Published online Jun 20, 2024. doi: 10.5662/wjm.v14.i2.91889
Revised: February 10, 2024
Accepted: March 28, 2024
Published online: June 20, 2024
Processing time: 157 Days and 20.4 Hours
However, the connection between smoking and the prognosis of patients with bladder cancer remains unclear.
To determine whether smoking is linked to the recurrence and progression of bladder cancer.
As of July 20, 2022, relevant English-language research was identified by sear
A total of 12 studies were included in this meta-analysis. The combined analysis revealed that tobacco exposure was associated with a significantly greater recurrence rate than nonsmoking status [odd ratios (OR) = 1.76, 95%CI: 1.84-2.93], and the progression of bladder cancer was significantly greater in smokers than in nonsmokers (OR = 1.21, 95%CI: 1.02-1.44). Stratified analysis further revealed that current smokers were more likely to experience relapse than never-smokers were (OR = 1.85, 95%CI: 1.11-3.07). Former smokers also had a greater risk of relapse than did never-smokers (OR = 1.73, 95%CI: 1.09-2.73). Subgroup analysis indi
This meta-analysis revealed that tobacco exposure may be a significant risk factor for both the recurrence and progression of bladder cancer.
Core Tip: In this meta-analysis, 12 studies were included to investigate the connection between smoking and the prognosis of bladder cancer patients. The results showed that tobacco exposure was associated with a significantly greater recurrence rate and faster progression of bladder cancer than nonsmoking status. Subgroup analysis further revealed that current and former smokers had a greater risk of relapse than did never smokers, and non-Caucasians may be more susceptible to bladder cancer recurrence than Caucasians are. Therefore, smoking is a major risk factor for bladder cancer recurrence and progression, and cessation of smoking is recommended. Regular follow-up and treatment are crucial for reducing the risk of smoking.
- Citation: Xiang L, Xie QQ, Xu SS, Ruan WJ, Xu DH, Gan YY, Zuo J, Xu WJ, Li ZP. Association between tobacco exposure and bladder cancer recurrence: A systematic review and meta-analysis. World J Methodol 2024; 14(2): 91889
- URL: https://www.wjgnet.com/2222-0682/full/v14/i2/91889.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i2.91889
Bladder cancer is a prevalent urological malignancy worldwide. It affects a significant number of individuals and remains a common cancer type, particularly in developed countries[1]. Bladder cancer leads to hundreds of thousands of deaths annually[2]. The incidence of bladder cancer is affected by sex-related factors. The occurrence of this disease is approximately three to four times greater in males than in females. However, women with bladder cancer often receive a dia
Tobacco smoking remains a significant global public health concern and leads to more than 5 million deaths each year[6]. In China alone, 2 million people lose their lives with smoking-related illnesses[7]. Smoking has been firmly estab
The study was carried out in accordance with the PRISMA[17] statement.
Given that this meta-analysis is based on previously published data and does not involve any individual-level data collection or analysis, ethical approval is not needed for this study. This study adheres to the ethical guidelines and best practices for meta-analyses, ensuring a rigorous and objective synthesis of the available evidence.
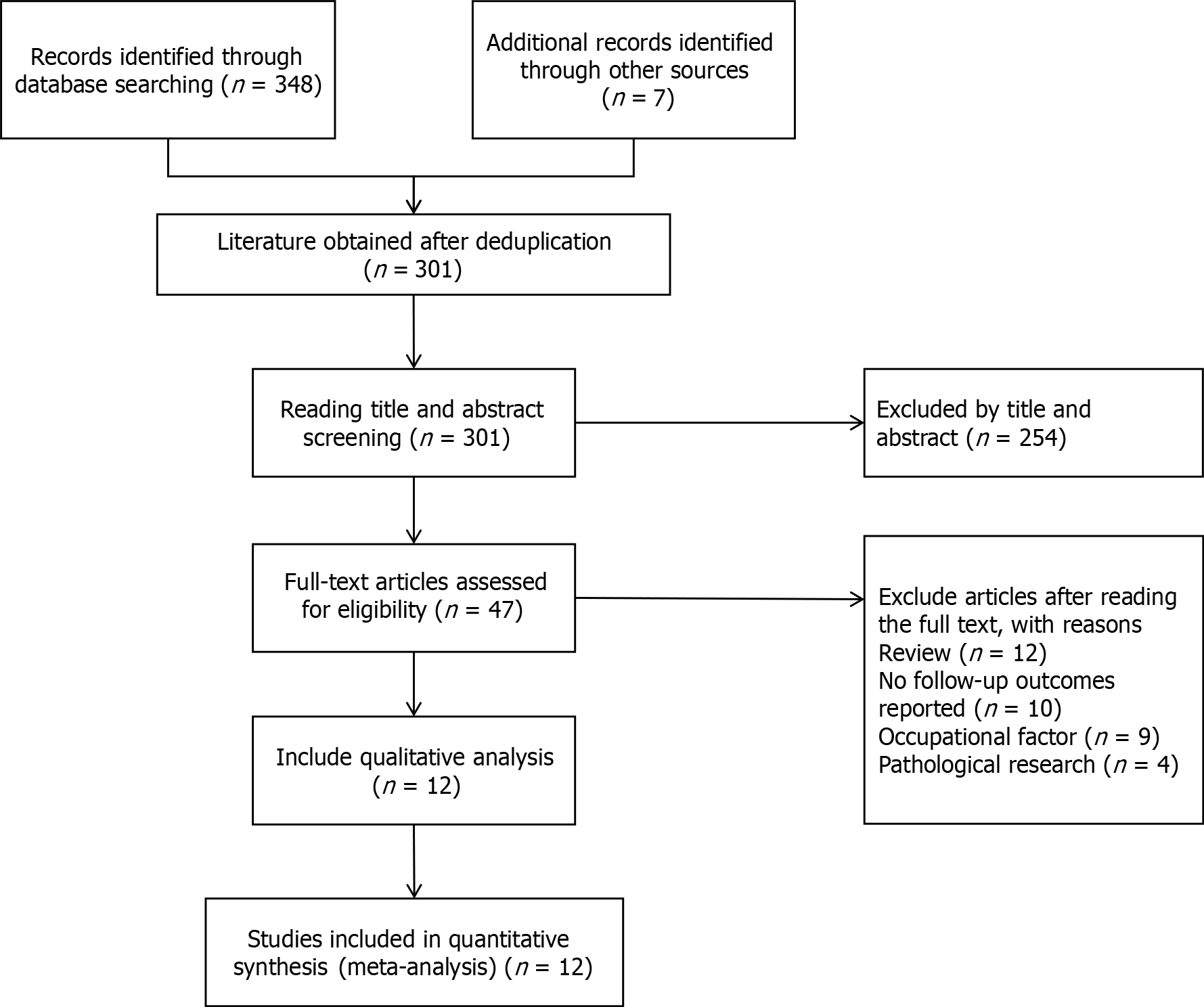
We conducted a comprehensive literature search of Medline (PubMed), Web of Science, and the Cochrane Library covering the period from the establishment of each database to July 2022. Our search strategy included a combination of keywords and medical subject headings terms related to smoking, urinary bladder neoplasms, malignant tumor of the urinary bladder, cancer, tumor, bladder, cancer recurrence, and various combinations of these terms. We also manually checked the reference lists of all identified studies and related reviews to ensure comprehensive coverage. Additionally, we searched the Clinical Trials website (clinicaltrial.gov) for relevant unpublished studies as of July 20, 2022. The flowchart in Figure 1 summarizes the identification and evaluation process of the studies included in this review.
