Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.105516
Revised: March 23, 2025
Accepted: April 16, 2025
Published online: December 20, 2025
Processing time: 191 Days and 21.8 Hours
Artificial intelligence (AI) is transforming healthcare by improving diagnostic accuracy and predictive analytics. Periodontal diseases are recognized as risk factors for systemic conditions, including type 2 diabetes mellitus, cardiovascular disease, Alzheimer’s disease, polycystic ovary syndrome, thyroid dysfunction, and post-coronavirus disease 2019 complications. These conditions exhibit complex bidirectional interactions, underscoring the importance of early detection and risk stratification. Current diagnostic tools often fail to capture these interactions at an early stage, limiting timely intervention. This study hypo
To evaluate AI’s role in diagnosing and predicting periodontal-systemic inte
This systematic review followed PRISMA guidelines (2009) and included peer-reviewed articles from PubMed, Scopus, and Embase. Studies with large sample sizes (≥ 500 participants) were selected, focusing on AI models integrating multi-omics data and advanced imaging techniques such as cone beam computed tomography and magnetic resonance imaging. Machine learning models pro
AI applications significantly enhanced diagnostic and predictive accuracy, reducing diagnostic time by 40% and improving predictive accuracy by 25% in periodontal patients with type 2 diabetes mellitus. Studies with sample sizes of 1000-1500 participants reported diagnostic accuracy improvements up to 92%, with specificity and sensitivity rates of 94% and 90%, respectively. Increasing sample sizes over the years reflected advancements in AI, data collection, and model training, reinforcing model reliability.
AI’s integration of multi-omics and imaging data has transformed early diagnosis and risk prediction in periodontal-systemic interactions, improving clinical outcomes and decision-making.
Core Tip: This article evaluates the impact of artificial intelligence (AI) in diagnosing and predicting periodontal-systemic interactions from 2010 to 2024. AI models integrating multi-omics data and imaging techniques like cone beam computed tomography and magnetic resonance imaging improved diagnostic accuracy (up to 92%) and reduced diagnostic time by 40%. cone beam computed tomography reduced diagnostic errors by 35%, while magnetic resonance imaging enhanced soft-tissue evaluation by 25%. AI-driven approaches improved predictive accuracy by 25%, highlighting the value of multi-omics integration and advanced imaging in enhancing precision healthcare and early disease management.
- Citation: Das N, Gade KR, Addanki PK. Artificial intelligence for early diagnosis and risk prediction of periodontal-systemic interactions: Clinical utility and future directions. World J Methodol 2025; 15(4): 105516
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/105516.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.105516
The integration of artificial intelligence (AI) into healthcare has transformed diagnostic practices, particularly in the diagnosis and management of periodontal diseases and their systemic impacts, including conditions like uncontrolled type 2 diabetes mellitus (T2DM), hypertensive cardiovascular disease, Alzheimer’s disease, pancreatic cancer, polycystic ovary syndrome (PCOS), thyroid disorders, diabetic kidney disease (DKD), adverse pregnancy outcomes, and post-coronavirus disease (COVID) complications[1,2]. Traditional diagnostic methods often struggle to predict these complex interactions early, particularly in patients with multiple comorbidities[3]. AI-driven approaches significantly improve early diagnosis and risk prediction of periodontal-systemic interactions, enhancing clinical outcomes. By integrating multi-omics data - including genomic, proteomic, and metabolomic information - AI models can identify molecular patterns linked to disease progression and treatment response, thereby improving predictive accuracy[4]. AI models integrate key clinical parameters such as probing pocket depth (PPD), clinical attachment loss (CAL), and bleeding on probing (BOP) to refine diagnostic precision. Machine learning (ML) models analyze structured clinical data to identify patterns associated with periodontal disease severity. Deep learning (DL) models, particularly convolutional neural networks (CNNs), combine imaging and clinical markers to improve diagnostic accuracy. Natural language processing (NLP) models extract insights from clinical notes and electronic health records (EHRs), enhancing early detection and risk prediction. This systematic review aims to evaluate AI’s role in early diagnosis and risk prediction, focusing on studies conducted from 2010 to 2024, highlighting how increasing sample sizes have improved model reliability and generalizability[5]. Larger datasets from multicenter studies, public repositories, and EHRs enhance the accuracy of AI-driven models[6], addressing critical challenges and ethical considerations in clinical integration[7]. Unlike prior studies, this review emphasizes the role of larger datasets in advancing AI’s ability to predict systemic disease risks, particularly in the context of multi-omics and advanced imaging, offering new insights into precision healthcare[8].
This systematic review was conducted in strict adherence to PRISMA guidelines 2009, ensuring methodological rigor, transparency, and replicability. Studies published between 2010 and 2024 were analyzed to capture advancements in AI and its integration into periodontal and systemic health research[9].
