Published online Jun 20, 2025. doi: 10.5662/wjm.v15.i2.99300
Revised: October 11, 2024
Accepted: November 15, 2024
Published online: June 20, 2025
Processing time: 131 Days and 6.4 Hours
Intrathecal bupivacaine is the traditional anesthetic drug used in spinal anesthesia for caesarean sections (CSs), but ropivacaine has emerged as a potential alter
To systematically evaluate and compare the efficacy and safety of intrathecal hyperbaric bupivacaine and hyperbaric ropivacaine for spinal anesthesia in CSs.
A thorough search of electronic databases was carried out to find pertinent randomized controlled trials (RCTs) comparing intrathecal hyperbaric ropi
Total 8 RCTs were selected from a pool of 119 search results for meta-analysis. The meta-analysis evaluated pooled effect sizes and assessed heterogeneity among the studies. The primary objective was to compare key outcomes to identify any significant variances in efficacy and safety profiles between two local anesthetics. The analysis revealed that the difference in the onset of sensory blockade between the two local anesthetics was statistically insignificant (P = 0.1586). However, the onset of motor blockade appeared to be faster with bupivacaine (P = 0.03589). Additionally, the regression of sensory and motor blockade occurred earlier in the ropivacaine group. Furthermore, the duration of the first analgesic effect was shorter with a significance level of P < 0.05. Regarding side effects profile, including hypotension, nausea, and shivering, the study did not observe any significant differences between the two groups.
This meta-analysis offers insights into the effectiveness and safety of hyperbaric bupivacaine vs ropivacaine for cesarean sections. Hyperbaric ropivacaine had a comparable safety profile and faster regression of sensory and motor blockade than hyperbaric bupivacaine, perhaps aiding early mobilization of parturient and facilitating mother-child bonding. Choosing ropivacaine may offer benefits beyond efficacy for cesarean section patients and short surgical procedures.
Core Tip: Hyperbaric bupivacaine had been the standard local anesthetic drug for spinal anesthesia in caesarean sections. Its drawbacks include high incidences of adverse events (hypotension, nausea) and delayed ambulation. Ropivacaine, a newer local anesthetic agent, offers potential advantages such as improved safety and shorter motor blockade duration. Recent marketing of heavy ropivacaine has reignited interest in this. This meta-analysis compares the hyperbaric preparation of ropivacaine and bupivacaine in the context of spinal anesthesia for cesarean sections. This analysis aims to shed light on the comparative benefits of these agents, paving the way for informed decision-making in obstetric anesthesia practices.
- Citation: Anand R, Nag DS, Patel R, Sharma P, Uppalapati VK, Singh UK. Comparative efficacy of hyperbaric bupivacaine vs hyperbaric ropivacaine in spinal anesthesia for cesarean section: A meta-analysis. World J Methodol 2025; 15(2): 99300
- URL: https://www.wjgnet.com/2222-0682/full/v15/i2/99300.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i2.99300
The latest data over the last 2 decades, encompassing 94.5% of global live births reveals significant increases in caesarean section (CS) rates worldwide. Approximately 21.1% of women underwent cesarean deliveries, with rates varying from 5% in sub-Saharan Africa to 42.8% in Latin America and the Caribbean. Notably, CS rates have been escalating in all regions since 1990, and projections indicate a further increase, with an estimated 28.5% of women globally expected to give birth via CS by 2030[1]. The CS contributes to a significant perioperative workload at many healthcare centers, underscoring the critical importance of their anesthetic management.
The choice between general anesthesia (GA) and neuraxial anesthesia (NA) for CS depends on various factors such as maternal co-morbidities, the urgency of the procedure, and the availability of equipment and expertise. NA, notably spinal anesthesia, is favored and widely adopted due to its ease of administration, rapid onset, high success rates, and documented improvements in maternal and fetal outcomes compared to GA[2]. Spinal anesthesia is widely acknowledged as a safe and efficient NA method for CS, providing benefits such as fewer neonatal complications, fewer block failures, and lower chances of aspiration pneumonia in compared to other NA techniques and GA respectively[3,4]. Bupivacaine and ropivacaine as amino amide local anesthetic agents have been widely used for spinal anesthesia. They are now available commercially as hyperbaric and isobaric preparations. Bupivacaine was discovered in 1957 and became available commercially in 1965. Its closely related compound, ropivacaine, underwent clinical trials starting in 1990 and was introduced clinically in 1996[5].
The ideal drug for spinal anesthesia should have minimal hemodynamic effects, provide appropriate anesthesia duration, ensure prompt return of sensation and movement, and have a favorable side effect profile[4]. For decades, bupivacaine, either hyperbaric or isobaric, had been the preferred choice for spinal anesthesia, backed by its extensive clinical history[6]. The baricity of local anesthetics is crucial in determining their distribution in the intrathecal space, influencing the anesthetic block level. Achieving a consistent sensory blockade with an isobaric solution may be unpredictable during pregnancy, which alters local anesthetic requirements and increases variability in sensory block extension. The hyperbaric solution was preferrable due to more predictable sensory blockade in spinal anesthesia[7]. However, significant hypotension occurs during its administration. It is manageable with fluids and vasopressors, but it can lead to nausea and vomiting, negatively impacting the patient experience during the perioperative period[8]. However, intrathecal bupivacaine associated with prolonged sensory blockade and delayed motor function recovery extends post-delivery stay in the post-anesthesia care unit. It may delay bonding with the neonate after childbirth[9]. Reducing the local anesthetic dose, incorporating adjuvants, and considering alternatives such as ropivacaine and levobupivacaine can help diminish side effects without compromising anesthesia efficacy[10].
Ropivacaine, an S-enantiomer amide local anesthetic like bupivacaine, has been studied for spinal anesthesia in obstetric patients undergoing cesarean deliveries[4,11,12]. Ropivacaine has shown comparable efficacy with minimal impact on hemodynamics and shorter duration of sensory and motor blocks, enhancing recovery and patient safety[13]. With the introduction of hyperbaric ropivacaine for commercial use, its application among anesthesiologists should rise. Notably, no prior meta-analysis has directly compared hyperbaric ropivacaine with hyperbaric bupivacaine without additional adjuvants in obstetrical setting, making this meta-analysis an important effort to fill this knowledge gap. This meta-analysis attempts to evaluate the comparative effectiveness of intrathecal bupivacaine vs intrathecal ropivacaine, focusing on hyperbaric preparations without adjuvants, in CS.
