Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.105305
Revised: April 7, 2025
Accepted: April 24, 2025
Published online: December 20, 2025
Processing time: 199 Days and 4.4 Hours
Colorectal polyps remain a significant health concern because they can develop into cancer. Therefore, accurate assessment and diagnosis of polyps, along with appropriate treatment decisions, are crucial in preventing complications or ma
Core Tip: Scarred and complex colorectal polyps remains a big concern because of fibrosis, technical complexity, and excessive recurrence rates. Traditional endoscopic techniques, which includes endoscopic mucosal resection, can be useless in these instances. Emerging alternatives, inclusive of endoscopic submucosal dissection, endoscopic full-thickness re
- Citation: Tawheed A, Hafez MM, Ismail A, Madkour A. Scarred and complex colorectal polyps: Traditional techniques and emerging alternatives. World J Methodol 2025; 15(4): 105305
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/105305.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.105305
Colorectal polyps are gastrointestinal polyps defined as abnormal growths that protrude in the lumens of the colon or rectum and are often discovered during colonoscopies. There are various types of polyps; they differ in size, morphology, and histological characteristics. The size and morphology of the polyps define the modality of management. In addition, the histological classification of polyps determines the follow-up plan, as described by Bujanda et al[1].
As explained in Repici et al[2] and Longcroft-Wheaton et al[3], most detected polyps during screening colonoscopies are small, benign, and easily managed endoscopically. However, around 10%-15% of colorectal polyps are classified as “complex”. Recent studies fail to make a clear differentiation between complicated polyps and scarred polyps. The WEO Consensus on Colorectal Lesions Classification describe complex polyps relied on certain characteristics, such as location, size (> 2 cm), and surface characteristics that make endoscopic removal hard. Scarred polyps distinguished by submucosal fibrosis, which might be due previous procedurues such as partial resections or biopsies. While complex polyps may need progressive resection techniques, such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), scarred polyps commonly need specific techniques to overcome fibrosis, such as traction techniques or full-thickness resection (EFTR). The word complex reflects the varying degrees of complexity in size, shape, extent, location, and scarring contributing ultimately to the resection complexity using conventional endoscopic methods. Complex polyps are generally defined as any lesion whose endoscopic resection is technically difficult because of its (1) size (> 20 mm); (2) morphology (sessile); (3) extent (polyps occupying greater than a third of the lumen circumference, wrapping a fold (clam-shell polyps), and polyps crossing over two haustral folds); (4) location (right side, ileocecal valve, appendiceal orifice, sigmoid colon, dentate line, and inner curve of hepatic and splenic flexures); or (5) presence of inflammatory bowel, disease (IBD), diverticulosis, strictures, or scarred polyps from previous biopsy or failed polypectomy as shown in Vormbrock et al[4], Jung et al[5], Sanchez-Yague et al[6], Hintze et al[7], Iishi et al[8], Waye et al[9] and Mönkemüller et al[10]. Technical difficulty can be assessed via the size/morphology/site/access (SMSA) scoring system, which helps predict the risk of incomplete resection, adverse events, and recurrence based on the scoring system (Table 1), prescribed by Sansone et al[11] and Sidhu et al[12]. Over the past decade, numerous attempts have been made to manage nearly all types of colorectal polyps including polyps with local invasive carcinoma. Baker et al[13] descriped different endoscopic techniques for managing scarred and complex polyps include EMR and submucosal ESD. These methods have an important role, especially when polyps are contained within the mucosa with no lymph nodal involvement. In situations where traditional endoscopic approaches fail in treating scarred polyps, more advanced techniques such as EFTR are used. In addition, innovative devices such as Zaghloul et al[14] introduced EndoRotor, which show a solution using endoscopic powered resection (EPR), which is a non-thermal device that provides a safe and effective alternative for removing scarred polyps with a success rate of 84% with minimal complications. The early resection of polyps including scarred polyps could prevent colorectal cancer. These polyps have a high recurrence rate, highlighting the importance of diagnostic and post-endoscopic resection surveillance strategies. Kandiah et al[15] emphasized that, managing scarred and complex polyps is challenging due to technical complexity and the high risk of complications. Usually managing them requires skilled endoscopists equipped with the necessary tools. The current advances in research and endoscopic techniques and technologies provide tools to ensure effective treatment while prioritizing patient safety. This review aimed to discuss the types, diagnosis, and management of scarred and complex colorectal polyps. Additionally, despite the fast progress of endoscopic technologies, recent studies on cutting-edge techniques as artificial intelligence (AI)-assisted classification and molecular imaging still understated in the current literature. Combining these advancements is crucial for diagnostic accuracy and therapeutic strategies.
| Factor | Benchmarks | Points |
| Size | < 1 cm | 1 |
| 1–1.9 cm | 3 | |
| 2–2.9 cm | 5 | |
| 3–3.9 cm | 7 | |
| > 4 cm | 9 | |
| Morphology | Pedunculated | 1 |
| Sessile | 2 | |
| Flat | 3 | |
| Site | Left | 1 |
| Right | 2 | |
| Access | Easy | 1 |
| Difficult | 3 |
Numerous unresolved concerns persevere despite significant advancements have been made in the management of scarred polyps. One key gap is the lack of a comprehensive, systematic approach adding multimodal treatment pathways for these lesions. This review aims to bond this gap by explaining the first organized integration of different therapeutic decisions and presenting decision-making framework relay on the SMSA-Scar Index.
According to D'Amico et al[16], Burgess et al[17], Nusko et al[18], and Myung et al[19], the first step in polyps’ classification is the endoscopic assessment of the lesion with high-definition white light imaging. This allows the evaluation of fold convergence, edge retraction, expansion/thickened folds, firm consistency, and erythema. However, the assessment of submucosal invasive cancer risk appears to be associated with increasing size, recto-sigmoid location, and surface morphology. A basic polyps classification is the lateral spreading lesions (LSL) classification. LSL classification generally classifies polyps as: (1) Granular Lateral spreading lesions (G-LSL), representing polyps that spread laterally and circumferentially rather than vertically; (2) Granular lateral spreading lesion with dominant nodule (G-LSL with dominant nodule); and (3) Non-granular lateral spreading lesion.
The difference between non-lifting polyps and scarred polyps is often neglected, leading to ineffective treatment strategies. Scarred polyps mainly occur due to submucosal fibrosis arising from prior events like biopsies, partial resections, or ablative therapy. As the changed tissue compliance caused by this fibrosis, traditional lifting techniques are less effective. Nevertheless, not every polyp that does not lift is scarred. Other causes of non-lifting behaviour contain deep invasive cancer, submucosal tumours (like lipomas or carcinoids), or inadequate submucosal injection. It is critical to differentiate between these causes since submucosal tumours might require different diagnostic and treatment approaches, such as endoscopic ultrasound (EUS) evaluation before resection, however fibrotic polyps may advantage from techniques such as EFTR or hybrid ESD. Non-lifting polyps are characterized by failure of elevation after injecting the submucosa, leading to failure of resection using endoscopic techniques, making the management a bit complicated. Due to submucosal fibrosis presenting with non-elevating polyps leading to lack of elevation during the procedure, methods such as EMR may fail to successfully manage these polyps completely, as explained in Friedland et al[20]. However, EPR using EndoRoto is recommended to treat non-lifting scarred polyps.
Polyps with fibrosis or scars often result from prior failed attempts of resection or less likely biopsies, leading to anchored bases with failure to elevate after injecting the base, making them less suitable for conventional endoscopic techniques There are different types of fibrosis mainly primary fibrosis, which is commonly linked to chronic inflammatory diseases like Crohn's disease or ulcerative colitis and occurs without any prior endoscopic or surgical procedures. Other forms of fibrosis can cause scarred polyps, which alters the causes and approaches of treatment. It may be essential to establish combine therapeutic treatment like biologics or anti-fibrotic drugs with specific endoscopic techniques to heal inflammatory-driven fibrosis. EMR, ESD, or surgery are samples of prior procedures that can result in secondary fibrosis. Due to the dense submucosal scarring, these lesions are usually challenging to eliminate endoscopically, require specific procedures such EFTR, traction-assisted ESD, or hybrid resection approaches as mentioned in Ayoub et al[21]. Ichkhanian et al[22] noted that, this lack of elevation could also lead to difficulty in containing the polyp in the polypectomy snare either in hot polypectomy, cold polypectomy, or EMR techniques, thus increasing the risk of diathermy-induced perforation. Ichkhanian et al[22] reported that, fibrosis increases the risk of adverse events during resection in general and is linked with high rates of recurrence ranging from 10-20% post-resection. Moreover, incomplete removal of these scarred polyps can elevate the risk of colon cancer.