Two independent reviewers (Li ZP and Xie QQ) evaluated the eligibility of each study using standardized criteria. The inclusion criteria were as follows: (1) Original research article; (2) had undergone bladder cancer surgery; (3) smoked long-term compared to never or former smokers or were currently smoking as an indicator of exposure; (4) had risk estimates [hazard ratios (HR), risk ratios (RR), odd ratios (OR)] with corresponding 95%CI for the study results or detailed baseline and follow-up data in the authors' report to be able to calculate the above indicators; and (5) papers are written and published in English. To minimize errors and biases in the pooled data, a unified standard for the definition of smoking was established. Any active exposure to tobacco was defined as "tobacco exposure". Patients who were still smoking at the time of bladder cancer diagnosis or who stopped smoking within one year of diagnosis were defined as "current smokers". "Former smoker" was defined as a patient who had quit smoking at least one year before bladder can
After analysis by researchers, articles were screened according to the following criteria to exclude the following: (1) Did not report available data, such as conference abstracts, expert opinions, comments, or letters to the editor; (2) were well-published articles or articles with data reuse; (3) had flawed research designs and low quality assessment scores; (4) had incorrect statistical methods that could not be corrected, could not be provided or could not be transformed into ORs (RR, HR) or their 95%CI, or measured data that did not provide means and standard deviations; and (5) had smoking exposure categories and corresponding effect values that were not clearly described. If two studies reported the same or overlapping populations, only the study with the largest sample size was included; if the studies included different and related exposures or stratified analyses, both studies were included.
Two researchers (Si-Si Xu and Wen-Jie Ruan) independently extracted information from the eligible studies using standardized data collection forms. The extracted information included the first author, year of publication, study design, database and country, follow-up time, sample size, outcome, age, sex, time of diagnosis, surgical method, interventions, and smoking status.
The quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale[18] for cohort studies. This scale rates the quality of selection, comparability, and outcome quality of the included articles. After independent scoring by two reviewers, the studies included in this study were identified as follows: 1 high-quality study, 10 medium-quality studies, and 1 low-quality study.
The RevMan (Review Manger 5.4.1) for assessing risk of bias was utilized to conduct an analysis of bias risk.
The combined analysis revealed that most studies classified smoking status as smoking, quitting smoking, or never smoking. To unify the standards and eliminate the influence of subtle differences in the definitions of smoking among different articles, we used current smoking, former smoking and never smoking to define smoking status.
Statistical heterogeneity was assessed using the Cochrane chi-square test (Q test) (P < 0.1 was considered to indicate significant statistical heterogeneity). HR and 95%CI were calculated using Cox regression for statistical analysis. Study-specific risk estimates were pooled by random or fixed effects meta-analyses. The association between smoking status and postoperative bladder cancer outcomes was assessed in detail using forest plots.
Subgroup analysis and meta-regression analysis were also conducted based on surgical method (radical cystectomy vs transurethral resection of the bladder), disease stage (muscle invasive bladder cancer, nonmuscle invasive bladder cancer and bladder cancer), geographic region (United States, Europe or Asia), and study design (single center and the center) to identify potential sources of heterogeneity. Sensitivity analyses were performed by the back-off method (removing one item at a) to test whether the results were influenced by a particular study.
Furthermore, a funnel plot assessment was applied to capture potential publication bias and to examine the impact of publication bias on the validity of the estimates. Statistical analyses were conducted using SPSS 28.0 (IBM, Chicago, IL, United States) and RevMan software, version 5 (http://ims.cochrane.org/revman). P < 0.05 was considered to indicate statistical significance unless the article specifically stated otherwise.
This passage is a summary of a meta-analysis that aimed to assess the impact of smoking status on bladder cancer outcomes. The researchers conducted a comprehensive literature search and identified 186 articles from PubMed, 123 articles from the Web of Science, 32 articles from the Cochrane Library, and 7 articles manually. After screening and evaluation by reviewers, 12 studies were included in the meta-analysis[11,12,14,16,19-26]. The included studies were conducted in different regions, including Europe (5 studies)[16,21-24], Asia (5 studies)[11,12,19,25,26], and North America (2 studies)[14,20]. A total of 5817 patients were included in the meta-analysis, and most of those studies provided detailed follow-up data on tobacco exposure. Yuruk et al[26] study recruited 187 participants and grouped them according to their smoking status[16]. On the other hand, Grotenhuis et al[16] included a total of 1459 patients from 1995 to 2006 after three expansions. However, only 66% of the patients responded, resulting in a cohort of 963 patients for the study. These figures demonstrate the extensive variation in participant demographics across various studies, emphasizing the significance of conducting research on diverse patient populations to achieve a more comprehensive understanding of the impact of smoking on bladder cancer outcomes.
The findings from the meta-analysis suggest that smoking status is linked to both the recurrence and progression of bladder cancer. These results from this study can offer valuable insights to healthcare professionals and patients about the impact of smoking on bladder cancer outcomes, thus informing future research in this area.