This meta-analysis adhered to the preferred reporting items for systematic reviews and meta-analyses guidelines. It focused on women scheduled for elective CSs under spinal anesthesia receiving either intrathecal hyperbaric bupivacaine or hyperbaric ropivacaine without any adjuvant.
To compare the efficacy and safety of hyperbaric intrathecal bupivacaine and hyperbaric intrathecal ropivacaine for spinal anesthesia in cesarean section by assessing: (1) Onset and duration of sensory and motor blockade; (2) Duration of analgesia; (3) Incidence of hypotension; and (4) Frequency of adverse effects (nausea and shivering).
What is the comparative efficacy and safety of hyperbaric intrathecal bupivacaine vs hyperbaric intrathecal ropivacaine in terms of onset, duration, and regression of sensory and motor blockade, duration of analgesia, incidence of hypotension, nausea and shivering in women undergoing cesarean section?
The study selection criteria comprised randomized controlled trials (RCTs) comparing hyperbaric intrathecal bupivacaine with hyperbaric intrathecal ropivacaine in patients undergoing cesarean sections.
Studies: RCTs.
Population: Adult women (age > 18 years) American Society of Anesthesiologists I-II, undergoing elective or semi-elective cesarean section.
Interventions: Hyperbaric intrathecal ropivacaine.
Comparison: Hyperbaric intrathecal bupivacaine.
Outcomes: Studies must report data on duration of the sensory blockade, motor blockade, duration of analgesia, incidence of hypotension, and frequency of side effects (e.g., nausea, vomiting, and shivering).
Studies not employing randomized controlled trial design. Studies including emergency cesarean sections. Studies with unavailable data on the specified outcomes. Studies with significant methodological flaws. Studies using additional medications alongside local anesthetics which could affect the outcomes.
A comprehensive search approach was undertaken, including a variety of search phrases across significant databases such as MEDLINE (via PubMed), Scopus, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from January 2000 to January 2024. The search terms covered aspects related to the population (including key words "Cesarean section", "cesarean delivery", "parturient", and "obstetrical"), interventions (such as "hyperbaric bupivacaine", "hyperbaric ropivacaine", "intrathecal bupivacaine", "intrathecal bupivacaine", and "spinal anesthesia"), outcomes (key words "sensory block", "motor block", "analgesia", "hypotension", and "side effects"), and randomized controlled trial requirements. The search procedure was deliberately refined using "Boolean operators" (AND, OR, NOT). Only English language studies were considered, and other language articles were excluded. Trial registers were also searched for trials at the completion stage.