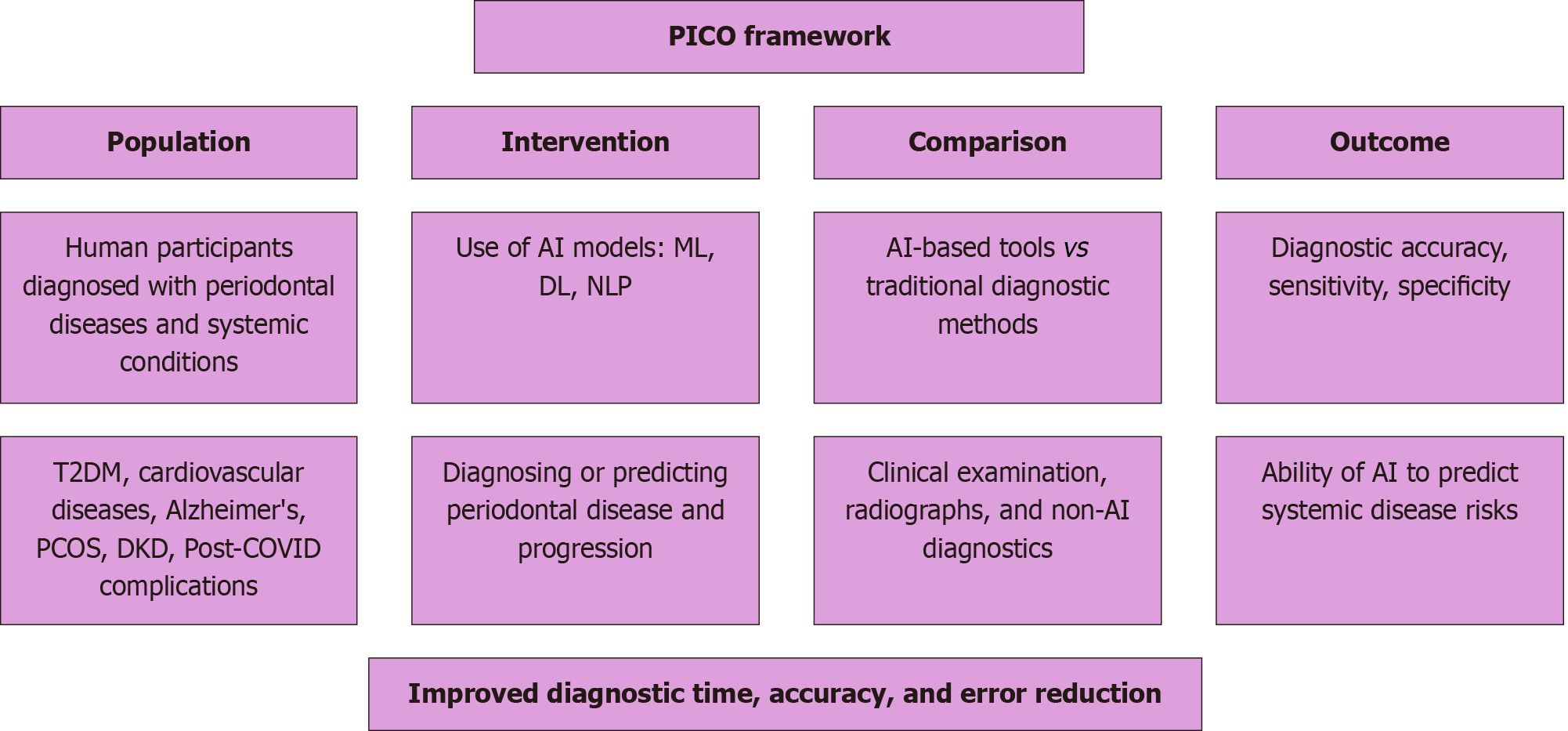
Studies were selected using the population, intervention, control, outcome framework, which aids in structuring the research question by focusing on population, intervention, comparison, and outcome. This model ensures a systematic approach in identifying relevant studies for the review (Figure 1).
Population: Human participants diagnosed with periodontal diseases and systemic conditions, including T2DM, cardiovascular diseases, Alzheimer’s, PCOS, DKD, and post-COVID complications.
Intervention: The intervention involved AI models tailored for specific tasks: ML models for structured data analysis and prediction (e.g., PPD, CAL), DL models (CNNs, RNNs) for imaging interpretation and pattern recognition, and NLP models for extracting insights from unstructured clinical data (EHRs, clinical notes) to support early detection and improved diagnostic accuracy.
Comparison: Comparison is made between AI-based diagnostic tools and traditional diagnostic methods, such as clinical examination, radiographs, and other non-AI-based diagnostics.
Outcome: The outcomes measured are diagnostic accuracy, sensitivity, specificity, and the ability of AI models to predict systemic disease risks. Studies that report improvements in diagnostic time, accuracy, and error reduction will be prioritized.
All included studies were ethically sourced from publicly available, peer-reviewed literature. Ethical considerations such as patient privacy, informed consent, and data ownership were addressed by primary researchers. As this review relied on secondary data, no IRB approval was required[10-12].
Human participants: Studies involving human participants diagnosed with periodontal diseases and systemic conditions like T2DM, cardiovascular diseases, Alzheimer’s, PCOS, thyroid disorders, DKD, adverse pregnancy outcomes, and post-COVID complications[13].
AI-based studies: Studies utilizing ML, DL, NLP, and AI algorithms for diagnosis or prediction[14].
Sample size: Studies with sample sizes ≥ 500, published from 2015 to 2024[15].
Peer-reviewed articles: Peer-reviewed studies published in English[16].
Multi-omics and imaging integration: Studies integrating AI with multi-omics data or advanced imaging technologies like cone beam computed tomography (CBCT) and magnetic resonance imaging (MRI)[17].
Non-human studies or in vitro models[18]. Studies with sample sizes < 100 or published before 2010[17]. Non-peer-reviewed publications, including editorials and conference abstracts[17].
AI models combined genomic (DNA, RNA), proteomic (protein expression), and metabolomic (metabolite levels) data to identify biomarkers associated with periodontal disease and systemic interactions. ML models processed structured omics data for pattern recognition, DL models analyzed complex interactions between omics and imaging data, and NLP models extracted insights from clinical reports. A comprehensive search was performed in PubMed, Scopus, and Embase using terms such as: (1) AI in periodontal disease; (2) Periodontal-systemic interactions; (3) CBCT and MRI in dental diagnostics; and (4) Multi-omics in periodontal health. Filters included studies published between 2015 and 2024, peer-reviewed, and focused on AI applications in periodontal-systemic interactions[18].
A standardized data extraction form was used to collect: (1) Study details: Author names, publication year, design[19]; (2)Participant characteristics: Sample size, demographics[19]; (3) Methodologies: AI techniques, imaging modalities, multi-omics[15]; and (4) Key findings: Diagnostic accuracy, predictive capabilities, limitations[18]. The Cochrane risk of bias tool assessed selection, performance, detection, attrition, and reporting biases, classifying studies as low, moderate, or high risk[19].