Hao et al[23] highlighted that, large polyps more than 20 mm in size can be flat or bulky in appearance, frequently extending across multiple haustral folds and in a large portion of lumen circumference. Managing these polyps is challenging due to their bulky nature and extent. Moreover, the difficulty in managing sessile polyps increases when these large polyps are present in certain areas such as the right side of the colon, ileocecal valve, or near the dentate line. Sessile and flat polyps should also be classified as granular (homogeneous or nodular-mixed) or non-granular (elevated or pseudodepressed), as these two different types are associated with certain risks of submucosal invasive cancer (SMIC). The SMIC risk was found to be higher in polyps of nongranular pseudodepressed nature, followed by polyps of granular nodular-mixed nature, then polyps with nongranular flat-elevated nature, and at last come granular-homogeneous types.
Integration between the scarred polyp classification and treatment approaches can improve clinical decision-making. For Low-Fibrosis Polyps (SMSA Score < 8, Minimal Scarring) EMR with submucosal lifting is the favoured technique. Cold snare resection may be beneficial for smaller lesions (Partial Submucosal Fibrosis, SMSA Score 8–10) Moderate-Fibrosis Polyps Traction-assisted ESD or hybrid ESD (ESD-assisted EMR) are the chosen approaches. Also if lifting still feasible Piecemeal EMR can be done. If restricted to superficial layers, EFTR (Endoscopic Full-Thickness Resection) is the favoured technique for high-fibrosis polyps (severe submucosal fibrosis, SMSA Score > 10). Other substitutes contain laparoscopic-assisted endoscopic resection for challenging colonic locations, like the flexures or right-sided colon.
Recently, to aid in diagnosing invasive lesions, the United States Multi-Society Task Force guidelines recommend a second step of assessment using aids such as the Paris classification, and virtual chromoendoscopy (such as Narrow Band Imaging, or chromoendoscopy).
Axon et al[24] described that, Paris classification takes the polyps’ morphology into focus, classifying polyps as protruding (0-Is—sessile, 0-Ip—pedunculated, and Isp—semi-pedunculated), flat (elevated 0-IIa, flat 0-IIb, and depressed 0-IIc) and excavated (Type 0-III). Excavated lesions are rarely seen in the colon. Depressed lesions have an increased risk of malignancy (30%–50% of cases). Recent researches highlighted the elevated malignancy risk linked to depressed-type colorectal lesions, mainly those showing asymmetrical surface patterns and Kudo pit pattern type V. Regarding recent ESGE guidelines (2024), risk factors comprise Irregular or interrupted vascular construction on narrow-band imaging (NBI), occurrence of deep submucosal invasion (SM3 or beyond) relyed on high-resolution magnification endoscopy, eventually histological features like high-grade dysplasia or initial submucosal carcinoma. AI models can improve real-time estimation of malignancy possibility in depressed-type lesions, enhancing sensitivity while preserving specificity. Integrating these risk stratification tools can significantly affect management choices, guiding the choice of en bloc resection via ESD against early surgical intervention when deep invasion is supposed.
Complex and scarred polyps are usually diagnosed in endoscopic procedures. This could be done with the help of some extra functions integrated into the processor or aids such as dye spray chromoendoscopy (Kudo classification). Buchner et al[25] described chromoendoscopy as a valuable tool for diagnosing colorectal abnormalities by applying a dye spray, like Indigo Carmine, to accentuate mucosal irregularities and outline the surface, border, and any depressions in polyps. Chromoendoscopy can diagnose flat or depressed lesions linked to invasive cancer, even if they are small.
Another helpful function to characterize colonic polyps is the magnification of endoscopy, which improves the visualization of pit patterns on surfaces of polyps that predict underlying pathology. Iwatate et al[26] reported that through magnifying chromoendoscopy, the pit patterns of the polyp could be identified, leading to risk stratification for the possibility of submucosal invasion. Type I polyps have the least risk of invasion, while type V polyps have the highest risk. This information can predict malignant changes and guide the selection of the most appropriate treatment modality.
NBI is another method that can be used to detect malignant transformation of colorectal polyps by assessment of microcapillary architecture – the meshed capillary pattern. Disorganization of this pattern indicates dysplasia. Sano classification depends on this pattern and classified polyps from I to III while III are the polyps with malignant changes. The Narrow Band Imaging International Colorectal Endoscopic (NICE) classification, also, allows examination of the surface characteristic of a polyp based on surface appearance, color, and vessel pattern. The NICE classification has a high specificity, but a low sensitivity for detecting invasive cancer, as menthioned in Hewett et al[27], Hayashi et al[28] and Zhou et al[29]. Sumimoto et al[30] described that Japanese Narrow Band Imaging Expert Team (JNET) further divides type 2 into JNET 2a (conventional adenoma) and JNET 2b (adenoma with high-grade dysplasia or superficial SMIC). The WASP criteria, based also on NBI findings, was developed to help identify sessile serrated lesions. Utsumi et al[31] described Flexible spectral imaging color enhancement (FICE) as an another imaging technique available with modern Fujinon colonoscopes, and detecting surface patterns can also differentiate between polyp types. FICE is quite similar to NBI, but some studies show that FICE could differentiate between neoplastic and non-neoplastic polyps without magnification.
Shibagaki et al[32] showed that acetic acid has been a promising addition to advanced imaging techniques for assessing colorectal polyps. When used during colonoscopy, acetic acid causes a temporary whitening of mucosal tissue, highlighting subtle changes that may indicate dysplasia or malignancy. This method facilitates the distinction between neoplastic and non-neoplastic lesions based on their reaction to acetic acid application.
According to Tombazzi et al[33], EUS holds great potential in characterizing colorectal polyps. Unlike CT and MRI, EUS provides T-stage assessment, particularly for rectal tumors. Its accuracy ranges from 55% to 91% for all stages of rectal cancer and from 25% to 98% for T1 tumors. According to a study by Hurlstone et al[34], demonstrated EUS’s high accuracy in staging malignant polyps. Their evaluation of Paris II sessile malignant polyps accurately staged the invasion depth in 93% of cases. Furthermore, EUS plays a crucial role in assessing for residual disease after polypectomy and can identify the location of previously excised polyps, as described in Marone et al[35]. Despite being a method for assessing submucosal lesions and assessing depth of invasion, EUS has certain boundaries on its application. Due to alterations in bowel wall thickness and gas interferences in right-sided colon polyps, EUS might not correctly measure deep submucosal or muscularis propria involvement. The tortuosity of the right colon and angulation can bound efficient probe placing, possibly decreasing diagnostic accurateness. Compared to high-resolution endoscopy with NBI or AI-assisted endoscopic techniques, EUS may be nominal in differentiating among fibrotic and neoplastic tissue in scarred polyps.