The Newcastle-Ottawa Quality Assessment Scale was used to evaluate the quality of each study, with scores ranging from 6 to 9 (with a mean of 7.42), indicating a generally acceptable methodological approach. Table 1 lists the scores for each individual study, while Table 2 provides a detailed breakdown of the scoring criteria.
| Ref. | Area | Sort | Period | Mean follow-up time (months) | Sample size | Disease stage | NOS score | Cur:For:Non |
| Michalek et al[14], 1985 | United States | Retrospective | 1963-1975 | N/A | 354 | NMIBC | 7 | 132:128:94 |
| Grotenhuis et al[16], 2015 | Netherlands | Forward-looking | 2007-2012 | 12 | 963 | NMIBC | 8 | 292:490:181 |
| Wyszynski et al[25], 2014 | Lebanon | Forward-looking | 1994-2002 | 37 | 857 | NMIBC | 8 | 214:379:123 |
| Chen et al[19], 2007 | Taiwan | Retrospective | 1997-2005 | N/A | 206 | NMIBC | 6 | 78:64:64 |
| Leibovici et al[20], 2005 | United States | Forward-looking | 1995-2003 | 15 | 519 | NMIBC | 7 | 185:239:95 |
| Yuruk et al[26], 2017 | Turkey | Forward-looking | 2013-2014 | 32 | 187 | NMIBC | 9 | 114:35:38 |
| van Osch et al[24], 2018 | United Kingdom | Forward-looking | 2005-2011 | 51 | 722 | NMIBC | 8 | 336:283:103 |
| Ogihara et al[12], 2015 | Japan | Retrospective | 1995-2012 | N/A | 634 | NMIBC | 8 | 181:154:299 |
| Rava et al[21], 2018 | Spain | Forward-looking | 1998-2001 | N/A | 936 | MIBC | 7 | 401:369:166 |
| Hagiwara et al[11], 2013 | Japan | Retrospective | 1994-2010 | N/A | 245 | NMIBC | 7 | 72:52:121 |
| Serretta et al[22], 2013 | Italy | Retrospective | 2002-2003 | 48 | 395 | NMIBC | 7 | 127:171:97 |
| Serretta et al[23], 2020 | Italy | Forward-looking | 2008-2012 | N/A | 194 | NMIBC | 7 | 67:127 |
| Ref. | Selection | Comparability | Outcome | Score | ||||||
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | 7 | 8 | ||
| Michalek et al[14], 1985 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Grotenhuis et al[16], 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Wyszynski et al[25], 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Chen et al[19], 2007 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||
| Leibovici et al[20], 2005 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Yuruk et al[26], 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| van Osch et al[24], 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Ogihara et al[12], 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Rava et al[21], 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Hagiwara et al[11], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Serretta et al[22], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| Serretta et al[23], 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
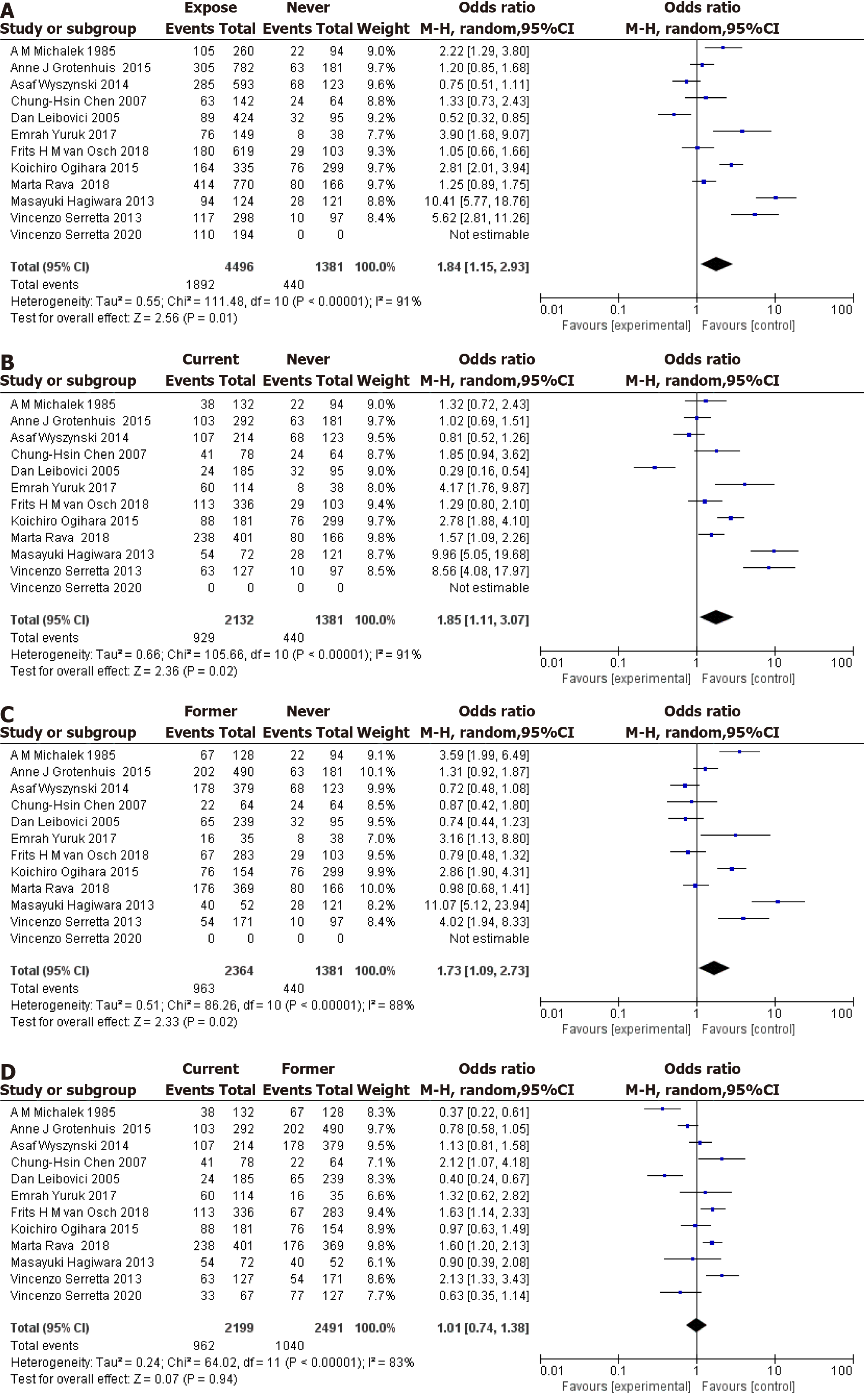
Twelve articles were included in the analysis, with only 11 articles considered for the nonsmoking comparison, as shown in Figure 2. The meta-analysis revealed a significant association between tobacco exposure and an increased risk of bladder cancer recurrence in patients who smoked compared to those who had never smoked, with an OR of 1.84 (95%CI: 1.15-2.93, Figure 2A). Notably, substantial heterogeneity was observed across the included studies [I-squared statistic (I²) = 91%, Q = 111.48, P < 0.00001 for heterogeneity]. Furthermore, current smoking status was also associated with an elevated risk of bladder cancer recurrence compared to never smoking status, with an OR of 1.85 (95%CI: 1.11-3.07, Figure 2B). Again, significant heterogeneity was observed across studies (I² = 91%, Q = 105.66, P < 0.00001 for heterogeneity). Additionally, previous smoking was found to be associated with an increased risk of bladder cancer recurrence compared to never smoking, with an OR of 1.73 (95%CI: 1.09-2.73, Figure 2C), albeit with substantial heterogeneity across studies (I² = 88%, Q = 86.26, P < 0.00001 for heterogeneity). However, when comparing previous smoking status to current smoking status, no significant association was observed with bladder cancer recurrence, yielding an OR of 1.01 (95%CI: 0.74-1.38, Figure 2D). Nonetheless, substantial heterogeneity was still evident across studies (I² = 83%, Q = 64.02, P < 0.00001 for heterogeneity).