Two reviewers conducted data extraction independently, with any discrepancies resolved through discussion or consultation with a third reviewer if needed. The extracted data encompassed study characteristics, participant demographics, intervention specifics, and outcomes systematically.
A random-effects model was employed to compute summary effect estimates for continuous outcomes (e.g., duration of sensory and motor block) and risk ratios for dichotomous outcomes (e.g., incidence of hypotension). Heterogeneity was assessed using the I2 statistic, while a qualitative evaluation of studies investigated potential sources of heterogeneity. Funnel plots and Egger's test assessed publication bias.
For data collection Microsoft Excel 365[14] was used. Subsequently, data analysis was performed using the online web tool[15] [based on the Meta and Metafor packages, R programming environment (R version 4.2.2)] and results verified with Jamovi statistical software package [Version 2.5.6][16].
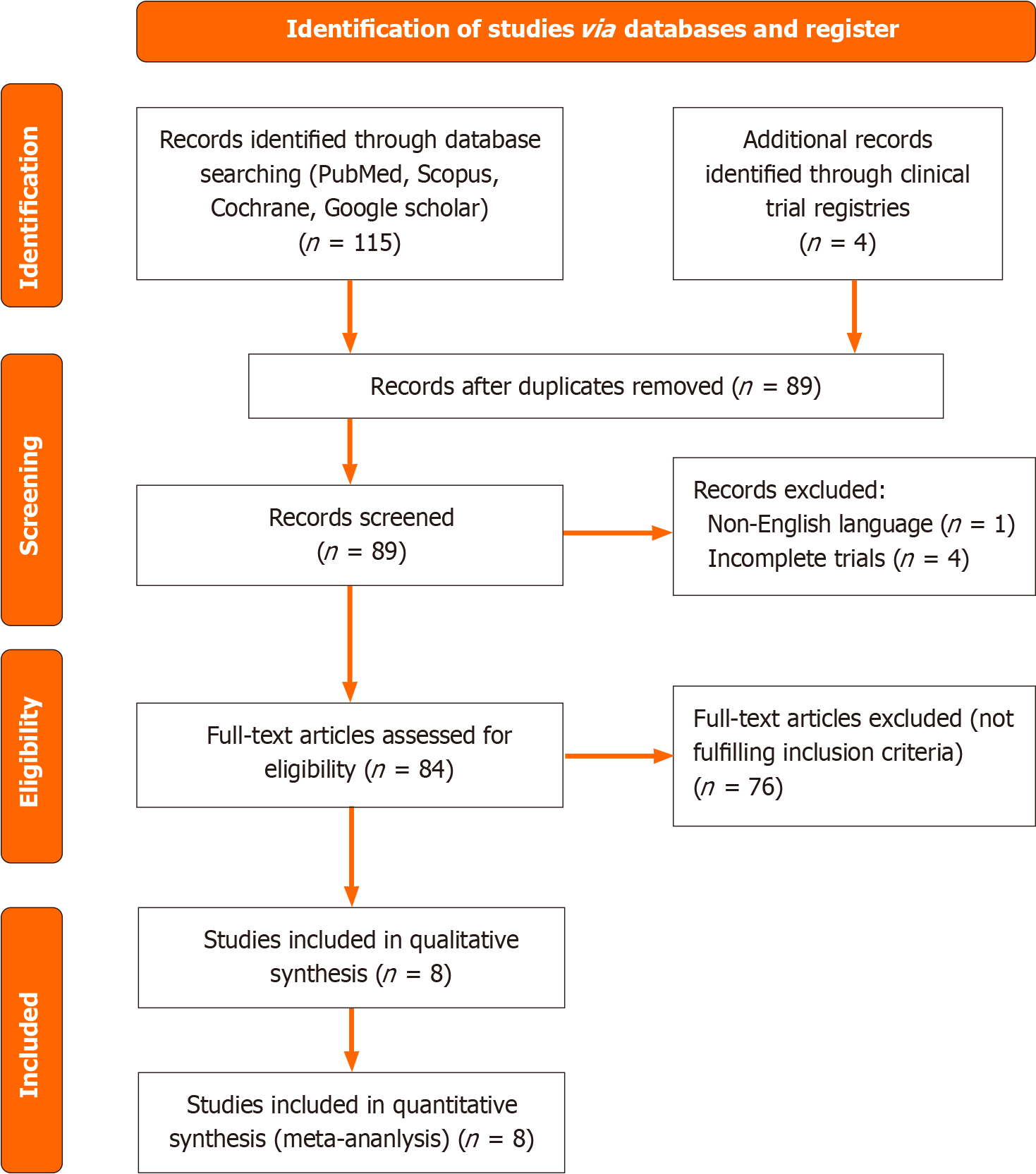
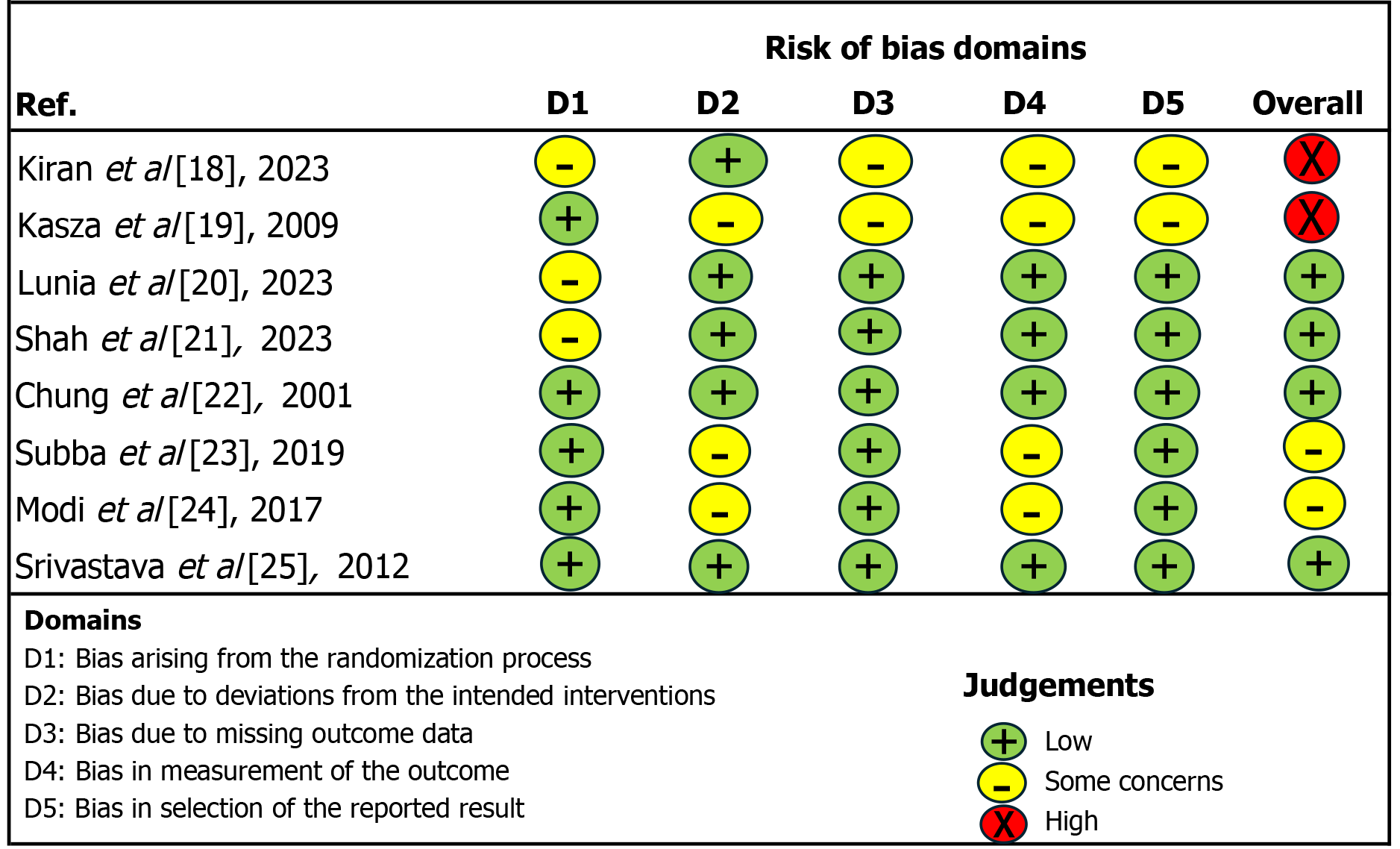
Two reviewers conducted a literature search between April 1, 2024, and May 31, 2024. They initially identified 115 records through database searching across platforms such as PubMed, Scopus, Cochrane, and Google Scholar. They found 4 more records through clinical trial registries. For a total of 119 records subjected to initial screening (Figure 1). After removing duplicates, the total number of records was reduced to 89. Among these records, 1 was excluded due to being in a non-English language, while 4 were excluded for being incomplete trials. Following the initial screening, 84 full-text articles were assessed for eligibility, out of which 76 articles were excluded as they did not meet the inclusion criteria. Finally, 8 studies were included in the quantitative synthesis, forming the basis for the meta-analysis findings (Table 1)[18-25]. All studies were assessed for risk of bias using the revised Cochrane risk of bias tool for randomized trials RoB 2.0[17] with assessment based on journal article content (Figure 2).