As explained in O’Brien et al[36], histological examination is critical to categorize polyps, including scarred and complex polyps, and to choose the appropriate treatment after differentiating between neoplastic and non-neoplastic polyps. Nearly 70% of colorectal polyps are of adenomatous origin and are characterized by dysplastic glandular epithelium, which might be low- or high-grade dysplasia. These adenomas can be classified into tubular, which is the most common type, with over 80% of adenomatous polyps, then tubulovillous, with nearly 15% of adenomatous polyps, and villous adenoma, with less than 5%, according to World Health Organization criteria. Also, it was found in Julka et al[37] that nearly 95% of cases of adenocarcinoma of the colon are of adenomatous polyp origin. Serrated lesions show distinct genetic features and structures when compared to traditional adenomas. Definite molecular pathways are closely associated with the probability of malignant transformation in serrated lesions. Recent researches have confirmed the importance of vital genetic and epigenetic adjustments, just like the strong relationship between BRAF mutations and sessile serrated lesions (SSLs) and the serrated neoplasia pathway that could give up with CpG island methylation and the development to colorectal most cancers. Typical serrated adenomas (TSAs), which have another carcinogenic path, are more likely to have KRAS mutations. The change from serrated polyps to colorectal most cancers can be augmented through high tiers of CpG island methylation that may silence tumor suppressor genes.
BRAF-mutated SSLs commonly reveal CIMP-high lesions, which are related to an increased danger of malignant conversion. Assessing theses genetic alterations may help in improving management strategies, as SSLs with BRAF mutations and CIMP-high status may need close intervals for surveillance as the risk of malignant transformation is high. KRAS-mutated TSAs might advantage from targeted therapeutic approaches.
The serrated polyps include sessile serrated adenomas, conventional serrated adenomas, hyperplastic polyps, and mixed hyperplastic/adenomatous polyps. These polyps have serration and have rapid malignant transformation when compared to others as described in Szylberg et al[38]. Histology of the biopsy sample from polyps is very important as it may show submucosal invasion. Qudah et al[39] noted that biopsy samples may not always suggest invasion into the submucosa, which can result in false-negative results when compared to the total specimen. Consequently, histopathology reports should include a requirement stating that there is no evidence of malignant transformation in the examined tissue. However, it should also alert that a more serious pathology cannot be ruled out if part of a larger lesion. So, histopathological examination of colorectal polyps is essential for making the ideal treatment decisions.
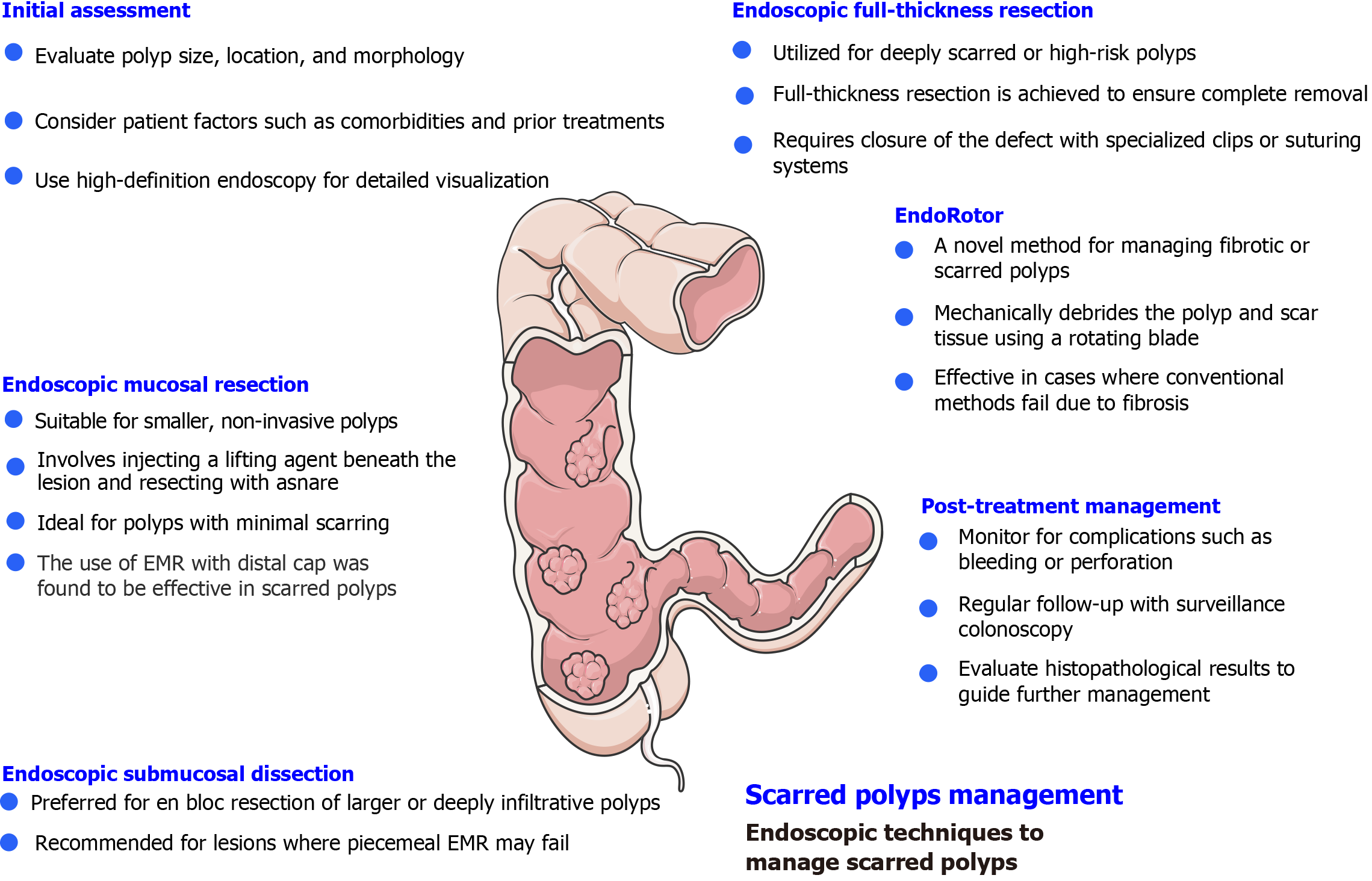
Management of scarred and complex polyps has always been challenging, and in the early days, surgical interventions were the sole option. However, with the recent advancements in endoluminal endoscopy and resection techniques for gastrointestinal polyps and tumors, the landscape has changed significantly (Figure 1).
Gaglia et al[40] mentioned that argon plasma coagulation is one of the currently used modalities to treat scarred polyps, but it has a high recurrence rate. On the contrary, ESD provides en-bloc resection, which is highly needed, it could be technically challenging and associated with high failure rates As described in Azzolini et al[41]. Also, Zaghloul et al[14] noted that EFTR allows for en-bloc resection, but it is limited to polyps up to 30mm. As noted in Wilson et al[42], EPR is a new method recently introduced and showed promising results in the management of scarred polyp. A study in Kandiah et al[15] exploring the feasibility of using an EndoRotor showed a success rate of up to 84% in managing scarred polyps in the rectosigmoid colon, indicating the potential of this tool to manage scarred polyps without the need for surgery.
EMR is a minimally invasive procedure used for the removal of colorectal polyps, particularly those without a stalk. Resection of the polyps by this technique is by injecting a solution into the submucosa to make a cushion which prevents deep thermal injury and reduces procedure duration. Saline is the most common agent used for injection. However, the American Society for Gastrointestinal Endoscopy recommended the use of more viscous agents such as succinylated gelatin, hydroxyethyl starch, Eleview, or glycerol as noted in Hwang et al[43]. These agents could help in shorter procedure duration, a lower rate of post-polypectomy bleeding, and a higher rate of successful and en-bloc resections. Chaptini et al[44] emphasized that diluted adrenaline can be used as it allows better visualization and induces vasoconstriction to reduce bleeding risk. After injection of the solution, the lesion is lifted from muscularis propria and dissected by polypectomy snare. Cold snare is also used, especially with serrated polyps sized between 10 mm and 20 mm. Mann et al[45] underlined that EMR can be performed either en-bloc or piecemeal techniques. However, en-bloc resection is preferred over piecemeal EMR because it allows for histological assessment of the lesion’s margin, especially when malignancy or deep invasion is suspected. Additionally, the recurrence rate for individuals who undergo piecemeal EMR is 20% higher compared to en-bloc resection.