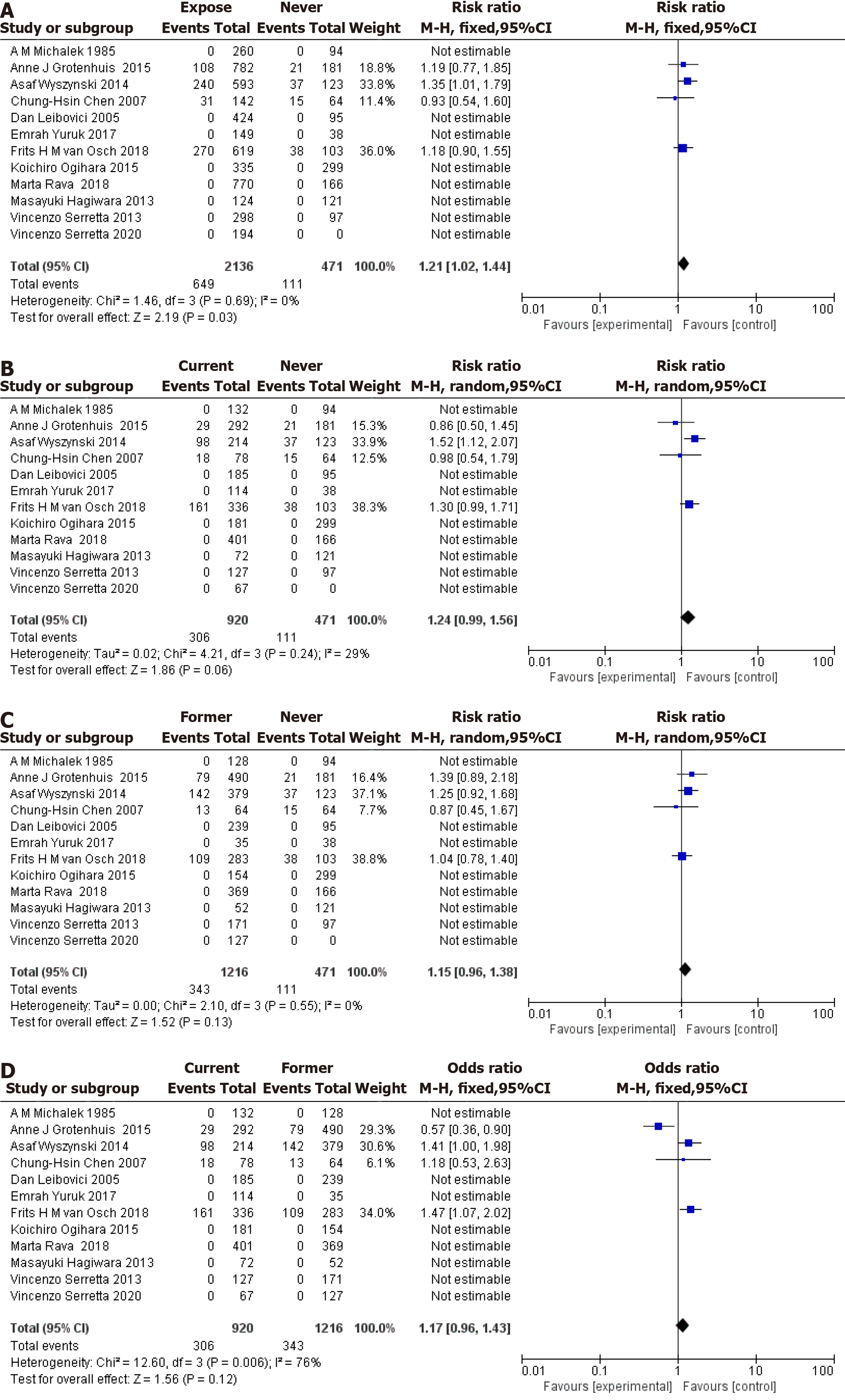
Twelve articles were included in the analysis, as shown in Figure 3. The meta-analysis revealed that tobacco exposure was associated with an increased risk of bladder cancer progression compared with never smoking status, with an OR of 1.21 (95%CI: 1.02-1.44, Figure 3A). No significant heterogeneity was observed across studies (I² = 0%, Q = 1.46, P = 0.69). Current smoking status was not associated with an increased risk of bladder cancer progression compared with never smoking status, with an OR of 1.24 (95%CI: 0.99-1.56, Figure 3B). No significant heterogeneity was observed across studies (I² = 29%, Q = 4.21, P = 0.24). Previous smoking status was not associated with an increased risk of bladder cancer progression compared with never smoking status, with an OR of 1.15 (95%CI: 0.96-1.38, Figure 3C). No significant heterogeneity was observed across studies (I² = 0%, Q = 2.10, P = 0.55). However, when comparing previous smoking status to current smoking status, no significant association was observed with bladder cancer progression, yielding an OR of 1.17 (95%CI: 0.96-1.43, Figure 3D). Substantial heterogeneity was observed across studies (I² = 76%, Q = 12.60, P = 0.006). In summary, the meta-analysis suggested that tobacco exposure is associated with an increased risk of bladder cancer progression compared to never smoking. However, the results for current and previous smoking statuses were inconclusive due to insufficient data and significant heterogeneity across studies. Additional research is needed to further explore the impact of smoking on bladder cancer progression and to address the limitations of the current analysis.