| Ref. | Type | Blinding | Randomisation | Sample size | Hyperbaric drug concentration | Dose (mg) | |||
| R | B | R | B | R | B | ||||
| Kiran et al[18], 2023 | RCT | Double | Unclear | 30 | 30 | 0.75 | 0.5 | 15 | 10 |
| Kasza et al[19],2006 | RCT | Single | Random allocation | 36 | 39 | 0.75 | 0.5 | 15 | 10 |
| Lunia et al[20], 2023 | RCT | Double | Unclear | 43 | 43 | 0.75 | 0.5 | 15 | 10 |
| Shah et al[21], 2023 | RCT | Double | Unclear | 40 | 40 | 0.75 | 0.5 | 15 | 10 |
| Chung et al[22], 2001 | RCT | Double | Random number table | 30 | 30 | 0.5 | 0.5 | 18 | 12 |
| Subba et al[23], 2019 | RCT | Single | Random number table | 30 | 30 | 0.5 | 0.5 | 15 | 12.5 |
| Modi et al[24], 2017 | RCT | Single | Random number table | 40 | 40 | 0.5 | 0.5 | 15 | 10 |
| Srivastava et al[25], 2012 | RCT | Double | Sealed envelop | 39 | 41 | 0.5 | 0.5 | 15 | 11 |
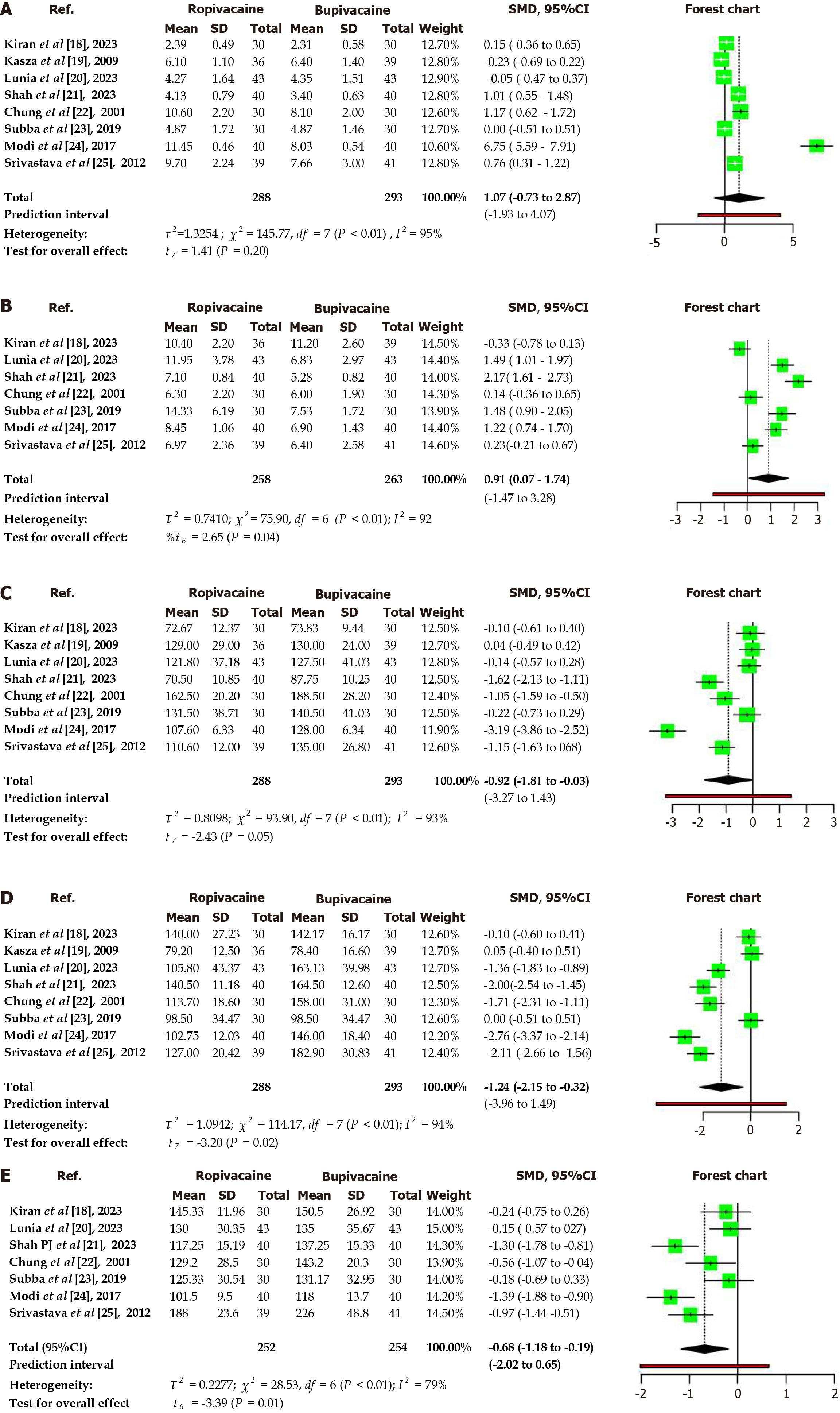
In the analysis of 8 studies[18-25] involving a total of 288 subjects in the ropivacaine cohort and 293 subjects in the Bupivacaine cohort, a comparison was made using a random effects model with the Inverse variance method (summarized standardized mean difference) for continuous variables (Table 2, Figure 3).