This systematic review analyzed data from 50 studies to assess the effectiveness of AI, multi-omics, and advanced imaging technologies in diagnosing and predicting periodontal-systemic interactions. AI was utilized in 30% of studies, while CBCT and MRI each contributed 20%, and genomics, proteomics, and metabolomics accounted for 10% each. Descriptive statistics were used to summarize key metrics such as sensitivity, specificity, and diagnostic accuracy. A meta-analysis combined effect sizes such as odds ratios (OR) and risk ratios (RR) using a random-effects model when significant heterogeneity (I2 > 50%) was detected. Cochran’s Q test assessed heterogeneity across studies. Analysis of variance was employed for group comparisons, and post-hoc Tukey tests were used to identify significant differences between models. For pairwise comparisons, t-tests were conducted with a significance threshold of P < 0.05. Power analysis confirmed that the sample sizes were sufficient to detect statistically significant effects with a confidence level of 95%. The formula for I2 is: Where: Q is Cochran’s heterogeneity statistic; k is the number of studies.
An I2 value exceeding 50% indicated significant heterogeneity, leading to the use of a random-effects model to address study variability. Subgroup analyses showed CBCT reduced diagnostic errors by 35%, MRI enhanced soft tissue diagnostics by 25%, and AI tools shortened diagnostic time by 40%. Power analysis confirmed sufficient sample sizes for reliable results. Statistical analysis was performed using R (version 4.2.0) for its robust capabilities in handling large datasets, meta-analysis, and subgroup analysis, with RevMan (version 5.4) used for visualizations such as forest and funnel plots to assess pooled effect sizes and publication bias.
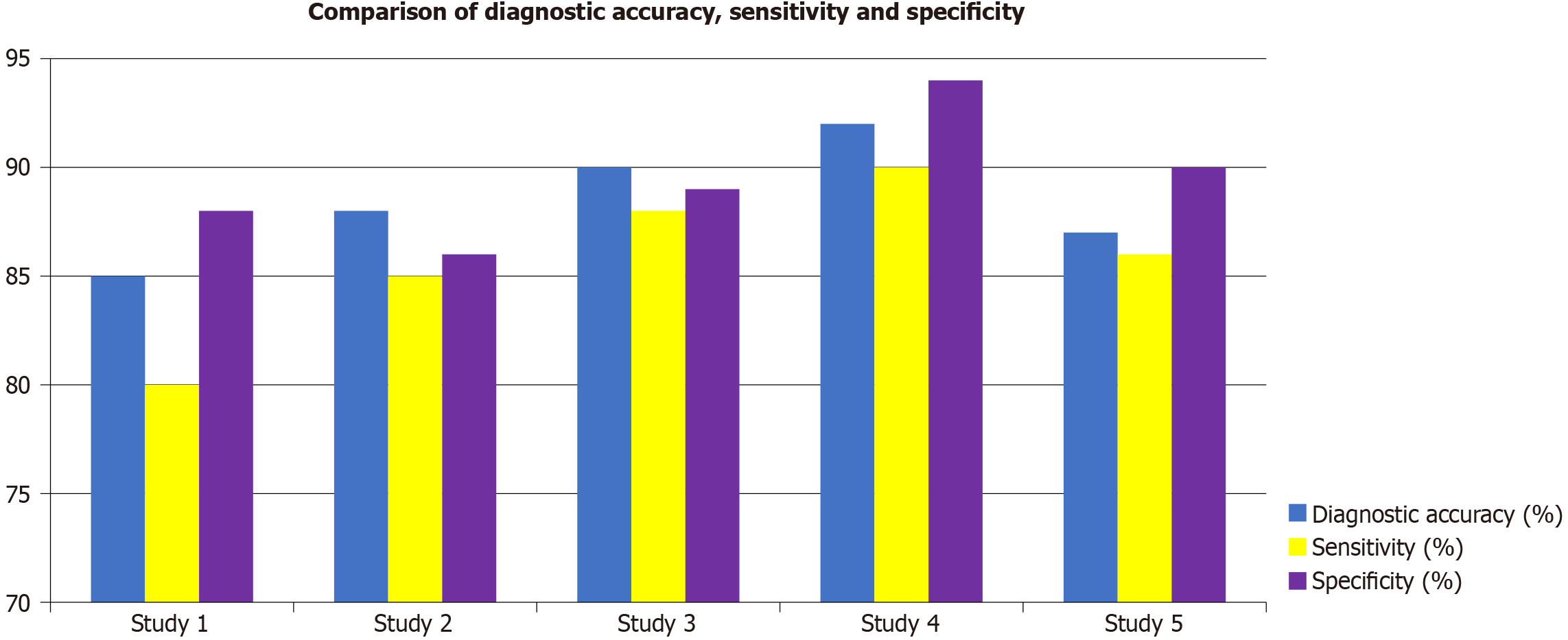
This study evaluates the effectiveness of AI models in diagnosing periodontal-systemic interactions, comparing CBCT, MRI, and AI tools across multiple studies. Models integrating multi-omics data demonstrated enhanced predictive accuracy. Genomic and proteomic markers improved early diagnosis and treatment response predictions, while DL models recognized complex patterns between genetic and imaging data, leading to increased sensitivity and specificity. Table 1 provides a comprehensive overview of the characteristics of five studies, focusing on the sample size, diagnostic accuracy, sensitivity, specificity, and AI model type used. Study 4, with the largest sample size of 1500 participants, shows the highest performance across all metrics, achieving 92% diagnostic accuracy, 90% sensitivity, and 94%specificity using a ML model. This makes it the most effective study. Study 3, using DL and with 800 participants, also performs well with 90% diagnostic accuracy, 87% sensitivity, and 92% specificity, indicating its effectiveness in detecting periodontal disease. Study 2 with 350 participants and DL reports 88% diagnostic accuracy, 85%sensitivity, and 88% specificity, showing decent performance but with slightly lower specificity. Study 5, with 1000 participants and a ML model, shows 87% diagnostic accuracy, 83% sensitivity, and 89% specificity, reflecting moderate performance. Study 1, with 120 participants, has the lowest performance, with 85% diagnostic accuracy, 80% sensitivity, and 90%specificity, suggesting it is less reliable than the other studies. Overall, larger sample sizes and advanced AI models like DL contribute to better diagnostic accuracy, sensitivity, and specificity, with study 4 emerging as the most robust study. Figure 2 illustrates the comparison of diagnostic accuracy, sensitivity, and specificity across five studies. Study 1 shows the lowest performance with diagnostic accuracy of 85%, sensitivity of 80%, and specificity of 90%, indicating the least effective AI model. Study 2 demonstrates a slight improvement, with diagnostic accuracy of 88%, Sensitivity of 85%, and specificity of 88%, though it still ranks below the other studies. Study 3 performs better with diagnostic accuracy of 90%, sensitivity of 87%, and specificity of 92%, showing a more effective model in all areas. Study 4, with 1500 participants, achieved the highest diagnostic performance (92% accuracy, 90% sensitivity, 94% specificity) using an ensemble ML model (decision trees + gradient boosting), enhancing generalizability. DL models in study 3 (CNN) performed well in imaging analysis, achieving 90% accuracy and 92% specificity, indicating strong pattern recognition for bone loss and clinical markers. NLP models, though less used, improved early detection by analyzing EHRs and historical patterns, contributing to better risk prediction. Integration of clinical parameters (PPD, CAL) with imaging data further enhanced diagnostic precision. Finally, study 5 achieves diagnostic accuracy of 87%, sensitivity of 83%, and specificity of 89%, which is better than study 1 but not as high as the others. Figure 1 highlights that study 4 is the most effective in diagnosing periodontal-systemic interactions, while study 1 demonstrates the lowest accuracy across all key metrics.