Subgroup and meta-regression analyses were also conducted to explore heterogeneity among studies examining the association between current smoking status and disease recurrence (Table 3). Notably, significant heterogeneity was observed. However, we performed a sensitivity analysis to assess the impact of individual studies on the pooled results by excluding each study in turn. The results indicated that the significant association between current smoking status and disease recurrence was consistent and robust (data not shown). Therefore, despite the observed heterogeneity, the conclusion that current smoking status is associated with an increased risk of disease recurrence is supported by the sensitivity analysis. Additional research is needed to further explore the impact of smoking on disease recurrence and to address the limitations of the current analysis.
| Analysis specification | n | Tobacco exposure vs never smoking | Current smoker vs never smoker | Former smoker vs never smoker | Current smoker vs former smoker | ||||
| OR (95%CI) | I2 (%) | OR (95%CI) | I2 (%) | OR (95%CI) | I2 (%) | OR (95%CI) | I2 (%) | ||
| All | 12 | 1.84 (1.15-2.93) | 91 | 1.85 (1.11-3.07) | 91 | 1.73 (1.09-2.73) | 88 | 1.01 (0.74-1.38) | 83 |
| Race | 91 | 90 | 88 | 83 | |||||
| Caucasian | 7 | 1.33 (1.12-1.58) | 1.32 (1.09-1.59) | 1.29 (1.07-1.56) | 1.03 (0.89-1.18) | ||||
| Noncaucasian | 5 | 2.13 (1.74-2.61) | 2.24 (1.78-2.83) | 1.76 (1.39-2.23) | 1.16 (0.93-1.45) | ||||
| Study design | 91 | 91 | 88 | 83 | |||||
| Multi | 6 | 1.98 (1.68-2.32) | 2.07 (1.73-2.48) | 1.67 (1.39-2.01) | 1.21 (1.04-1.40) | ||||
| Single | 5 | 1.09 (0.87-1.37) | 1.00 (0.78-1.30) | 1.12 (0.87-1.43) | 0.81 (0.65-1.00) | ||||
| Sort | 91 | 88 | 84 | ||||||
| Retrospective | 5 | 3.23 (2.59-4.02) | 3.23 (2.52-4.14) | 3.15 (2.44-4.08) | 1.04 (0.83-1.32) | ||||
| Forward | 6 | 1.05 (0.89-1.24) | 1.09 (0.91-1.32) | 0.97 (0.81-1.17) | 1.10 (0.95-1.28) | ||||
| Disease stage | 91 | 91 | 88 | 83 | |||||
| NMBIC | 10 | 1.93 (1.65-2.26) | 1.83 (1.54-2.18) | 1.81 (1.52-2.15) | 0.94 (0.81-1.09) | ||||
| Others | 2 | 1.00 (0.78-1.29) | 1.20 (0.91-1.59) | 0.85 (0.65-1.12) | 1.38 (1.11-1.72) | ||||
| Research cycle | 91 | 91 | 88 | 83 | |||||
| End of before 2010 | 5 | 1.26 (1.01-1.56) | 1.22 (0.95-1.56) | 1.25 (0.99-1.59) | 0.93 (0.76-1.14) | ||||
| End after 2010 | 7 | 1.88 (1.59-2.21) | 1.92 (1.60-2.31) | 1.59 (1.32-1.92) | 1.14 (0.98-1.33) | ||||
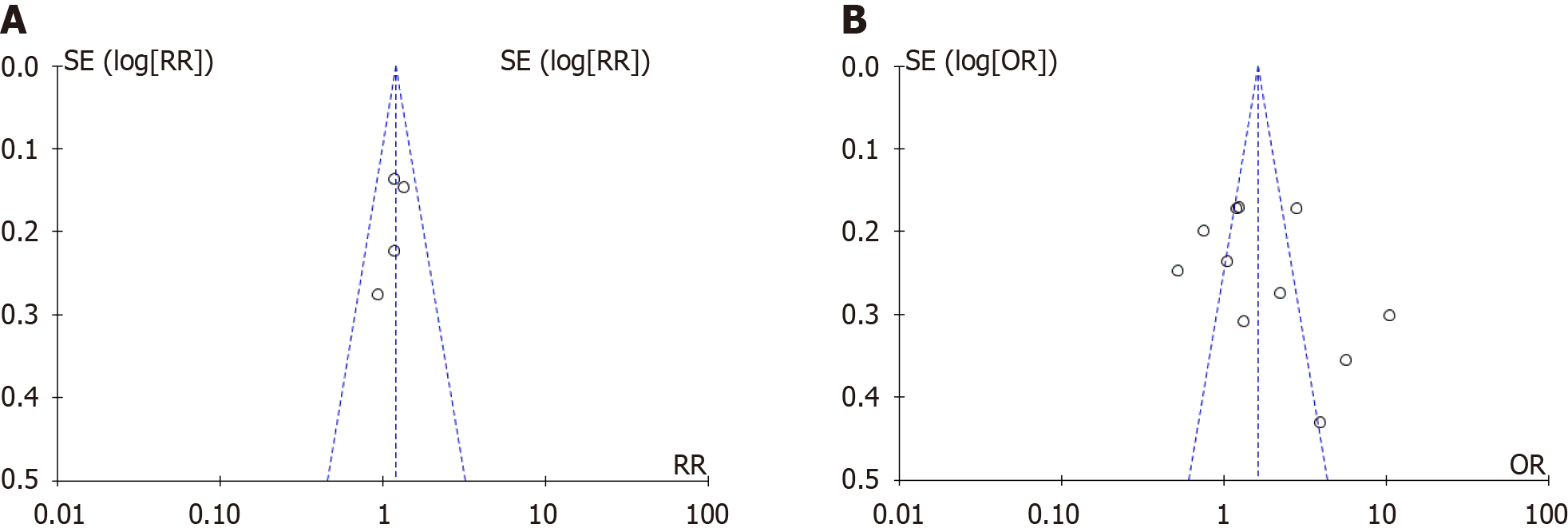
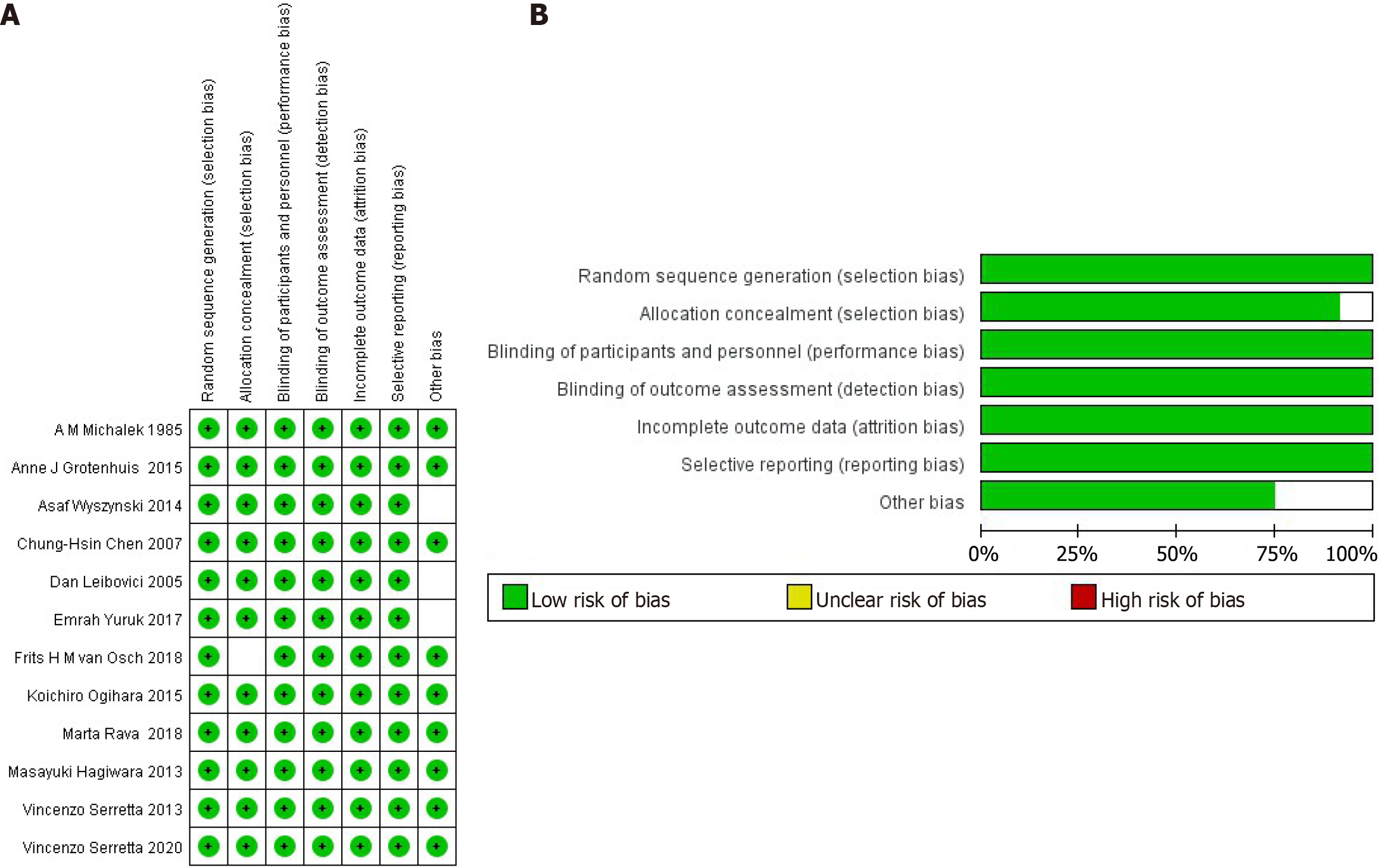
By creating and analyzing funnel plots, it was observed that there was potential publication bias between tobacco exposure and bladder cancer recurrence (Figure 4A), which may have affected the reliability of the pooled results. However, no obvious publication bias was observed between tobacco exposure and bladder cancer progression (Figure 4B), indicating that the pooled results are robust. These findings suggest that additional studies are needed to confirm the association between smoking and bladder cancer recurrence, particularly to address potential publication bias and other sources of heterogeneity. We used the RevMan bias risk tool to carry out risk assessment, and the results showed that all the articles included in this study had a low risk (Figure 5). The findings revealed that no significant high risk of bias was identified across any of the included studies. Notably, three studies exhibited unclear risk of bias with regard to other potential biases, while one study demonstrated unclear risk of bias, specifically in terms of selection bias.