Underwater EMR (uEMR) is a specialized technique that enables polyps removal by filling the colon’s lumen with fluid after suctioning the air. Once underwater, due to the high fat density of the polyp, it is separated from the muscularis propria, reducing the risk of snare entrapment of the muscle layer. Intraluminal water also serves as a heat sink, preventing the deeper colonic wall from thermal damage. Additionally, it is believed that uEMR reduces the risk of recurrence, perforation, and post-polypectomy syndrome.
The primary advantage of uEMR lies in the low distension of the bowel lumen, which allows for the capture of a significantly larger mucosal surface area by the snare. Furthermore, fluid immersion reduces the colonic haustral folds, making both sides of the fold more planar, facilitating easier en-bloc resection, and making the management of complex polyps in terms of size achievable.
In a retrospective study in Schenck et al[46] comparing uEMR and standard EMR, uEMR demonstrated a higher rate of complete macroscopic resection (98.6% vs 87.1%) and a lower rate of recurrence (7.3% after uEMR compared to 28.3% after standard EMR), especially for large lesions > 1.5 cm. A meta-analysis prescribed in Chandan et al[47] that included a total of 7 trials investigating the efficacy and safety of uEMR compared to conventional EMR, reported a higher efficacy in terms of en-bloc resection and lower recurrence rates for uEMR.
Another EMR technique that has been reported to be effective in resecting scarred polyps is the distal cap-assisted EMR (EMR-DC) technique. In Douglas et al[48], a study that included 61 patients who underwent EMR-DC for scarred lesions. The authors reported a clinical success of 100% while the 6-month recurrence rate was as low as 9.8%.
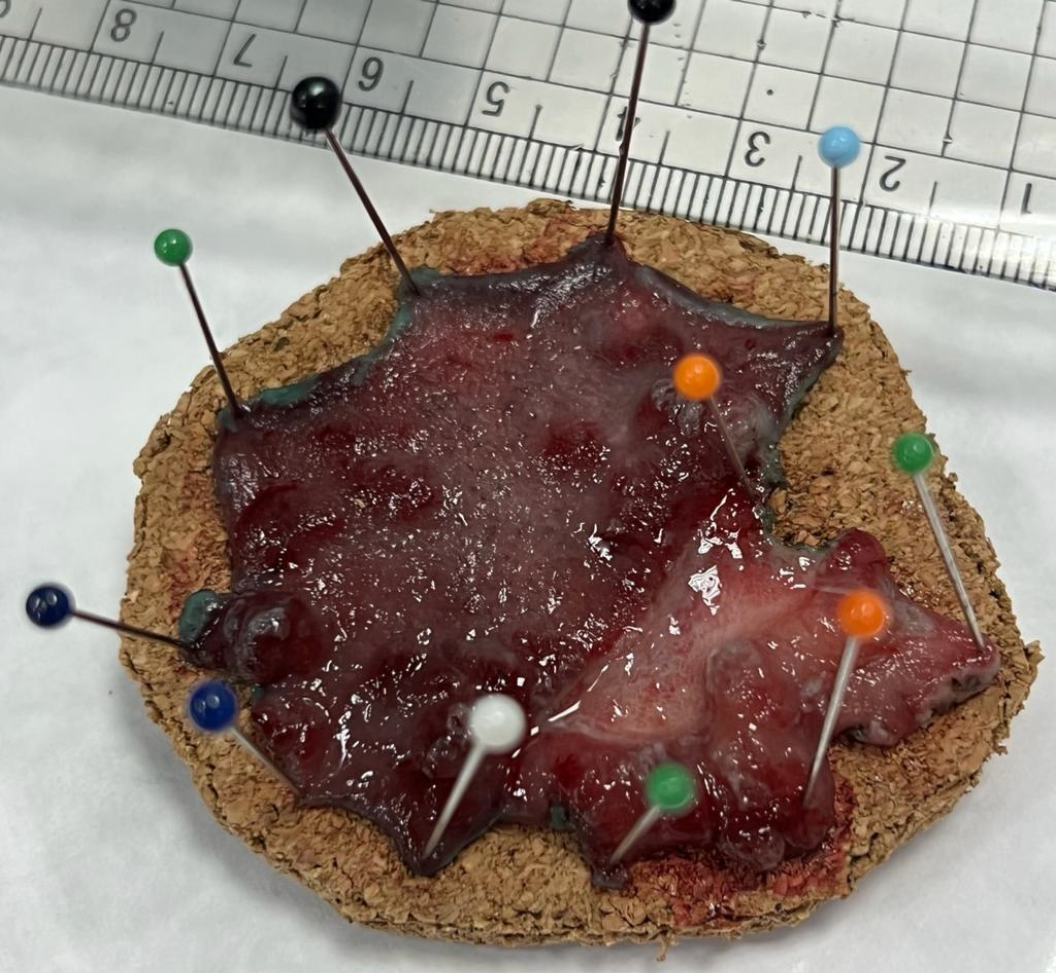
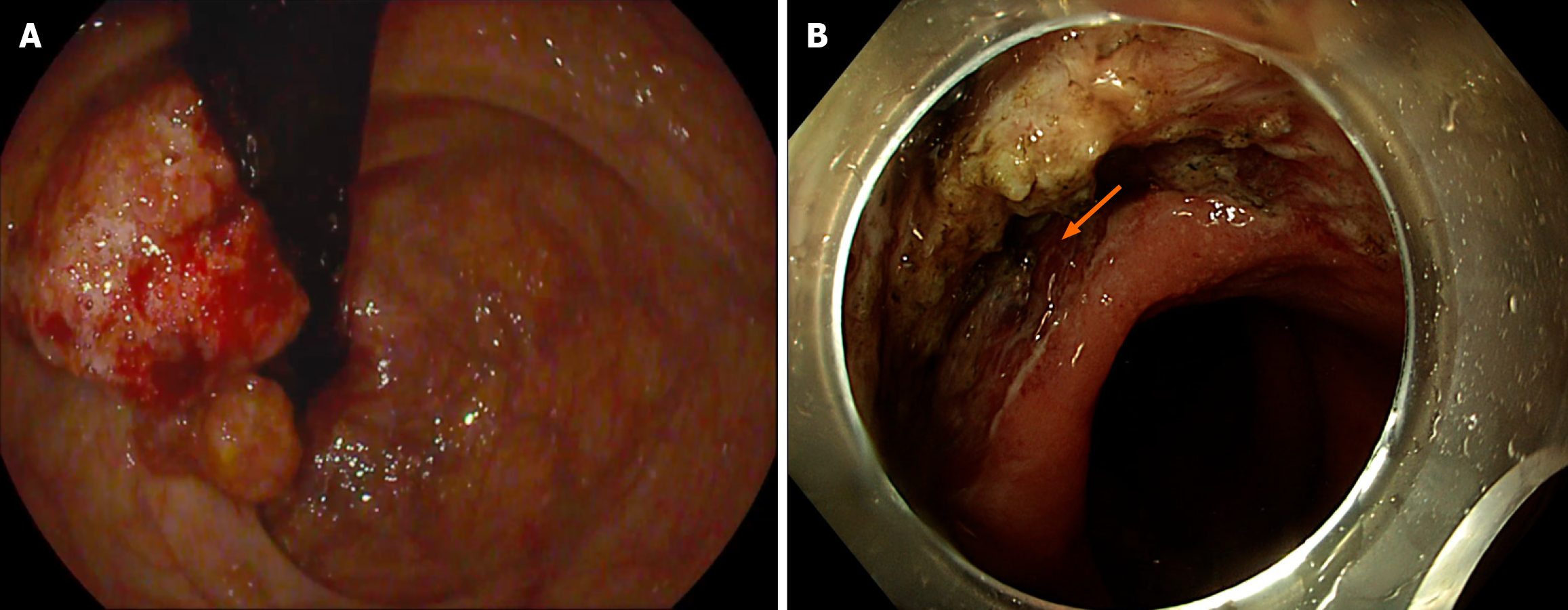
ESD, a technique that can be used to resect large polyps en-bloc (Figure 2), especially flat polyps, has proven to be a rescue therapy. If not for ESD, these polyps would have been piecemeal resected using EMR, making them at higher risk of recurrence. Moreira et al[49] described that ESD involves submucosal injection to lift the lesion, followed by a circumferential incision of the mucosa (Figure 3). After that, the submucosa above the muscularis propria is dissected. While ESD is an effective technique for managing polyps, it requires considerable technical skill and experience to be successfully and safely performed. In addition, in cases such as scarred polyps, it may not be a viable option since lifting after injection usually fails. The American Gastroenterology Association recommends ESD for large polyps to ensure complete resection or those polyps with a high risk of carcinoma involvement as noted in Draganov et al[50]. ESD is highly effective in managing early rectal cancers (T1, where cancer is confined to the submucosa) and offers a minimally invasive approach. Jung et al[51] underlined that ESD has shown promising results in the management of complex polyps, such as recurrent polyps after EMR, tumors associated with IBD, and large colorectal polyps.