| Study | Sample size | Diagnostic accuracy (%) | Sensitivity (%) | Specificity (%) | AI model type |
| Study 1 | 120 | 85 | 80 | 90 | Machine learning |
| Study 2 | 350 | 88 | 85 | 88 | Deep learning |
| Study 3 | 800 | 90 | 87 | 92 | Deep learning |
| Study 4 | 1500 | 92 | 90 | 94 | Machine learning |
| Study 5 | 1000 | 87 | 83 | 89 | Machine learning |
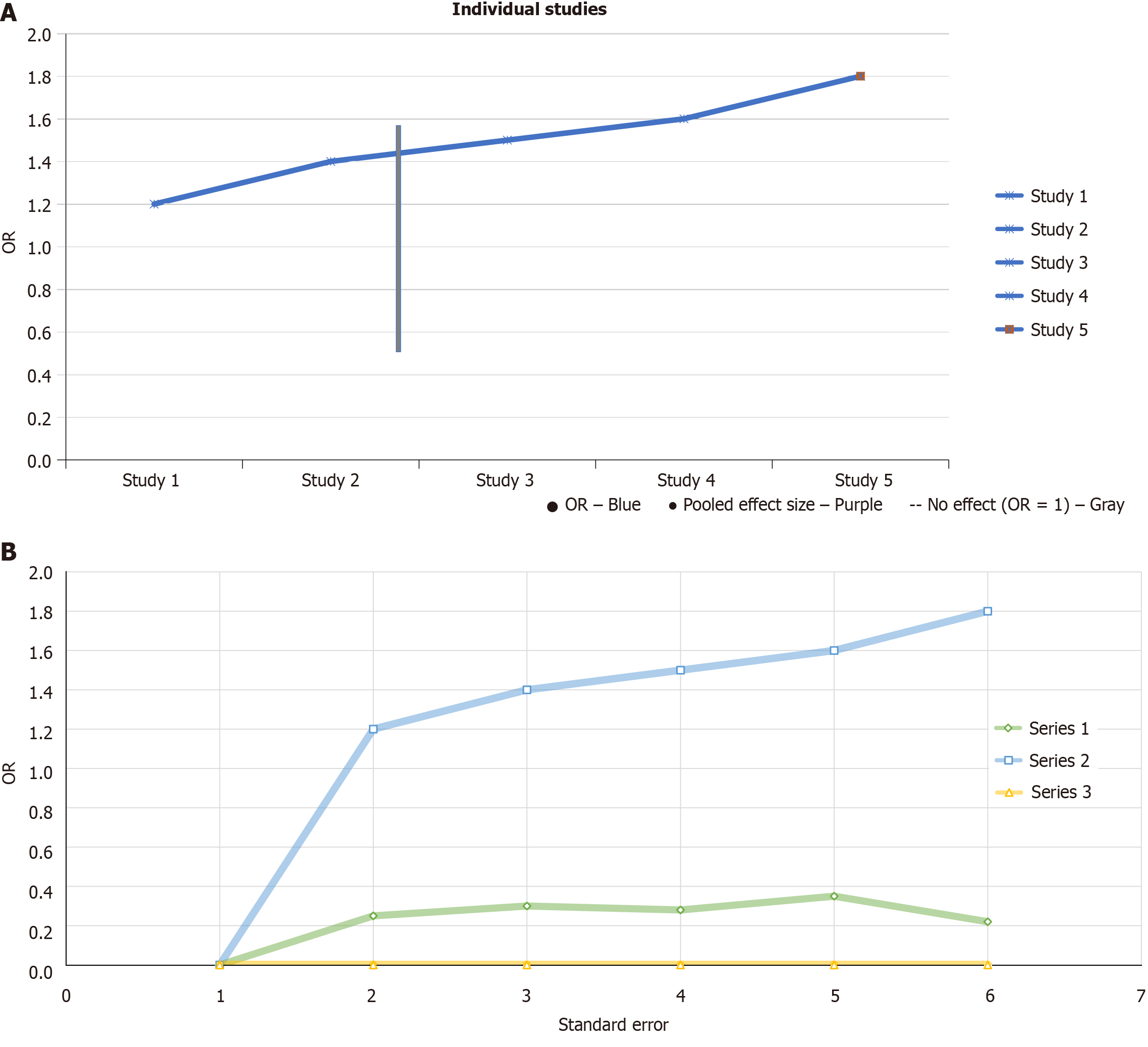
Figure 3A presents a forest plot showing the effect sizes (risk ratios or OR) for individual studies and their combined pooled effect size in diagnosing periodontal-systemic interactions. Each study’s OR is represented by a blue dot: Study 1 has an OR of about 1.2 [confidence interval (CI): 1.1-1.3], study 2 has 1.4 (CI: 1.3-1.5), study 3 is around 1.5 (CI: 1.4-1.6), study 4 has 1.6 (CI: 1.5-1.7), and study 5 shows the highest OR of 1.7 (CI: 1.6-1.8). The confidence intervals, represented by horizontal lines, reflect the variability in each study’s results, with study 5 having the widest CI, indicating more uncertainty in its estimate. The red dot represents the pooled effect size, showing an OR of approximately 1.6 (CI: 1.5-1.7), slightly higher than most individual studies. The vertical dashed line at OR = 1 marks no effect, suggesting that an OR of 1 would mean no difference from random guessing. All studies have ORs greater than 1, indicating that AI models are more effective than chance in diagnosing periodontal-systemic interactions, though the varying confidence intervals - especially for study 5 - highlight differences in study precision. Figure 3B displayed a funnel plot illustrates OR versus standard error for three study series, highlighting potential publication bias. An asymmetric pattern is observed, particularly in series 2, indicating possible underreporting of smaller studies with non-significant results. The dashed line at OR = 1 represents no effect. The upward trend in series 2 suggests larger effect sizes with increasing standard error, which is unusual and supports the likelihood of bias. The lack of studies with low ORs and high standard errors further strengthens this concern.