| Parameter | Standardized mean difference | 95%CI | P value | I² | Interpretation |
| Onset of sensory blockade | 1.07 | -0.73 to 2.87 | 0.2 | 0.95 | Statistically insignificant. High heterogeneity. Ropivacaine group is comparable to bupivacaine |
| Onset of complete motor block | 0.91 | 0.07-1.74 | < 0.05 | 0.92 | Significant difference. High heterogeneity. Bupivacaine is faster in onset than ropivacaine |
| Sensory blockade regression | -0.92 | -1.81 to -0.03 | < 0.05 | 0.93 | Significant difference. High heterogeneity. Regression is faster with ropivacaine |
| Motor blockade recovery | -1.24 | -2.15 to -0.32 | < 0.05 | 0.94 | Significant difference. High heterogeneity. Recovery is faster with ropivacaine |
| Duration of analgesia | -0.68 | -1.18 to -0.19 | < 0.05 | 0.79 | Significant difference. High heterogeneity. Duration of analgesia is longer with bupivacaine |
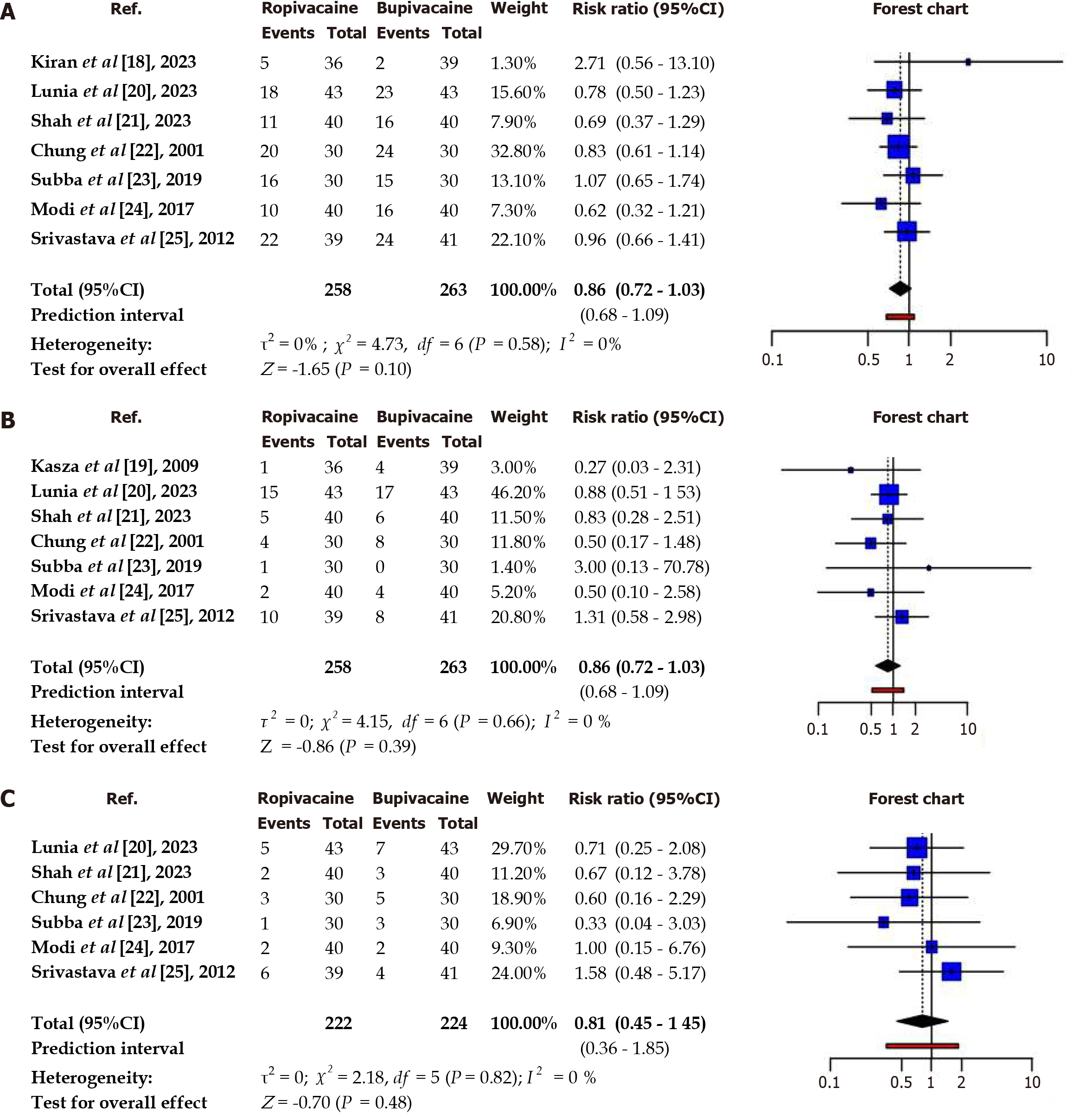
A comparison was made between the ropivacaine and bupivacaine cohorts, using the Mantel-Haenszel method to determine the risk ratio (Table 3, Figure 4). The results showed no significant difference between the two cohorts. Ropivacaine had a lower incidence of hypotension, nausea and shivering as suggested by risk ratio analysis, but the results lack statistical significance. No heterogeneity was indicated by I2 values, which indicated consistency in results among studies.
| Parameter | Risk ratio | 95%CI | P value | Heterogeneity (I²) | Interpretation |
| Hypotension | 0.86 | 0.72-1.03 | 0.58 | 0 | No significant difference was observed in hypotension risk |
| Nausea and Vomiting | 0.85 | 0.58-1.23 | 0.86 | 0 | No significant difference was observed in nausea and vomiting risk |
| Shivering | 0.81 | 0.45-1.45 | 0.58 | 0 | No significant difference was observed in shivering risk |
Onset of blockade: Intrathecal ropivacaine and bupivacaine exhibited a similar onset of sensory blockade, but ropivacaine showed delayed onset of complete motor blockade.
Duration of block: Ropivacaine demonstrated a significantly shorter time to achieve sensory regression, complete motor blockade recovery, and analgesia duration compared to bupivacaine.
Side effects: The frequency of side effects such as hypotension, nausea, and shivering appeared lower with ropivacaine, although statistical significance was not established in this analysis.