Endoscopic full-thickness resection represented a new hope for the treatment of colorectal polyps, especially complex polyps or those that have previously been manipulated. Schurr et al[52] noted that Over-the-scope clips have significantly enhanced the safety and practicality of EFTR. EFTR enables the resection of lesions extending deep to the submucosa, addressing challenges commonly encountered by conventional endoscopic methods or specific anatomical locations (close to a diverticulum or appendiceal orifice) as described in Mueller et al[53]. Zwager et al[54] underlined that the success rate of EFTR is approximately 83.3%, and an R0 resection rate indicates the complete removal of malignant tissue, approaching 75%. These data underscore EFTR’s ability to cure certain tumors classified as T1 or T2, particularly those smaller than 3 cm. As explained in Aslanian et al[55], EFTR could present a rescue therapy when conventional techniques such as EMR and ESD fail to remove the polyp due to the presence of scar tissue or worries about deeper neoplastic involvement. It has demonstrated effectiveness in removing scarred polyps that exhibit the “non-lifting sign,” which complicates resections. However, careful individual assessment based on the characteristics of the lesion and the patient’s health remains crucial. While EFTR provides numerous benefits, clinicians must be cautious about depth. The recommendation to use EFTR depends on the depth of fibrosis which can be assessed by endoscopic visualization as fibrotic tissue frequently looks as a inflexible, non-distensible part with decreased submucosal lifting capacity. Another method is EUS can aid define whether fibrosis is limited to the submucosa or spreads into the muscularis propria, guiding EFTR possibility. These recommendations of assessment affect the treatment strategy as in superficial fibrosis EFTR remains a viable option but in Deep Fibrosis Involving the Muscularis Propria there is high risk of perforation.
Mun et al[56] highlighted the potential complications associated with its use, including the risk of bleeding, perforation, appendicitis when the lesion is near the appendiceal orifice, and dehiscence due to the OVESCO clip falling off the colonic mucosa, which could lead to peritonitis and sepsis. With ongoing technological progress, the indications for procedures like EFTR are thought to expand, potentially leading to enhanced patient outcomes, not only for those with intricate polyps but also contributing to a reduction in the overall incidence of colorectal cancer.
The last five years have witnessed significant research on the application of EPR in the management of gastrointestinal polyps. Zaghloul et al[14] reported that EPR has emerged as a promising approach that addresses some of the current challenges associated with scarred polyps. Its reported efficacy and safety in treating scarred polyps, either alone or in combination with other modalities provide an additional defense against the need for surgery for such complex polyps. In 2019, Kandiah et al[15], reported a cohort of 19 patients who had scarred polyps and were treated by EndoRotor. The cure rate after EndoRotor reached up to 84% of their cohort without any notable complications and with only three cases of recurrence. The first international multicenter experience of EPR was reported by Knabe et al[57] where the authors recruited 45 patients with a total lesion number of 48 from all over the gastrointestinal tract treated by EPR alone. The technical success was 98%, yet nearly 47% of cases had residual/recurrence after the first treatment session. The limitations of EndoRotor include the need for multiple treatment sessions, the stiffness of the device catheter, which makes it challenging to maneuver inside the lumen and impossible to retract the scope, the difficulty in using it in the upper gastrointestinal tract since it requires a working channel of 3.2 mm, which is not present in gastroscopes, and finally, the poor quality of collected samples for histopathological examination, EndoRotor usage in the upper gastrointestinal tract is limited by the rigidity of its catheter in exact anatomical positions, mainly at colonic flexures and angulated segments, like the hepatic and splenic flexures. The stiffness of the EndoRotor catheter decreases its flexibility, making navigation over sharp angulations harder. Poor catheter placing at flexures might cause partial resection, increasing recurrence rates. Excessive force applied in highly angulated regions may raise the danger of mucosal damage or perforation. To ease these challenges, other strategies can be considered, containing pre-procedural positioning changes by improving patient positioning during colonoscopy to enhance endoscopic admission. Usage of auxiliary endoscopic devices as extra accessories like stiffening overtubes or rotatable sheaths to improve device mechanism. Laparoscopic direction with endoscopic resection might be necessary, mainly for proximal colonic lesions. As highlighted in Zaghloul et al[14].
Johnson et al[58] reported that the role of surgery has been significantly diminished over the past few years due to the remarkable advancements in natural orifice transluminal surgeries. Nevertheless, surgical management can still be beneficial when endoscopic techniques fail or when the characteristics of polyps exhibit complexity or deep invasive carcinoma, especially with the higher risk of colon cancer in polyps exhibiting these characteristics. Consideration of the management plan is very crucial, especially with those who have a high risk of recurrence. Laparoscopic techniques have offered management by providing less invasive compared to open surgery in terms of short recovery time, less postoperative pain, and lower incidence of complications.
Rates of incomplete resection and recurrence: The nightmare of incomplete resection rates for complex colorectal polyps poses a significant challenge in clinical practice. Various anatomical and histological factors influence the outcomes. As reported in Taghiakbari et al[59], research reveals that incomplete resections occur in between 2% to 27% of cases. Larger lesions, particularly those exceeding 20 mm or involving multiple colonic folds, exhibit even higher rates of residual tissue post-removal. It was also found that piecemeal resection was associated with a local recurrence rate between 10% and 20%, highlighting the importance of comprehensive pre-procedural assessments and careful selection of techniques. As noted in Tholoor et al[60], incomplete resections have been associated with specific polyp characteristics, which can be identified using scoring systems such as SMSA. These systems help in identifying patients at higher risk of developing residual disease and complications during endoscopic excision. An Australian multicenter retrospective study mentioned in Mangira et al[61], involving patients undergoing piecemeal resection of 204 polyps larger than 20 mm revealed a recurrence rate of up to 5.5% at the initial colonoscopy, and 3.5% at the second colonoscopy. Piecemeal EMR comes with higher rates of incomplete resection. Knabe et al[62] reported a study of 252 polyps over 20 mm found that 88.5% of polyps removed in a piecemeal fashion had a 17.1% complication rate. At 6 and 12-month follow-up colonoscopies, nearly 36% and 33% of cases showed incomplete resection and residual tissue, respectively. Additionally, a study in Desomer et al[63] noted that used HD white light endoscopy and NBI technique demonstrated a 26% improvement in sensitivity for detecting local recurrence.
Bleeding is the most common adverse event classified to associate with endoscopic techniques used to manage scarred polyps. It is classified into two types: Intra-procedural bleeding (IPB) and post-procedural bleeding (PPB). IPB, described as any active bleeding that occurs at some point of the endoscopic procedure, necessitating direct haemostatic inter
To reduce bleeding risks during EMR, Bendall et al[66] reported that hot-snare techniques are widely used due to their effectiveness in controlling immediate hemorrhage. However, they may lead to increased delayed bleeding. Resection of pedunculated polyps can result in severe intraprocedural bleeding that can be managed by placing a clip or a detachable snare “endoloop”. Changing patients’ positions can also improve visualization of the bleeding point.