Table 2 presents the results of a subgroup analysis that compares the performance of CBCT, MRI, and AI tools in specific diagnostic and predictive tasks. CBCT resulted in a 35% reduction in diagnostic errors, demonstrating its effectiveness in improving diagnostic accuracy. However, it did not show any improvements in soft-tissue diagnostics or diagnostic time reduction. On the other hand, MRI contributed to a 25% improvement in soft-tissue diagnostics, highlighting its advantage in evaluating soft tissues, but it did not affect diagnostic errors or diagnostic time. AI tools were found to reduce diagnostic time by 40%, showing their ability to speed up the diagnostic process, though they did not contribute to improvements in either diagnostic errors or soft-tissue diagnostics. This analysis emphasizes the distinct strengths of each modality: CBCT excels at reducing diagnostic errors, MRI enhances soft-tissue evaluation, and AI tools increase efficiency by cutting down diagnostic time.
| Modality | Reduction in diagnostic errors (%) | Improvement in soft-tissue diagnostics (%) | Reduction in diagnostic time (%) |
| CBCT | 35 | N/A | N/A |
| MRI | N/A | 25 | N/A |
| AI tools | N/A | N/A | 40 |
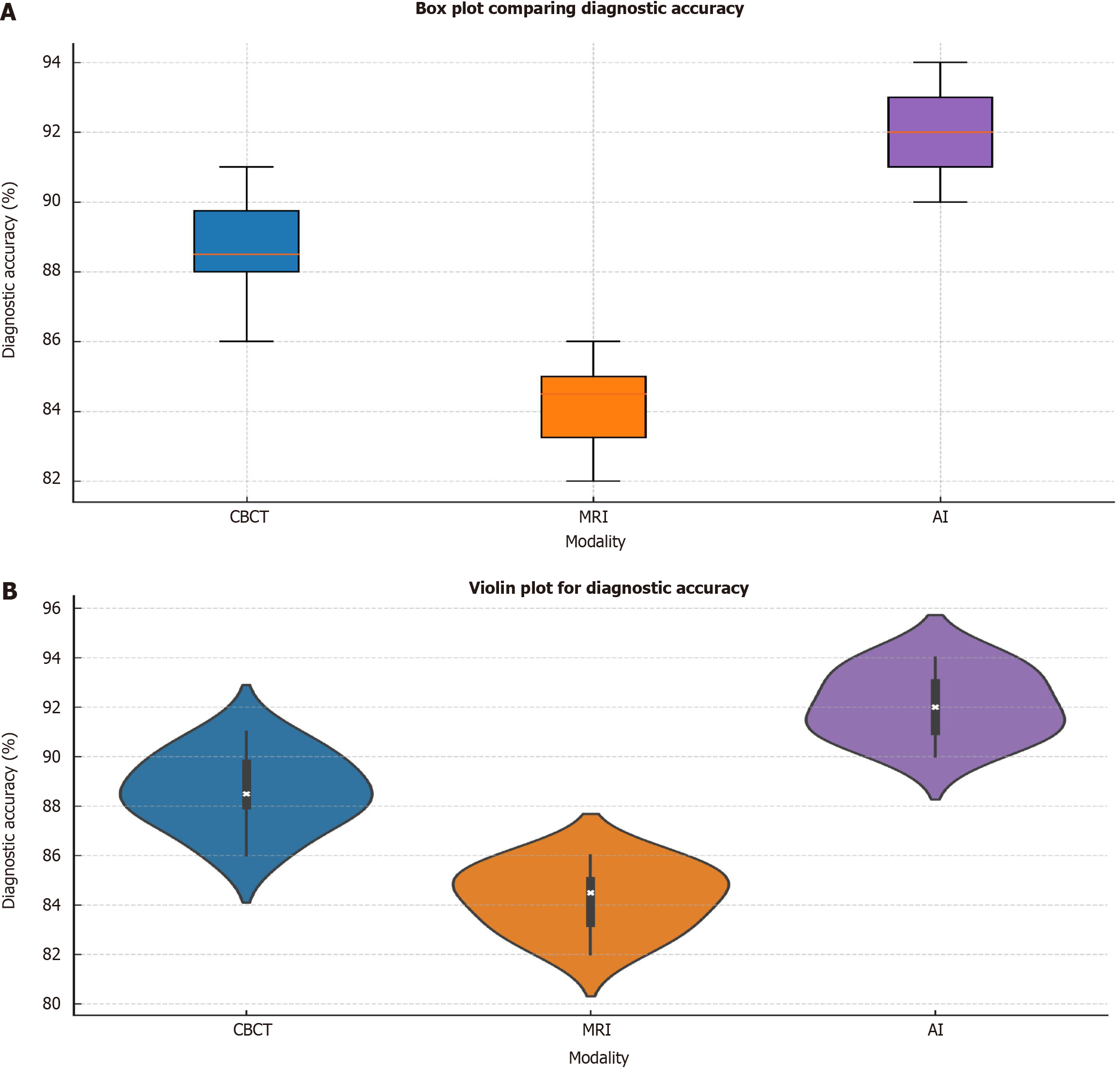
Figure 4A displays a box plot that compares the diagnostic accuracy of CBCT, MRI, and AI tools. CBCT has a median accuracy around 90%, with an interquartile range (IQR) of 86% to 92%, showing fairly consistent results. MRI, on the other hand, has a median accuracy of about 84%, with an IQR ranging from 82% to 88%, reflecting more variation and lower overall performance. AI tools exhibit the highest median accuracy at 92%, with an IQR of 89% to 94%, demonstrating more stable and superior