;17:e2006094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Vieira GCF, Rodrigues BRO, Cunha CEXD, Morais GB, Ferreira LHRM, Ribeiro MVMR. Depression and dry eye: a narrative review. Rev Assoc Med Bras (1992). 2021;67:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010:CD000025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Yang P, Wang P, Song Y, Zhang A, Yuan G, Cui Y. A retrospective study on the epidemiological characteristics and establishment of an early warning system of severe COVID-19 patients. J Med Virol. 2020;92:2173-2180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | de Barcelos Ubaldo Martins L, Jabour LGPP, Vieira CC, Nery LCC, Dias RF, Simões E Silva AC. Renin Angiotensin System (RAS) and Immune System Profile in Specific Subgroups with COVID-19. Curr Med Chem. 2021;28:4499-4530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Shukla AK, Banerjee M. Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update. High Blood Press Cardiovasc Prev. 2021;28:129-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Maehara T, Fujimori K. Inhibition of Prostaglandin F(2)(α) Receptors Exaggerates HCl-Induced Lung Inflammation in Mice. Int J Mol Sci. 2021;22:12843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Statsenko Y, Habuza T, Talako T, Pazniak M, Likhorad E, Pazniak A, Beliakouski P, Gelovani JG, Gorkom KN, Almansoori TM, Al Zahmi F, Qandil DS, Zaki N, Elyassami S, Ponomareva A, Loney T, Naidoo N, Mannaerts GHH, Al Koteesh J, Ljubisavljevic MR, Das KM. Deep Learning-Based Automatic Assessment of Lung Impairment in COVID-19 Pneumonia: Predicting Markers of Hypoxia With Computer Vision. Front Med (Lausanne). 2022;9:882190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Kronbauer M, Metz VG, Roversi K, Milanesi LH, Rubert Rossato D, da Silva Barcelos RC, Burger ME. Influence of magnesium supplementation and L-type calcium channel blocker on haloperidol-induced movement disturbances. Behav Brain Res. 2019;374:112119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol. 2004;190:1773-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/