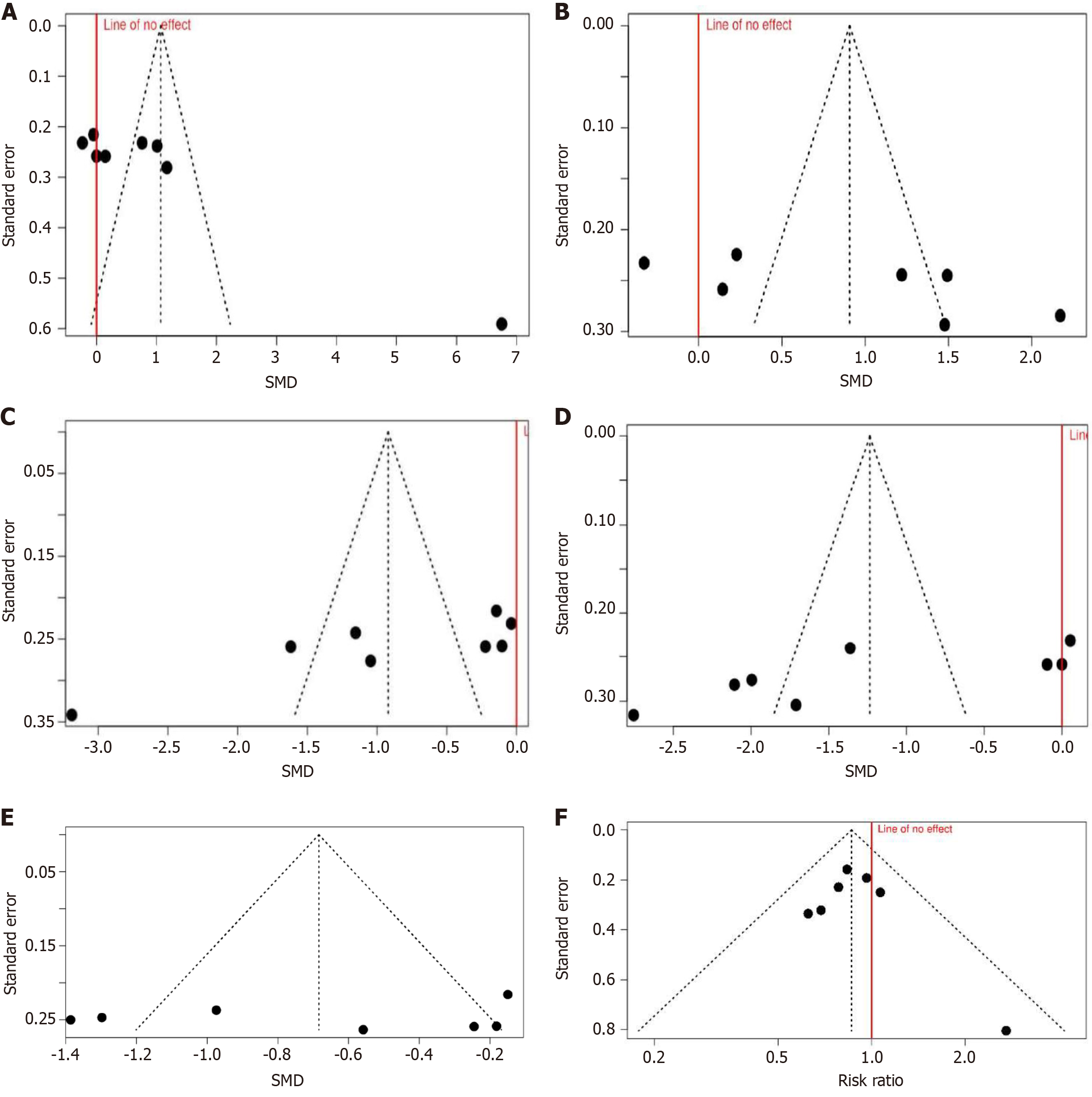
Multiple parameters were subjected to funnel chart tests and Eggers tests. The analysis suggests publication bias in the onset and regression of sensory blockade and regression of motor blockade. However, funnel charts of the onset of motor blockade, analgesia duration and incidence of hypotension suggest lack of bias (Figure 5). Egger’s test results supported the findings (Table 4).
| Parameter | Publication bias | Publication bias (Egger's test) |
| Onset of sensory blockade | Yes | Yes (t = 4.914, P = 0.003) |
| Onset of complete motor block | No | No (t = 2.02, P = 0.099) |
| Sensory blockade regression | Yes | Yes (t = -3.199, P = 0.019) |
| Motor blockade recovery | Yes | Yes (t = -2.754, P = 0.033) |
| Duration of analgesia | No | No (t = -0.345, P = 0.744) |
This meta-analysis, which included 8 research studies with 288 participants in the ropivacaine group and 293 participants in the Bupivacaine group, examined the effects of spinal anesthesia on parameters during cesarean sections using hyperbaric solutions. All findings were subjected to appropriate statistical analysis.
This meta-analysis aimed to compare the onset and duration of sensory and motor blockade, duration of analgesia and side effect profile, between hyperbaric solutions of ropivacaine and bupivacaine in the clinical context of spinal anesthesia for cesarean section. Our findings indicate that while ropivacaine exhibited a faster onset of sensory blockade, this difference did not reach statistical significance. However, there was a significantly longer onset time for complete motor blockade with ropivacaine compared to bupivacaine. Conversely, ropivacaine demonstrated a quicker and more significant regression of both blockade and motor blockade. The post-spinal duration of analgesia was lower with statistical significance in the case of ropivacaine.
The quicker onset of motor blockade observed with bupivacaine is consistent with prior research and is probably due to its higher lipid solubility when compared to ropivacaine. While the disparity in onset times for sensory and motor blockade may not be clinically significant during elective surgical procedures due to minor variations, the faster regression time could promote early mobility and shorten recovery. In obstetric anesthesia, this faster regression time might aid in early bonding between mother and child and facilitate breastfeeding, potentially leading to higher satisfaction levels. Conversely, the shorter duration of analgesia provided by ropivacaine could lead to an earlier need for analgesic medication. For short-duration procedures, ropivacaine can be used with equivalent efficacy to bupivacaine in terms of block characteristics. However, cases of prolonged surgery may require higher conversion rates to GA. Anesthesiologists may prefer either drug depending on their clinical needs, as local anesthetics have advantages and downsides.
Ropivacaine, an S-enantiomer amide local anesthetic, exhibits a distinct nerve block profile by causing less motor nerve blockade compared to bupivacaine, possibly due to its lower potency, reduced lipid solubility, and selective blockade of sodium channels specific to pain-sensing neurons[26]. Reduced duration of a motor blockade could offer postoperative benefits such as a decreased immobilization period and a more favorable recovery profile. Another meta-analysis by Malhotra et al[12] compared ropivacaine with bupivacaine irrespective of the nature of the solution (hyperbaric/isobaric) and adjuvants. They also interpreted that intrathecal ropivacaine results in a shorter duration of motor block (P value < 0.0001) than intrathecal bupivacaine and this reduction in motor block duration is consistent regardless of the doses of ropivacaine and bupivacaine used. Their subgroup analysis further suggested that patients receiving intrathecal ropivacaine in CS experienced motor block resolution approximately 35.7 minutes earlier than those receiving intrathecal bupivacaine.