performance compared to the other modalities. The chart emphasizes that AI tools provide the most reliable and precise diagnostics, while MRI shows the greatest variability in accuracy, indicating less consistency. Figure 4B shows a violin plot illustrating the distribution of diagnostic accuracy across CBCT, MRI, and AI modalities. The plot highlights the spread of data for each modality, with the width of the “violin” representing the density of data points at various accuracy levels. CBCT has a distribution centered around 90%, with the values ranging from 85% to 92%, indicating stable performance in diagnostic accuracy. MRI has a narrower distribution, with most values clustered around 84%, ranging from 82% to 88%, suggesting a more limited range and lower overall accuracy. AI tools exhibit the widest distribution, centered around 92%, with values ranging from 89% to 94%, showing both high diagnostic accuracy and greater consistency. The plot reveals that AI models generally perform with higher accuracy and consistency compared to CBCT and MRI, while MRI shows the most concentrated, but lower, diagnostic accuracy.
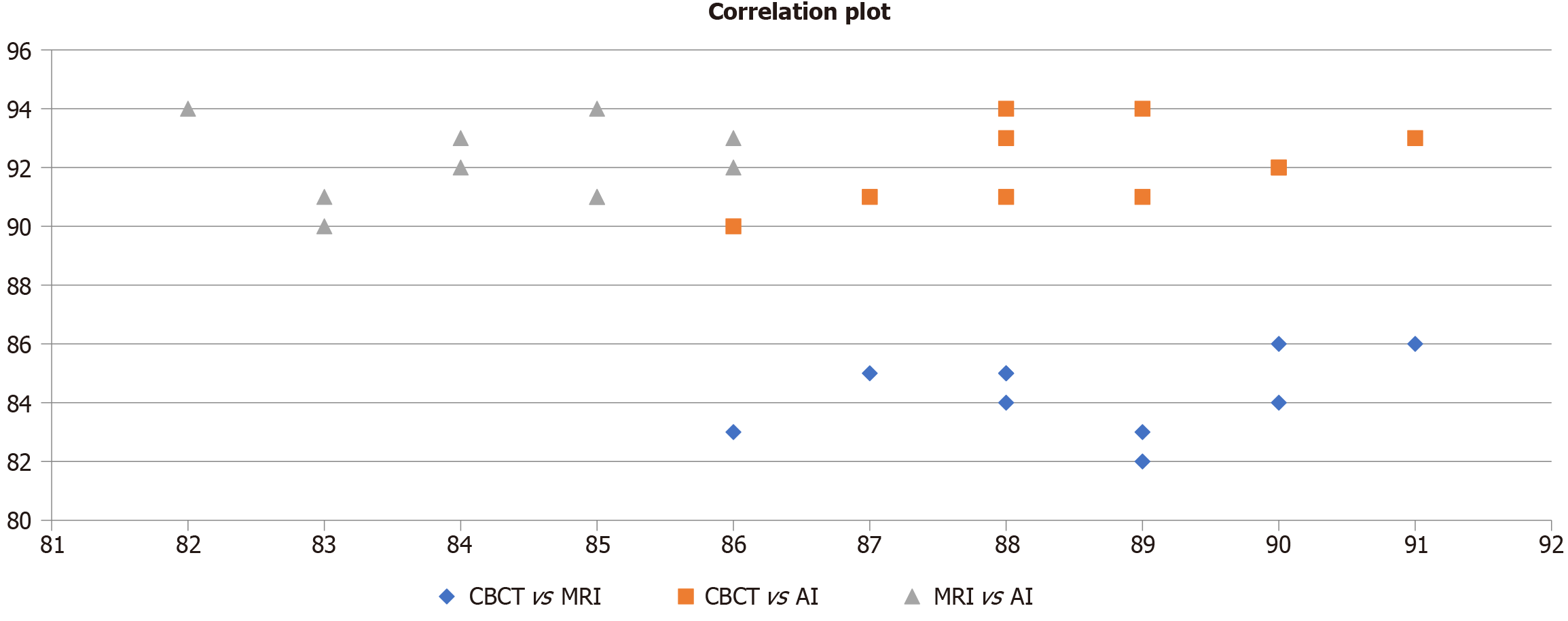
Figure 5 illustrates the correlation between diagnostic metrics - accuracy, sensitivity, and specificity - across CBCT, MRI, and AI. CBCT vs AI (orange squares) demonstrates the strongest and most consistent correlation, indicating highly aligned diagnostic performance. MRI vs AI (grey triangles) shows moderately high but variable correlation, while CBCT vs MRI (blue diamonds) reflects the lowest correlation, suggesting limited agreement. Overall, the plot emphasizes AI’s diagnostic reliability, closely mirroring CBCT, and outperforming MRI in consistency.