Bladder cancer is a common malignancy worldwide, and there are significant sex differences in its incidence[27,28]. Tobacco smoking is a well-established risk factor for bladder cancer, as exposure to tobacco carcinogens increases the morbidity and mortality associated with this disease[29]. However, most of those previous studies focused on prevention and clinical treatment, and there is limited research on the prognosis of bladder cancer, particularly the impact of tobacco smoking on patients after surgical treatment[30]. Currently, there is no conclusive evidence that tobacco smoking in
To our knowledge, this is the first systematic epidemiological assessment of the association between smoking status and bladder cancer patient outcomes in the past five years. A meta-analysis of 12 cohort studies with a total of 5817 bladder cancer patients was conducted to provide stable and reliable results. The outcomes of surgical treatment for bladder cancer include disease recurrence, disease progression, and cancer-specific mortality. The pooled results suggest that tobacco exposure may increase the risk of bladder cancer recurrence after surgery. Both current smoking and previous smoking were found to be independent risk factors for bladder cancer recurrence, but there was no significant difference between current smoking and previous smoking in terms of bladder cancer recurrence after surgery (OR = 1.01, 95%CI: 0.74-1.38). Additionally, we found that tobacco exposure was significantly associated with bladder cancer progression, but when patients were stratified, the associations of current smoking status and previous smoking status with postoperative bladder cancer progression were not statistically significant. These findings suggest that smoking cessation may be beneficial for improving the prognosis of bladder cancer patients. However, further research is needed to confirm these results and to explore the impact of smoking cessation on bladder cancer outcomes.
The pooled results from the meta-analysis showed that current and former smokers had a significantly greater risk of experiencing disease relapse than patients who had never smoked. Additionally, bladder cancer progression was significantly faster in individuals exposed to tobacco. However, the differences in postoperative recurrence between patients who were current smokers and those who were previously smoking were not well characterized, suggesting that the relationship between tobacco exposure and bladder cancer recurrence may not be dose dependent. Furthermore, tobacco exposure was significantly associated with cancer progression, but the roles of current and previous smoking in cancer progression have not been well validated. The mechanisms underlying the association between tobacco exposure and bladder cancer recurrence and progression are thought to be complex and multifactorial. Strong carcinogens in tobacco, such as coal tar, polycyclic aromatic hydrocarbons, aromatic amines, and nitrosamines[31,32], are absorbed into the bloodstream through inhalation and transported to the kidneys, where they are concentrated in the urine. This process results in exposure of the bladder epithelium to carcinogens, leading to cellular damage and an increased risk of bladder cancer development. Additionally, long-term exposure to smoking carcinogens may lead to cumulative molecular alterations that adversely affect bladder cancer biology and clinical behavior, promoting growth and motility. Furthermore, continuing smoking may weaken the immune response to bladder cancer, leading to an increased risk of recurrence and death. These findings suggest that smoking cessation may be beneficial for improving the prognosis of bladder cancer patients. However, further research[32] is needed to confirm these results and to explore the impact of smoking cessation on bladder cancer outcomes.