Khalil et al[27] conducted a meta-analysis comparing intrathecal ropivacaine with bupivacaine in non-obstetrical surgeries, yielding comparable results. Their study indicated that sensory and motor blockade duration was notably shorter in the ropivacaine group. Additionally, they observed a reduced hypotension and a decreased need for vasopressors. While our results align with theirs, the limited number of studies in our analysis hindered the attainment of statistical significance regarding the incidences of hypotension, nausea and shivering.
There was significant heterogeneity across studies for all outcomes except for the hypotension, nausea, and shivering. Heterogeneity suggests that other factors beyond the local anesthetic type might influence the results. It underscores the variability in study methodologies, patient populations, and drug concentrations, highlighting the need for caution when interpreting the findings. Studies were conducted with different ethnic populations, with single blinding in 3 studies[19,23,24] and double blinding in others. Four studies[22-25] in our metanalysis used 0.5 % ropivacaine whereas the rest used 0.75% ropivacaine heavy. In these studies, ropivacaine heavy was prepared by using dextrose instead of commercial preparation, except by Kiran et al[18] and Shah et al[21]. The dose varied among studies. These factors may influence block characteristics and may have contributed to heterogeneity. Further research is needed with due consideration for potential sources of this variation, such as differences in study populations, dosage regimens, or assessment techniques.
Evidence of publication bias was detected for the onset and regression of sensory blockade but not with the onset of motor blockade and categorical data. This may suggest variation in the assessment and reporting of sensory and motor blockade by authors as these are subjective, and variation may occur due to differences in assessment, time interval for observation, patient knowledge, and cooperation. Despite the wide time selection, only 8 studies fulfilled the inclusion criteria. It may have introduced time lag-bias, too. As 3 out of 8 studies were single-blinded, researcher bias and publication practice bias cannot be ruled out. Only English language paper selection is another source of potential bias. The presence of publication bias could potentially overestimate the observed effects. Although suggestive of publication bias, funnel plot asymmetry, should be interpreted with caution due to its limitations.
There were no significant differences between ropivacaine and bupivacaine regarding the incidence of hypotension, nausea, vomiting, or shivering. However, incidences appear to be lower with ropivacaine. This meta-analysis suggests that both drugs have a comparable safety profile regarding these common adverse effects. Further studies with larger sample sizes will help in definitive interpretation regarding the incidence of adverse effects.
The meta-analysis highlights several key findings regarding hyperbaric ropivacaine as a drug in spinal anesthesia. Ropivacaine has shown a slower onset of motor blockade, which can enhance maternal comfort and safety during surgery by allowing for careful monitoring and time to manage adverse events. However, in obstetrical emergencies small difference in duration may affect neonatal outcome. It also features a faster regression of sensory blockade, enabling quicker recovery and early engagement with newborns, which is vital for bonding. Additionally, its faster motor blockade recovery helps mothers regain mobility sooner, facilitating skin-to-skin contact. Although ropivacaine offers a shorter duration of analgesia compared to bupivacaine, this can be managed with supplemental analgesics, promoting quicker postoperative activity. Favorable risk ratios for side effects suggest that ropivacaine may lead to fewer adverse effects, such as hypotension, nausea, and shivering. This is crucial for enhancing maternal satisfaction and ensuring a positive experience during the perioperative period.
These findings emphasize the importance of individualized patient care, tailoring anesthetic choices based on individual needs and preferences. Anesthesiologists can make an informed decision after considering the benefits and trade-offs associated with each agent. Using ropivacaine as an alternative to bupivacaine in obstetrical anesthetic management may improve maternal experiences by providing effective anesthesia experience while minimizing adverse effects, supporting quicker recovery times and promoting early bonding between mother and neonate.
Future research should focus on research areas to enhance the understanding of hyperbaric ropivacaine and its effects in obstetric anaesthesia. First, comparative studies on dosage variations are needed to investigate how different dosages of hyperbaric ropivacaine and bupivacaine impact maternal and neonatal outcomes, with an emphasis on finding optimal dosing strategies that maximize analgesic efficacy while minimizing side effects such as hypotension and nausea. Additionally, longitudinal studies assessing maternal satisfaction over time post-cesarean delivery with various anaesthetic agents could provide insights into how anaesthetic choices influence long-term maternal well-being, bonding experiences, and psychological outcomes.
Moreover, research should focus on special populations, including patients with preeclampsia or comorbidities that complicate anesthesia management, to better understand their responses to different anesthetics. Finally, exploring patient-centred outcomes beyond satisfaction-such as quality of life measures, recovery times, and psychological impacts-will help inform more effective and individualized anaesthetic strategies in clinical practice.
Heterogeneity: The included studies displayed significant heterogeneity, potentially impacting the outcomes of the meta-analysis.
Publication bias: There is a possibility of publication bias, particularly concerning sensory blockade results, which could have led to overestimating the observed effects.
Language restriction: Only English language papers were considered for inclusion, possibly limiting the diversity of data sources.
Incomplete trials: Registered ongoing trials utilizing commercially available preparations were incomplete, reducing the available sample size for analysis.
Future research: Further investigations with larger sample sizes and standardized methodologies must validate these findings and explore potential patient subgroups that may respond differently to ropivacaine or bupivacaine.
Unassessed parameters: The meta-analysis did not specifically evaluate postoperative pain scores, severity of hypotension, other side effects, and patient satisfaction, indicating areas for future research and analysis.
Absence of subgroup and sensitivity analyses: Outcome may differ due to variation in dose and concentration. However, we could not conduct subgroup analyses due to the limited number of studies and smaller subgroup sizes. Smaller subgroups may result in underpowered analysis with possibility of the false positive results leading to incorrect interpretation.