PPB occurs hours to days after surgery and accounts for 2% and 11% of cases. Halme et al[67] described that clinically significant bleeding occurs in 6% of cases. Risk factors for delayed bleeding include lesions in the right colon, large lesions with a size greater than 40 mm, age over 75 years, antiplatelets or anticoagulants taken within seven days of the procedure, and intraprocedural bleeding. Not all patients with PPB require urgent colonoscopy. However, patients who show a response to hemodynamic resuscitation should be clinically observed first. If bleeding persists, adequate bowel preparation and a repeat colonoscopy should be performed.
Perforation is another complication of endoscopic resection, particularly with advanced techniques like ESD and EFTR. A meta-analysis reported in Hassan et al[68], 50 studies revealed that 1.5% of patients experienced complications due to perforation post-EMR for colorectal polyps larger than 20 mm. In contrast, the perforation risk increases significantly to a range of 3.3% to 10% in patients undergoing ESD. A meta-analysis of 97 studies involving patients undergoing standard ESD showed a perforation rate of 5.2%. Notably, this meta-analysis also included 12 studies with colorectal lesions resected using hybrid ESD, resulting in a perforation rate of 4.8%. The risk of perforation further increases with complex polyps and factors such as bulky lesions exceeding 20 mm, proximal lesions, submucosal fibrosis, and inexperienced operators as explained in Fuccio et al[69]. Regarding EFTR, a meta-analysis reported in Dolan et al[70], showed a lower perforation risk of 4.4% in cases. Initially, this finding may seem surprising considering the invasive nature of the procedure. However, it can be explained by the fact that EFTR involves closing the lumen after resection during every procedure, either manually or using a device. Management of endoscopic perforation varies according to multiple factors such as the size, site, bowel preparation, time of detection, and general condition of the patient. For small perforations, there is no need for surgery. Tawheed et al[71] reported that endoscopists can manage these small perforations less than 1 cm using through-the-scope clips, while lesions in the left colon and up to 3 cm could be closed using over-the-scope clips. For larger perforations requiring more extensive closure, advanced suturing devices are necessary. Recently, endoscopic endovacuum therapy has been reported to be beneficial in certain situations where perforation occurs. The concept behind this technique is to maintain negative pressure and suction in the presence of antibacterial tap, thereby promoting healing by preventing bacterial infection, reducing edema, and enhancing blood flow as described in Tawheed et al[71].
Post-polypectomy coagulation syndrome (PPCS) is a rare complication that occurs after polypectomy procedures, especially when electrosurgical current is used. However, with the recent advancements in endoscopic management of colorectal polyps, the incidence of PPCS may be on the rise. Arimoto et al[72] noted that the risk of developing PPCS ranges from 0.03% to 1.2% of polypectomy procedures. PPCS typically presents with similar symptoms to bowel perforation, such as fever, abdominal pain, and signs of peritonitis. However, it has a better prognosis. Early diagnosis is crucial to prevent unnecessary surgery, as PPCS usually responds well to medical treatment. Several factors increase the risk of developing PPCS, including the presence of complex polyps (in terms of size, location, and scarring), and a history of hypertension as explained in Arimoto et al[72] and Ito et al[73].
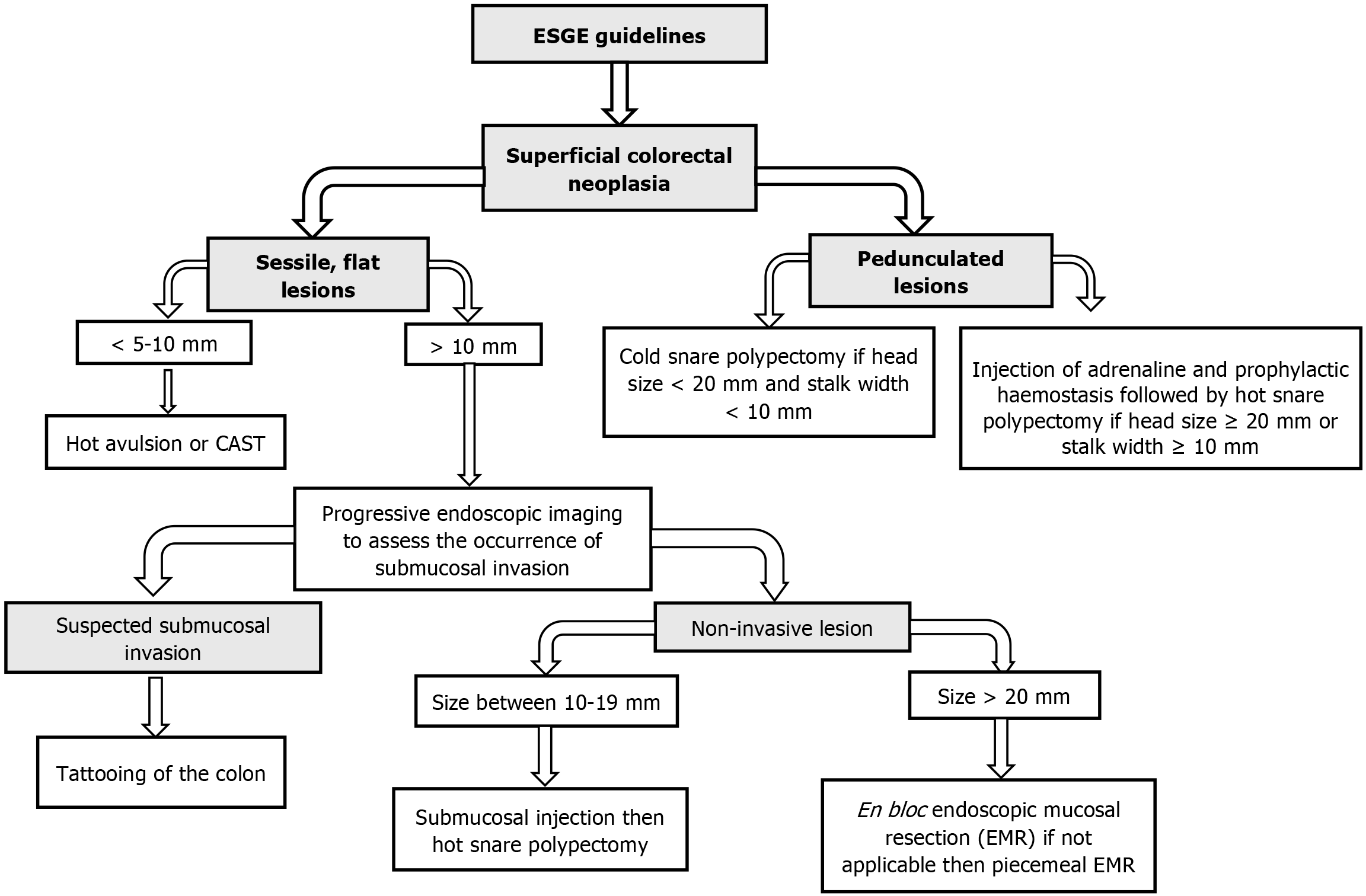
The ESGE 2024 guidelines offer critical updates on the control of complicated and scarred polyps (Figure 4), emphasizing threat stratification and proof-based medication selection. Key suggestions include risk-based Approach to Polypectomy as ESGE now advocates for a dependent type machine to determine whether EMR, ESD, or EFTR is the suitable technique for a given lesion. Expanded Role of AI and Molecular Markers by highlighting the combination of AI-assisted endo
Scarred polyps of the colon and rectum pose a significant challenge in clinical practice. Due to their high association with recurrence and increased risk of developing adenocarcinoma, successful resection is essential. However, there is no clear definition for the term “scarred” or “complex” polyps, and various scoring systems have been introduced to stratify the difficulty of polyp resection. Additionally, there is a lack of consensus or guidelines providing endoscopists with a clear framework for managing these polyps. Currently, EMR, ESD, EFTR, and EPR are used to treat these polyps, but none of these modalities have been extensively studied to determine their effectiveness in managing the challenging morphology of these polyps. Therefore, research should focus on refining the criteria for utilizing the currently used techniques to manage scarred polyps. Establishing a systematic framework is essential to assess the effectiveness of these advanced methodologies compared to traditional approaches. Also, a multidisciplinary approach is crucial throughout the entire process, from initial diagnosis to treatment planning and post-procedural follow-up care. Collaboration among gastroenterologists, surgeons, pathologists, and oncologists is essential to ensure the effectiveness of the management plan, which is tailored to each patient’s condition and lesion characteristics. Even while endoscopic resection strategies have advanced, current research is regularly confined as several research assessing EFTR, ESD, and other modern techniques are done at high-extent centres with professional operators, which introduces the first bias. This restricts generalisability because real-global results might vary in contexts with lower volume. The second element is operator dependency; methods like hybrid ESD and EFTR necessitate an excessive stage of technical talent and have an effect on reproducibility in numerous medical contexts. Because of their choice bias, studies generally omit situations which are technically hard, which ends up in published findings which can be unduly positive. The long-term efficacy of extra current modalities, especially AI-assisted diagnostic method, is in the long run understudied due to a loss of longitudinal statistics. Multicenter trials and standardised training procedures should be given top priority in future with a view to increase statistics dependability and expand the use of modern technology.