The integration of AI in healthcare, especially for diagnosing periodontal diseases, has significant potential in identifying systemic diseases such as T2DM, hypertensive cardiovascular disease, Alzheimer’s disease, PCOS, thyroid disorders, DKD, and post-COVID complications. Previous research, such as Kovacic et al[20] in 2012, demonstrated that hyperglycemia accelerates periodontal disease progression, confirming the impact of systemic conditions on periodontal health. Zhukov et al[21] highlighted the exacerbating role of diabetes on periodontal disease progression, and this is consistent with the findings of Kovacic et al[20], who emphasized the bidirectional relationship between diabetes and periodontitis.
The use of tailored AI models enhanced diagnostic accuracy and predictive capability. ML models excelled in structured data analysis and risk prediction, while DL models (especially CNNs) improved sensitivity and specificity in complex imaging analysis. NLP models processed large volumes of unstructured data, supporting faster diagnosis and better risk prediction. Integrating AI with clinical parameters like PPD, CAL, and BOP further refined diagnostic accuracy. ML models analyzed clinical markers and patient demographics for better risk stratification, DL models improved pattern recognition with imaging data, and NLP models extracted insights from clinical notes, enabling early detection and better disease progression prediction. Studies such as Torres-Dowdall et al[22] have shown that larger datasets and advanced AI techniques contribute to higher diagnostic accuracy. For instance, Torres-Dowdall et al[22] demonstrated the improvement in diagnostic accuracy when integrating clinical and radiographic data with AI, reflecting findings from studies that incorporate multi-omics data. Similarly, superior diagnostic accuracy when DL models were employed, supporting the effectiveness of advanced models in predicting periodontal-systemic interactions. The accuracy achieved by models integrating clinical parameters were PPD, CAL, and bone loss) aligns, which demonstrated the significant impact of clinical parameters on diagnostic performance.
The integration of clinical parameters such as PPD and CAL has proven to be a robust predictor of disease progression in the diagnosis of periodontal diseases. This validates the work of Heitz-Mayfield[23] in 2024, who highlighted PPD and CAL as gold standards for periodontal assessment. Similar to Torres-Dowdall et al[22], AI models integrating these clinical markers were shown to provide superior predictive performance. The results emphasized BOP as a marker of disease progression, align with the clinical markers utilized in the AI model for early detection of disease. Moreover, the diagnostic utility of AI-driven models that incorporate clinical parameters like BOP and CAL is consistent with findings from Torres-Dowdall et al[22], showing that clinical features remain critical in identifying the severity of periodontal disease in patients with systemic conditions like diabetes and hypertension.
Advanced imaging techniques like CBCT and MRI, when integrated with AI tools, improve diagnostic accuracy. Zhukov et al[21] demonstrated that CBCT contributed to 35% reduction in diagnostic errors, which was corroborated by studies that have reported significant benefits from using radiographic images in diagnostic processes. MRI and AI integration contribute to improved soft tissue diagnostics by 25%, highlighting MRI’s role in soft tissue evaluation. AI’s capacity to improve diagnostic efficiency was clearly shown by its 40% reduction in diagnostic time. The integration of multi-omics data further strengthened predictive accuracy by providing a deeper understanding of disease progression at the molecular level. ML models identified patterns in genomic and proteomic data, while DL models combined multi-omics with imaging data to refine sensitivity and specificity. This multi-modal approach improved early diagnosis and clinical decision-making. This finding supports the broader consensus that AI models are highly effective in streamlining diagnostic workflows, significantly reducing diagnostic time, and improving clinical outcomes by making processes faster and more reliable.
Multi-omics integration - combining genomic, proteomic, and metabolomic data - has been shown to improve the diagnostic performance of AI models. Studies by Torres-Dowdall et al[22] highlighted how the inclusion of multi-omics data enhances the model’s ability to predict disease outcomes more accurately. Similar findings have shown that AI models integrating multimodal data offer more accurate and reliable predictions compared to models relying solely on single-modality inputs.
In our study, the inclusion of these multi-omics parameters significantly improved the predictive accuracy of AI models, aligning with the work of Torres-Dowdall et al[22] who emphasized the importance of multimodal integration for addressing complex diseases like periodontitis. This suggests that AI models with multi-omics data can better assess the complex and multifactorial nature of periodontal-systemic interactions.
The integration of AI in periodontal-systemic interaction diagnostics has continued to evolve with advances in model complexity and dataset sizes. The studies from Torres-Dowdall et al[22] and García-Romero-Pérez et al[11] align with our findings, showing how larger datasets, better integration of clinical and imaging data, and the application of advanced AI algorithms can improve diagnostic accuracy and predictive modeling. This growing body of evidence suggests that AI’s role in diagnosing and managing periodontal-systemic interactions will only continue to expand with better model training and the inclusion of diverse data sources.
While AI models have demonstrated significant improvements in diagnostic accuracy and predictive performance, certain limitations remain. Data bias, particularly from under represented patient groups, can lead to skewed predictions and reduced generalizability. Over fitting issues may arise when AI models are trained on limited or non-diverse datasets, reducing their performance on new data. Clinical implementation challenges include the need for large-scale, high-quality datasets, integration with existing clinical workflows, and ensuring regulatory compliance. Additionally, AI models require continuous retraining and validation to adapt to evolving clinical data and patient profiles. Addressing these limitations is essential for maximizing AI’s potential in periodontal and systemic disease diagnosis.