These findings suggest that tobacco exposure may have a longer-term effect on bladder cancer recurrence, even after smoking cessation. This highlights the importance of smoking cessation for bladder cancer patients to improve their prognosis. However, the exact mechanism by which smoking cessation reduces subsequent tumor recurrence in patients with bladder cancer remains to be elucidated. Recent studies have shown that nicotine, a major component of tobacco smoke, activates multiple signaling pathways through nicotinic acetylcholine receptors. The MAPK/ERK, PI3K/Akt, and JAK/STAT pathways are associated with tumorigenesis, tumor progression, and acquisition of treatment resistance. These findings suggest that the effects of tobacco exposure on bladder cancer recurrence may be complex and multifactorial and involve various signaling pathways and mechanisms. Therefore, further research is needed to explore the detailed mechanisms by which smoking cessation reduces subsequent tumor recurrence in bladder cancer patients. This may lead to the development of effective strategies for improving the prognosis of bladder cancer patients who smoke or have a history of smoking. Ogihara et al[12] suggested that nicotine exposure in tobacco may induce tumor cell proliferation by activating the PI3K/Akt/mTOR pathway both in vitro and in vivo. This activation of signaling pathways by nicotine can lead to irreversible harmful cell activation, indicating that smoking cessation may not completely prevent the progression and recurrence of bladder cancer. The metabolic cycle of nicotine in the body is relatively short, lasting only 2-6 h[33], but the activation of these cancer-promoting signaling pathways by nicotine can have long-term effects on tumor growth and recurrence. Therefore, smoking cessation is still an important measure for reducing the risk of bladder cancer recurrence, but smoking cessation may not completely eliminate this risk. Future research should focus on exploring the detailed mechanisms by which smoking cessation reduces subsequent tumor recurrence in patients with bladder cancer. This may lead to the development of effective strategies for improving the prognosis of bladder cancer patients who smoke or have a history of smoking. The limitations of this study include the retrospective nature of some of the included studies, which may introduce recall bias. Additionally, there was significant heterogeneity in the studies regarding tobacco exposure, including different types of tobacco products, exposure modalities, and surgical techniques. This heterogeneity may have influenced the overall results, leading to a potential futility association between tobacco exposure and bladder cancer outcomes. It should also be noted that the effects of current and previous smoking on bladder cancer progression were not statistically significant, possibly due to the limited number of studies addressing this topic. The lack of adjustment for potential confounders in some studies may have also affected the results. In conclusion, the results of this meta-analysis suggest that tobacco exposure is significantly associated with postoperative recurrence and progression of bladder cancer. However, larger epidemiological studies with longer follow-up periods are needed to confirm these findings and further explore the mechanisms underlying the effects of tobacco exposure on bladder cancer outcomes.
This meta-analysis suggested that tobacco exposure may increase the risk of bladder cancer recurrence and progression after surgery. However, there was no significant association between current or previous smoking and postoperative cancer progression. Smoking is a major risk factor for bladder cancer, and cessation of smoking is recommended. Notably, quitting smoking may not completely eliminate this risk. Regular follow-up and treatment are crucial for reducing the risk of bladder cancer recurrence and progression in smokers. Additionally, genetic, environmental, and lifestyle factors may also influence bladder cancer risk, and smokers should consider these factors when taking preventive measures.
| 1. | Dobruch J, Oszczudłowski M. Bladder Cancer: Current Challenges and Future Directions. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Gu C, Wang Z, Zhou N, Li G, Kou Y, Luo Y, Wang Y, Yang J, Tian F. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer. 2019;18:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 3. | Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 480] [Article Influence: 43.6] [Reference Citation Analysis (3)] |
| 4. | Witjes JA. Follow-up in non-muscle invasive bladder cancer: facts and future. World J Urol. 2021;39:4047-4053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, Konety BR, Saigal CS; Urologic Diseases in America Project. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Jensen KP, Smith AH, Herman AI, Farrer LA, Kranzler HR, Sofuoglu M, Gelernter J. A protocadherin gene cluster regulatory variant is associated with nicotine withdrawal and the urge to smoke. Mol Psychiatry. 2017;22:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Muggli ME, Lee K, Gan Q, Ebbert JO, Hurt RD. "Efforts to Reprioritise the Agenda" in China: British American Tobacco's Efforts to Influence Public Policy on Secondhand Smoke in China. PLoS Med. 2008;5:1729-1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Erzurumluoglu AM, Liu M, Jackson VE, Barnes DR, Datta G, Melbourne CA, Young R, Batini C, Surendran P, Jiang T, Adnan SD, Afaq S, Agrawal A, Altmaier E, Antoniou AC, Asselbergs FW, Baumbach C, Bierut L, Bertelsen S, Boehnke M, Bots ML, Brazel DM, Chambers JC, Chang-Claude J, Chen C, Corley J, Chou YL, David SP, de Boer RA, de Leeuw CA, Dennis JG, Dominiczak AF, Dunning AM, Easton DF, Eaton C, Elliott P, Evangelou E, Faul JD, Foroud T, Goate A, Gong J, Grabe HJ, Haessler J, Haiman C, Hallmans G, Hammerschlag AR, Harris SE, Hattersley A, Heath A, Hsu C, Iacono WG, Kanoni S, Kapoor M, Kaprio J, Kardia SL, Karpe F, Kontto J, Kooner JS, Kooperberg C, Kuulasmaa K, Laakso M, Lai D, Langenberg C, Le N, Lettre G, Loukola A, Luan J, Madden PAF, Mangino M, Marioni RE, Marouli E, Marten J, Martin NG, McGue M, Michailidou K, Mihailov E, Moayyeri A, Moitry M, Müller-Nurasyid M, Naheed A, Nauck M, Neville MJ, Nielsen SF, North K, Perola M, Pharoah PDP, Pistis G, Polderman TJ, Posthuma D, Poulter N, Qaiser B, Rasheed A, Reiner A, Renström F, Rice J, Rohde R, Rolandsson O, Samani NJ, Samuel M, Schlessinger D, Scholte SH, Scott RA, Sever P, Shao Y, Shrine N, Smith JA, Starr JM, Stirrups K, Stram D, Stringham HM, Tachmazidou I, Tardif JC, Thompson DJ, Tindle HA, Tragante V, Trompet S, Turcot V, Tyrrell J, Vaartjes I, van der Leij AR, van der Meer P, Varga TV, Verweij N, Völzke H, Wareham NJ, Warren HR, Weir DR, Weiss S, Wetherill L, Yaghootkar H, Yavas E, Jiang Y, Chen F, Zhan X, Zhang W, Zhao W, Zhou K, Amouyel P, Blankenberg S, Caulfield MJ, Chowdhury R, Cucca F, Deary IJ, Deloukas P, Di Angelantonio E, Ferrario M, Ferrières J, Franks PW, Frayling TM, Frossard P, Hall IP, Hayward C, Jansson JH, Jukema JW, Kee F, Männistö S, Metspalu A, Munroe PB, Nordestgaard BG, Palmer CNA, Salomaa V, Sattar N, Spector T, Strachan DP; Understanding Society Scientific Group, EPIC-CVD, GSCAN, Consortium for Genetics of Smoking Behaviour, CHD Exome+ consortium, van der Harst P, Zeggini E, Saleheen D, Butterworth AS, Wain LV, Abecasis GR, Danesh J, Tobin MD, Vrieze S, Liu DJ, Howson JMM. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry. 2020;25:2392-2409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Flint-Richter P, Mandelzweig L, Oberman B, Sadetzki S. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro Oncol. 2011;13:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 744] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 11. | Hagiwara M, Kikuchi E, Tanaka N, Matsumoto K, Ide H, Miyajima A, Masuda T, Nakamura S, Oya M. Impact of smoking status on bladder tumor recurrence after radical nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2013;189:2062-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Ogihara K, Kikuchi E, Yuge K, Ito Y, Tanaka N, Matsumoto K, Miyajima A, Asakura H, Oya M. Refraining from Smoking for 15 Years or More Reduced the Risk of Tumor Recurrence in Non-muscle Invasive Bladder Cancer Patients. Ann Surg Oncol. 2016;23:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur Urol. 2011;60:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Michalek AM, Cummings KM, Pontes JE. Cigarette smoking, tumor recurrence, and survival from bladder cancer. Prev Med. 1985;14:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Wakai K, Ohno Y, Obata K, Aoki K. Prognostic significance of selected lifestyle factors in urinary bladder cancer. Jpn J Cancer Res. 1993;84:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Grotenhuis AJ, Ebben CW, Aben KK, Witjes JA, Vrieling A, Vermeulen SH, Kiemeney LA. The effect of smoking and timing of smoking cessation on clinical outcome in non-muscle-invasive bladder cancer. Urol Oncol. 2015;33:65.e9-65.17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51705] [Article Influence: 10341.0] [Reference Citation Analysis (2)] |
| 18. | Admiraal WM, Celik F, Gerdes VE, Dallal RM, Hoekstra JB, Holleman F. Ethnic differences in weight loss and diabetes remission after bariatric surgery: a meta-analysis. Diabetes Care. 2012;35:1951-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Chen CH, Shun CT, Huang KH, Huang CY, Tsai YC, Yu HJ, Pu YS. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100:281-6; discussion 286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, Gu J, Dong Q, Wu X. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J Clin Oncol. 2005;23:5746-5756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Rava M, Czachorowski MJ, Silverman D, Márquez M, Kishore S, Tardón A, Serra C, García-Closas M, Garcia-Closas R, Carrato A, Rothman N, Real FX, Kogevinas M, Malats N. Asthma status is associated with decreased risk of aggressive urothelial bladder cancer. Int J Cancer. 2018;142:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Serretta V, Altieri V, Morgia G, Di Lallo A, Carrieri G, Allegro R; Gruppo Studi Tumori Urologici (GSTU) Foundation. Cigarette smoking status at diagnosis and recurrence in intermediate-risk non-muscle-invasive bladder carcinoma. Urology. 2013;81:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Serretta V, Di Maida F, Baiamonte D, Vella M, Pavone C, Cacciatore L, Valerio MR, Scalici Gesolfo C, Sanfilippo C. Does Smoking Cessation at Primary Diagnosis Reduce the Recurrence Risk of Nonmuscle-Invasive Bladder Cancer? Results of a Prospective Study. Urol Int. 2020;104:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | van Osch FHM, Jochems SHJ, Reulen RC, Pirrie SJ, Nekeman D, Wesselius A, James ND, Wallace DMA, Cheng KK, van Schooten FJ, Bryan RT, Zeegers MP. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: a prospective cohort study. Cancer Causes Control. 2018;29:675-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, Pendleton EM, Celaya MO, Zens MS, Karagas MR, Andrew AS. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Yuruk E, Tuken M, Colakerol A, Serefoglu EC. The awareness of patients with non - muscle invasive bladder cancer regarding the importance of smoking cessation and their access to smoking cessation programs. Int Braz J Urol. 2017;43:607-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1615] [Cited by in RCA: 1829] [Article Influence: 203.2] [Reference Citation Analysis (0)] |
| 28. | Janisch F, Shariat SF, Schernhammer E, Rink M, Fajkovic H. The interaction of gender and smoking on bladder cancer risks. Curr Opin Urol. 2019;29:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Stern MC, Lin J, Figueroa JD, Kelsey KT, Kiltie AE, Yuan JM, Matullo G, Fletcher T, Benhamou S, Taylor JA, Placidi D, Zhang ZF, Steineck G, Rothman N, Kogevinas M, Silverman D, Malats N, Chanock S, Wu X, Karagas MR, Andrew AS, Nelson HH, Bishop DT, Sak SC, Choudhury A, Barrett JH, Elliot F, Corral R, Joshi AD, Gago-Dominguez M, Cortessis VK, Xiang YB, Gao YT, Vineis P, Sacerdote C, Guarrera S, Polidoro S, Allione A, Gurzau E, Koppova K, Kumar R, Rudnai P, Porru S, Carta A, Campagna M, Arici C, Park SS, Garcia-Closas M; International Consortium of Bladder Cancer. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: findings from the international consortium of bladder cancer. Cancer Res. 2009;69:6857-6864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Tellini R, Mari A, Muto G, Cacciamani GE, Ferro M, Stangl-Kremser J, Campi R, Soria F, Rink M, Xylinas E, Minervini A, Briganti A, Montorsi F, Roupret M, Shariat SF, Moschini M; European Association of Urology–Young Academic Urologists Urothelial Carcinoma Working Group (EAU-YAU). Impact of Smoking Habit on Perioperative Morbidity in Patients Treated with Radical Cystectomy for Urothelial Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Oncol. 2021;4:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 359] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Pande M, Lynch PM, Hopper JL, Jenkins MA, Gallinger S, Haile RW, LeMarchand L, Lindor NM, Campbell PT, Newcomb PA, Potter JD, Baron JA, Frazier ML, Amos CI. Smoking and colorectal cancer in Lynch syndrome: results from the Colon Cancer Family Registry and the University of Texas M.D. Anderson Cancer Center. Clin Cancer Res. 2010;16:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, Ouellette MM, Batra SK. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32:1384-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0