| 1. | Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16:3103-3111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 2. | Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E, Pagano N, Rando G, Strangio G, Carlino A, Romeo F, de Paula Pessoa Ferreira D, Zullo A, Ridola L, Malesci A. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Longcroft-Wheaton G, Bhandari M, Alkandari A, Bhandari P. Recent advances in the management of large and complex colonic polyps. F1000Res. 2018;7:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Vormbrock K, Mönkemüller K. Difficult colon polypectomy. World J Gastrointest Endosc. 2012;4:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Jung M. The 'difficult' polyp: pitfalls for endoscopic removal. Dig Dis. 2012;30 Suppl 2:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sanchez-Yague A, Kaltenbach T, Raju G, Soetikno R. Advanced endoscopic resection of colorectal lesions. Gastroenterol Clin North Am. 2013;42:459-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Hintze RE, Adler A, Veltzke W. Endoscopic resection of large colorectal adenomas: a combination of snare and laser ablation. Endoscopy. 1995;27:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Iishi H, Tatsuta M, Iseki K, Narahara H, Uedo N, Sakai N, Ishikawa H, Otani T, Ishiguro S. Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc. 2000;51:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Waye JD. Advanced polypectomy. Gastrointest Endosc Clin N Am. 2005;15:733-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Mönkemüller K, Neumann H, Fry LC, Ivekovic H, Malfertheiner P. Polypectomy techniques for difficult colon polyps. Dig Dis. 2008;26:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Sansone S, Ragunath K, Bianco MA, Manguso F, Beg S, Bagewadi A, Din S, Rotondano G. Clinical utility of the SMSA grading tool for the management of colonic neoplastic lesions. Dig Liver Dis. 2017;49:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Sidhu M, Tate DJ, Desomer L, Brown G, Hourigan LF, Lee EYT, Moss A, Raftopoulos S, Singh R, Williams SJ, Zanati S, Burgess N, Bourke MJ. The size, morphology, site, and access score predicts critical outcomes of endoscopic mucosal resection in the colon. Endoscopy. 2018;50:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Baker G, Vadaketh J, Kochhar GS. Endoscopic Full-Thickness Resection for the Management of a Polyp in a Patient With Ulcerative Colitis. Cureus. 2022;14:e24688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Zaghloul M, Rehman H, Sansone S, Argyriou K, Parra-Blanco A. Endoscopic treatment of scarred polyps with a non-thermal device (Endorotor): A review of the literature. World J Gastroenterol. 2024;30:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (2)] |
| 15. | Kandiah K, Subramaniam S, Chedgy F, Thayalasekaran S, Venetz D, Aepli P, Bhandari P. A novel non-thermal resection tool in endoscopic management of scarred polyps. Endosc Int Open. 2019;7:E974-E978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | D'Amico F, Amato A, Iannone A, Trovato C, Romana C, Angeletti S, Maselli R, Radaelli F, Fiori G, Viale E, Di Giulio E, Soriani P, Manno M, Rondonotti E, Galtieri PA, Anderloni A, Fugazza A, Ferrara EC, Carrara S, Di Leo M, Pellegatta G, Spadaccini M, Lamonaca L, Craviotto V, Belletrutti PJ, Hassan C, Repici A; Bowell Group. Risk of Covert Submucosal Cancer in Patients With Granular Mixed Laterally Spreading Tumors. Clin Gastroenterol Hepatol. 2021;19:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 18. | Nusko G, Mansmann U, Altendorf-Hofmann A, Groitl H, Wittekind C, Hahn EG. Risk of invasive carcinoma in colorectal adenomas assessed by size and site. Int J Colorectal Dis. 1997;12:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Myung DS, Kweon SS, Lee J, Shin IS, Kim SW, Seo GS, Kim HS, Joo YE. Clinicopathological features of laterally spreading colorectal tumors and their association with advanced histology and invasiveness: An experience from Honam province of South Korea: A Honam Association for the Study of Intestinal Diseases (HASID). PLoS One. 2017;12:e0184205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Friedland S, Shelton A, Kothari S, Kochar R, Chen A, Banerjee S. Endoscopic management of nonlifting colon polyps. Diagn Ther Endosc. 2013;2013:412936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ayoub F, Kim GE, Wang W, Chen D, Siddiqui UD. Delays in definitive endoscopic resection of previously manipulated colorectal polyps as a risk factor for inferior resection outcomes. Gastrointest Endosc. 2024;100:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Ichkhanian Y, Zuchelli T, Watson A, Piraka C. Evolving management of colorectal polyps. Ther Adv Gastrointest Endosc. 2021;14:26317745211047010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 23. | Hao XW, Li P, Wang YJ, Ji M, Zhang ST, Shi HY. Predictors for malignant potential and deep submucosal invasion in colorectal laterally spreading tumors. World J Gastrointest Oncol. 2022;14:1337-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (2)] |
| 25. | Buchner AM. The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia. Gastroenterol Hepatol (NY). 2017;13:336-347. [PubMed] |
| 26. | Iwatate M, Ikumoto T, Hattori S, Sano W, Sano Y, Fujimori T. NBI and NBI Combined with Magnifying Colonoscopy. Diagn Ther Endosc. 2012;2012:173269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 28. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 29. | Zhou QJ, Yang JM, Fei BY, Xu QS, Wu WQ, Ruan HJ. Narrow-band imaging endoscopy with and without magnification in diagnosis of colorectal neoplasia. World J Gastroenterol. 2011;17:666-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, Hayashi N, Oka S, Arihiro K, Yoshihara M, Chayama K. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Utsumi T, Iwatate M, Sano W, Sunakawa H, Hattori S, Hasuike N, Sano Y. Polyp Detection, Characterization, and Management Using Narrow-Band Imaging with/without Magnification. Clin Endosc. 2015;48:491-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Shibagaki K, Ishimura N, Yuki T, Taniguchi H, Aimi M, Kobayashi K, Kotani S, Yazaki T, Yamashita N, Tamagawa Y, Mishiro T, Ishihara S, Yasuda A, Kinshita Y. Magnification endoscopy in combination with acetic acid enhancement and narrow-band imaging for the accurate diagnosis of colonic neoplasms. Endosc Int Open. 2020;8:E488-E497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Tombazzi CR, Loy P, Bondar V, Ruiz JI, Waters B, Tombazzi CR. Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer. Fed Pract. 2019;36:S26-S29. [PubMed] |
| 34. | Hurlstone DP, Brown S, Cross SS, Shorthouse AJ, Sanders DS. High magnification chromoscopic colonoscopy or high frequency 20 MHz mini probe endoscopic ultrasound staging for early colorectal neoplasia: a comparative prospective analysis. Gut. 2005;54:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Marone P, de Bellis M, D'Angelo V, Delrio P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc. 2015;7:688-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 36. | O’Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, Dickersin GR, Ewing S, Geller S, Kasimian D. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 391] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Julka M, Cherukuri M, Lameh R. Screening for cancerous and precancerous conditions of the colon. Prim Care. 2011;38:449-68; viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Szylberg Ł, Janiczek M, Popiel A, Marszałek A. Serrated polyps and their alternative pathway to the colorectal cancer: a systematic review. Gastroenterol Res Pract. 2015;2015:573814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Al Qudah M, Haboubi N. Pitfalls in the reporting of neoplastic and pseudo neoplastic lesions in the colon and rectum. Folia Med (Plovdiv). 