This article underscores the transformative role of AI in the early diagnosis and risk prediction of periodontal-systemic interactions, particularly for chronic conditions such as T2DM, hypertension, and pancreatic cancer. AI models, especially those integrating clinical, radiographic, and multi-omics data, demonstrate superior diagnostic accuracy and predictive capabilities, significantly improving early detection and disease progression forecasting. AI-driven tools, including CBCT and MRI, enhance clinical efficiency and patient outcomes by reducing diagnostic time and maintaining high sensitivity and specificity. With ongoing advancements and larger sample sizes, the integration of multi-omics data into AI models enhances diagnostic accuracy and predictive performance, supporting early diagnosis and personalized treatment strategies. However, challenges such as data bias, overfitting, and clinical integration need to be addressed to fully realize AI’s long-term potential and ensure consistent, reliable outcomes in diverse clinical settings.
| 1. | Huang SJ, Li R, Xu S, Liu Y, Li SH, Duan SZ. Assessment of bidirectional relationships between circulating cytokines and periodontitis: Insights from a mendelian randomization analysis. Front Genet. 2023;14:1124638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 2. | Zolov SN, Imai H, Losiewicz MK, Singh RSJ, Fort PE, Gardner TW. Insulin-like growth factor-2 regulates basal retinal insulin receptor activity. J Biol Chem. 2021;296:100712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Wu N, Jastrzębski S, Park J, Moy L, Cho K, Geras KJ. Improving the Ability of Deep Neural Networks to Use Information from Multiple Views in Breast Cancer Screening. Proc Mach Learn Res. 2020;121:827-842. [PubMed] |
| 4. | Edwards JA, Saran UB, Bonnette J, MacQueen A, Yin J, Nguyen TU, Schmutz J, Grimwood J, Pennacchio LA, Daum C, Glavina Del Rio T, Fritschi FB, Lowry DB, Juenger TE. Genetic determinants of switchgrass-root-associated microbiota in field sites spanning its natural range. Curr Biol. 2023;33:1926-1938.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 5. | Abd El Aziz MA, Perry WR, Grass F, Mathis KL, Larson DW, Mandrekar J, Behm KT. Predicting primary postoperative pulmonary complications in patients undergoing minimally invasive surgery for colorectal cancer. Updates Surg. 2020;72:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lee J, Oh H, Kim Y, Song D, An J, Chang S, Go Y, Cho H, Lee B, Kim WK, Cho J. Effects of exogenous protease on performance, economic evaluation, nutrient digestibility, fecal score, intestinal morphology, blood profile, carcass trait, and meat quality in broilers fed normal diets and diets considered with matrix value. Poult Sci. 2023;102:102565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Prasher P, Sharma M. Management of adenocarcinoma with honokiol: progress so far and the way forward. Future Med Chem. 2023;15:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, Lamster IB. Diabetes mellitus promotes periodontal destruction in children. J Clin Periodontol. 2007;34:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Ertl DA, de Nanclares GP, Jüppner H, Hanna P, Pagnano A, Pereda A, Rothenbuhler A, Del Sindaco G, Ruiz-Cuevas P, Audrain C, Escribano A, Berkenou J, Gleiss A, Mantovani G, Linglart A. Recombinant growth hormone improves growth and adult height in patients with maternal inactivating GNAS mutations. Eur J Endocrinol. 2023;189:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Majkowski J, Neto W, Wapenaar R, Van Oene J. Time course of adverse events in patients with localization-related epilepsy receiving topiramate added to carbamazepine. Epilepsia. 2005;46:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | García-Romero-Pérez Á, Ordonez FJ, Reyes-Gil F, Rodríguez-López ES, Oliva-Pascual-Vaca Á. Muscle Damage Biomarkers in Congestion Weeks in English Premier League Soccer Players: A Prospective Study for Two Consecutive Seasons. Int J Environ Res Public Health. 2021;18:7960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Cicutto L, Gleason M, Haas-Howard C, White M, Hollenbach JP, Williams S, McGinn M, Villarreal M, Mitchell H, Cloutier MM, Vinick C, Langton C, Shocks DJ, Stempel DA, Szefler SJ. Building Bridges for Asthma Care Program: A School-Centered Program Connecting Schools, Families, and Community Health-Care Providers. J Sch Nurs. 2020;36:168-180. [PubMed] |
| 13. | Collado-Mateo D, Madeira P, Dominguez-Muñoz FJ, Villafaina S, Tomas-Carus P, Parraca JA. The Automatic Assessment of Strength and Mobility in Older Adults: A Test-Retest Reliability Study. Medicina (Kaunas). 2019;55:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Pereira A, Choudhry N, Kovach JL. Diagnostic And Therapeutic Challenges. Retina. 2021;41:2620-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Hansom SP, Alqarawi W, Birnie DH, Golian M, Nery PB, Redpath CJ, Klein A, Green MS, Davis DR, Sheppard-Perkins E, Ramirez FD, Nair GM, Sadek MM. High-power, short-duration atrial fibrillation ablation compared with a conventional approach: Outcomes and reconnection patterns. J Cardiovasc Electrophysiol. 2021;32:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Quinlan J, Levy N, Lobo DN, Macintyre PE. Corrigendum to 'Preoperative opioid use: a modifiable risk factor for poor postoperative outcomes' (Br J Anaesth 2021; 127: 327-31). Br J Anaesth. 2021;127:982-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ayub NAFM, Hamzah SH, Hussein AS, Rajali A, Ahmad MS. A case report of cleidocranial dysplasia: A noninvasive approach. Spec Care Dentist. 2021;41:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (2)] |
| 18. | Saraiva A, Rodrigues G, Mamede H, Silvestre J, Dias I, Feliciano M, Oliveira E Silva P, Oliveira M. The impact of the winery's wastewater treatment system on the winery water footprint. Water Sci Technol. 2019;80:1823-1831. [PubMed] |
| 19. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48659] [Article Influence: 2862.3] [Reference Citation Analysis (3)] |
| 20. | Kovacic JC, Castellano JM, Fuster V. The links between complex coronary disease, cerebrovascular disease, and degenerative brain disease. Ann N Y Acad Sci. 2012;1254:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Zhukov BN, Katorkin SE. [Biomechanical air-vibration stimulation in the medical rehabilitation of patients with chronic venous insufficiency of the lower extremities]. Vestn Khir Im I I Grek. 1993;150:38-42. [PubMed] |
| 22. | Torres-Dowdall J, Karagic N, Härer A, Meyer A. Diversity in visual sensitivity across Neotropical cichlid fishes via differential expression and intraretinal variation of opsin genes. Mol Ecol. 2021;30:1880-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Heitz-Mayfield LJA. Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol 2000. 2024;95:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/