In conclusion, our meta-analysis suggests that ropivacaine has a comparable onset of sensory blockade, but a significantly longer onset time for complete motor blockade and a quicker regression of both sensory and motor blockade. Both drugs demonstrated a comparable safety profile regarding hypotension, nausea and vomiting, and shivering. The presence of heterogeneity and potential publication bias underscores the need for a cautious interpretation of the results and further research to clarify the clinical implications of these findings. However, based on this meta-analysis ropivacaine appears to be an acceptable alternative to bupivacaine heavy, particularly for short-duration surgeries where early ambulation may be beneficial.
| 1. | Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6:e005671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 815] [Article Influence: 163.0] [Reference Citation Analysis (37)] |
| 2. | Watson SE, Richardson AL, Lucas DN. Neuraxial and general anaesthesia for caesarean section. Best Pract Res Clin Anaesthesiol. 2022;36:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (37)] |
| 3. | Olapour A, Akhondzadeh R, Rashidi M, Gousheh M, Homayoon R. Comparing the Effect of Bupivacaine and Ropivacaine in Cesarean Delivery with Spinal Anesthesia. Anesth Pain Med. 2020;10:e94155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (37)] |
| 4. | Jaafarpour M, Vasigh A, Najafi F, Sayadi H, Shafiei E. A Comparative Study on the Effect of Intrathecal Bupivacaine vs. Ropivacaine on Maternal and Neonatal Outcomes After Cesarean Section: A Systematic Review and Meta-analysis. Anesth Pain Med. 2023;13:e134732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (36)] |
| 5. | Ruetsch YA, Böni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (37)] |
| 6. | Sng BL, Han NLR, Leong WL, Sultana R, Siddiqui FJ, Assam PN, Chan ES, Tan KH, Sia AT. Hyperbaric vs. isobaric bupivacaine for spinal anaesthesia for elective caesarean section: a Cochrane systematic review. Anaesthesia. 2018;73:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (37)] |
| 7. | Besha A, Zemedkun A, Tadesse M, Hailu S, Mossie A, Shiferaw A, Angasa D, Adamu Y. Effects of Hyperbaric and Isobaric Bupivacaine on Hemodynamic Profiles and Block Characteristics Among Parturients Undergoing Elective Cesarean Section Under Spinal Anesthesia: A Randomized Controlled Trial. J Pain Res. 2023;16:3545-3558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (37)] |
| 8. | Burns SM, Cowan CM, Wilkes RG. Prevention and management of hypotension during spinal anaesthesia for elective Caesarean section: a survey of practice. Anaesthesia. 2001;56:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (37)] |
| 9. | Al-Abdulhadi O, Biehl D, Ong B, Boker A. Hyperbaric spinal for elective Cesarean section--ropivacaine vs bupivacaine. Middle East J Anaesthesiol. 2007;19:385-396. [PubMed] |
| 10. | Swain A, Nag DS, Sahu S, Samaddar DP. Adjuvants to local anesthetics: Current understanding and future trends. World J Clin Cases. 2017;5:307-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (45)] |
| 11. | Fadlalmola HA, Elhusein AM, Albadrani MS, Masada HK, Abdalla AM, Elhussain MY, El-Amin EI, Azeem FM. Safety and efficacy of combined ropivacaine and sufentanil compared with ropivacaine for cesarean sections: A systematic review and meta-analysis. Afr J Reprod Health. 2023;27:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (37)] |
| 12. | Malhotra R, Johnstone C, Halpern S, Hunter J, Banerjee A. Duration of motor block with intrathecal ropivacaine versus bupivacaine for caesarean section: a meta-analysis. Int J Obstet Anesth. 2016;27:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Luck JF, Fettes PD, Wildsmith JA. Spinal anaesthesia for elective surgery: a comparison of hyperbaric solutions of racemic bupivacaine, levobupivacaine, and ropivacaine. Br J Anaesth. 2008;101:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Microsoft Corporation. Microsoft Excel [Computer Software]. 2024. Available from: https://www.microsoft.com/en-us/microsoft-365/excel. |
| 15. | Metaanalysisonline. Metaanalysisonline.com. Available from: https://metaanalysisonline.com/. |
| 16. | The jamovi project. Jamovi (Version 2.5) [Computer Software]. 2024. Available from: https://www.jamovi.org. |
| 17. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18486] [Article Influence: 2640.9] [Reference Citation Analysis (0)] |
| 18. | Kiran K, Sirisha C, Aasim SA. Comparison of readymade 0.75% hyperbaric ropivacaine vs conventional 0.5% hyperbaric bupivacaine for elective C-sections. EJCM. 2023;13:742-753. |
| 19. | Kasza T, Knapik P, Misiolek H, Knapik D. Spinal anaesthesia using hyperbaric 0.75% ropivacaine vs hyperbaric 0.5% bupivacaine for elective caesarean section. Eur J Anaesth. 2006;23:178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Lunia A, Kumar M, Kumar D, Sharda ML. Comparison of hyperbaric 0.75% ropivacaine with 0.5% bupivacaine for elective caesarean section under spinal anaesthesia: A double blind randomised controlled study. Int J Acad Med Pharm. 2023;5:58-61. |
| 21. | Shah PJ, Bhuaarya RN, Sheikh MF. Block characteristics of hyperbaric bupivacaine versus hyperbaric ropivacaine in lower segment cesarean section: A randomized experimental study. Int J Med Anesthesiology. 2023;6:109-114. [DOI] [Full Text] |
| 22. | Chung CJ, Choi SR, Yeo KH, Park HS, Lee SI, Chin YJ. Hyperbaric spinal ropivacaine for cesarean delivery: a comparison to hyperbaric bupivacaine. Anesth Analg. 2001;93:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Subba S, Arjun Chhetri A, Bhattarai R. Comparison of effects of Bupivacaine and Ropivacaine in patients undergoing elective Caesarean section. BJHS. 2020;4:859-863. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Modi YC, Meena R. Hyperbaric Spinal Ropivacaine for Elective Cesarean Section: A Comparison to Hyperbaric Bupivacaine. IJSR. 2017;6. |
| 25. | Srivastava U, Joshi K, Gupta A, Dwivedi Y, Anand H, Kannaujia A, Pilendram S. Comparison of intrathecal hyperbaric ropivacaine and bupivacaine for caesarean delivery. Int J Anesthesiology. 2012;30. |
| 26. | Oda A, Ohashi H, Komori S, Iida H, Dohi S. Characteristics of ropivacaine block of Na+ channels in rat dorsal root ganglion neurons. Anesth Analg. 2000;91:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Khalil RS, Mehmud A, Banerjee R, Malhotra R, Banerjee A. Intrathecal ropivacaine versus bupivacaine in a non-obstetric population- A meta-analysis and trial sequential analysis. Indian J Anaesth. 2024;68:129-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/