2022;64:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Gaglia A, Sarkar S. Evaluation and long-term outcomes of the different modalities used in colonic endoscopic mucosal resection. Ann Gastroenterol. 2017;30:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Azzolini F, Camellini L, Sassatelli R, Sereni G, Biolchini F, Decembrino F, De Marco L, Iori V, Tioli C, Cavina M, Bedogni G. Endoscopic submucosal dissection of scar-embedded rectal polyps: a prospective study (Esd in scar-embedded rectal polyps). Clin Res Hepatol Gastroenterol. 2011;35:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Wilson N, Are VS, Osorio Cintron R, Azeem N, Bilal M. Use of the endoscopic powered resection device for the management of scarred polyps. VideoGIE. 2023;8:211-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | ASGE Technology Committee; Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, Komanduri S, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Chaptini LA, Jalloul S, Karam K. Cold snare polypectomy: A closer look at the efficacy and limitations for polyps 10-20 mm in size. World J Gastrointest Endosc. 2024;16:445-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Mann R, Gajendran M, Umapathy C, Perisetti A, Goyal H, Saligram S, Echavarria J. Endoscopic Management of Complex Colorectal Polyps: Current Insights and Future Trends. Front Med (Lausanne). 2021;8:728704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Schenck RJ, Jahann DA, Patrie JT, Stelow EB, Cox DG, Uppal DS, Sauer BG, Shami VM, Strand DS, Wang AY. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017;31:4174-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Chandan S, Bapaye J, Khan SR, Mohan BP, Ramai D, Dahiya DS, Bilal M, Draganov PV, Othman MO, Rodriguez Sánchez J, Kochhar GS. Safety and efficacy of underwater versus conventional endoscopic mucosal resection for colorectal polyps: Systematic review and meta-analysis of RCTs. Endosc Int Open. 2023;11:E768-E777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Douglas SR, Rex DK, Repici A, Kelly M, Heinle JW, Spadaccini M, Moyer MT. Distal Cap-assisted Endoscopic Mucosal Resection for Non-lifting Colorectal Polyps: An International, Multicenter Study. TIGE. 2023;25:236-242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Moreira P, Cardoso PM, Macedo G, Santos-Antunes J. Endoscopic Submucosal Dissection, Endoscopic Mucosal Resection, and Transanal Minimally Invasive Surgery for the Management of Rectal and Anorectal Lesions: A Narrative Review. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 51. | Jung DH, Youn YH, Kim JH, Park H. Endoscopic submucosal dissection for colorectal lateral spreading tumors larger than 10 cm: is it feasible? Gastrointest Endosc. 2015;81:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Schurr MO, Baur FE, Krautwald M, Fehlker M, Wehrmann M, Gottwald T, Prosst RL. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc. 2015;29:2434-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 53. | Mueller J, Kuellmer A, Schiemer M, Thimme R, Schmidt A. Current status of endoscopic full-thickness resection with the full-thickness resection device. Dig Endosc. 2023;35:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Zwager LW, Bastiaansen BAJ, van der Spek BW, Heine DN, Schreuder RM, Perk LE, Weusten BLAM, Boonstra JJ, van der Sluis H, Wolters HJ, Bekkering FC, Rietdijk ST, Schwartz MP, Nagengast WB, Ten Hove WR, Terhaar Sive Droste JS, Rando Munoz FJ, Vlug MS, Beaumont H, Houben MHMG, Seerden TCJ, de Wijkerslooth TR, Gielisse EAR, Hazewinkel Y, de Ridder R, Straathof JA, van der Vlugt M, Koens L, Fockens P, Dekker E; Dutch eFTR Group. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy. 2022;54:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 55. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 56. | Mun EJ, Wagh MS. Recent advances and current challenges in endoscopic resection with the full-thickness resection device. World J Gastroenterol. 2023;29:4009-4020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 57. | Knabe M, Maselli R, Cesbron-Metivier E, Hollerbach S, Petruzziello L, Prat F, Khara HS, Pioche M, Hartmann D, Cesaro P, Barbaro F, Berger A, Spada C, Diehl DL, May A, Ponchon T, Repici A, Costamagna G. Endoscopic powered resection device for residual colonic lesions: the first multicenter, prospective, international clinical study. Gastrointest Endosc. 2024;99:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Johnson GGRJ, Helewa R, Moffatt DC, Coneys JG, Park J, Hyun E. Colorectal polyp classification and management of complex polyps for surgeon endoscopists. Can J Surg. 2023;66:E491-E498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 59. | Taghiakbari M, Kim DHD, Djinbachian R, von Renteln D. Endoscopic resection of large non-pedunculated colorectal polyps: current standards of treatment. eGastroenterology. 2024;2:e100025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | holoor S, Tsagkournis O, Basford P, Bhandari P. Managing difficult polyps: techniques and pitfalls. Ann Gastroenterol. 2013;26:114-121. [PubMed] |
| 61. | Mangira D, Cameron K, Simons K, Zanati S, LaNauze R, Raftopoulos S, Brown G, Moss A. Cold snare piecemeal EMR of large sessile colonic polyps ≥20 mm (with video). Gastrointest Endosc. 2020;91:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 63. | Desomer L, Tutticci N, Tate DJ, Williams SJ, McLeod D, Bourke MJ. A standardized imaging protocol is accurate in detecting recurrence after EMR. Gastrointest Endosc. 2017;85:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Burgess NG, Metz AJ, Williams SJ, Singh R, Tam W, Hourigan LF, Zanati SA, Brown GJ, Sonson R, Bourke MJ. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651-61.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 65. | Naseer M, Lambert K, Hamed A, Ali E. Endoscopic advances in the management of non-variceal upper gastrointestinal bleeding: A review. World J Gastrointest Endosc. 2020;12:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 66. | Bendall O, James J, Pawlak KM, Ishaq S, Tau JA, Suzuki N, Bollipo S, Siau K. Delayed Bleeding After Endoscopic Resection of Colorectal Polyps: Identifying High-Risk Patients. Clin Exp Gastroenterol. 2021;14:477-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 67. | Halme ALE, Roshanov PS, Tornberg SV, Lavikainen LI, Devereaux PJ, Tikkinen KAO; VISION Investigators. Timing of Major Postoperative Bleeding Among Patients Undergoing Surgery. JAMA Netw Open. 2024;7:e244581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 69. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 70. | Dolan RD, Bazarbashi AN, McCarty TR, Thompson CC, Aihara H. Endoscopic full-thickness resection of colorectal lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2022;95:216-224.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Tawheed A, Bahcecioglu IH, Yalniz M, Ozercan M, Oral AC, El-Kassas M. Summary of the current guidelines for managing iatrogenic colorectal perforations and the evolving role of endoluminal vacuum therapy. World J Clin Cases. 2025;13:97545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Arimoto J, Higurashi T, Kato S, Fuyuki A, Ohkubo H, Nonaka T, Yamaguchi Y, Ashikari K, Chiba H, Goto S, Taguri M, Sakaguchi T, Atsukawa K, Nakajima A. Risk factors for post-colorectal endoscopic submucosal dissection (ESD) coagulation syndrome: a multicenter, prospective, observational study. Endosc Int Open. 2018;6:E342-E349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Ito S, Hotta K, Imai K, Yamaguchi Y, Kishida Y, Takizawa K, Kakushima N, Tanaka M, Kawata N, Yoshida M, Ishiwatari H, Matsubayashi H, Ono H. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J Gastroenterol Hepatol. 2018;33:2001-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/