Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.428
Peer-review started: December 13, 2021
First decision: March 24, 2022
Revised: April 1, 2022
Accepted: July 24, 2022
Article in press: July 24, 2022
Published online: September 20, 2022
Processing time: 277 Days and 4.2 Hours
Glioma is the most common primary tumor in the brain originating from glial cells. In spite of extensive research, the overall survival rate is not enhanced. A number of published articles observed differentially circulating levels of cytokines in glioma. Interleukin-6 (IL-6) protein coded by IL-6 gene is regulated by the immune system and it has been found to have a significant role in progression and apoptosis resistance of glioma.
To review the role of circulatory IL-6 in the development and progression of glioma and its utility as a biomarker.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were applied to filter the relevant studies based on inclusion and exclusion criteria. We used a combination of keywords and the Reference Citation Analysis (RCA) tool to search the potential studies and performed data extraction from selected studies.
The published results were inconsistent; however, most studies showed a significantly higher IL-6 level in glioma cases as compared to controls. Com
IL-6 level significantly differed between cases and controls, and among different cancer stages, which shows its potential as a diagnostic and prognostic marker.
Core Tip: In spite of extensive research in the field of brain oncology, the overall survival is not much improved. There is an urgent need to explore the circulatory markers for diagnosis and prognosis. This systematic review focused on the role of interleukin-6 in brain cancer development and progression and its utility as a diagnostic or prognostic biomarker.
- Citation: Singh M, Raghav A, Gautam KA. Role of the circulatory interleukin-6 in the pathogenesis of gliomas: A systematic review. World J Methodol 2022; 12(5): 428-437
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/428.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.428
Gliomas are the most common primary brain tumors in adults, accounting for 80% of malignant brain tumors originating from glial cells[1]. Globally, gliomas show a wide variation in incidence, and it is 0.01–12.7 in males and 0.01–10.7 in females per 100 000 people[2]. The lowest incidence is in Africa and highest in Northern Europe[2]. Gliomas are an increasing cause of death in children and the third most common in adolescents and adults[2]. According to the World Health Organization (WHO) classification, the most common occurring histological grade of gliomas is astrocytic tumors (grades I–III) and oligodendroglial tumors (grades II–III), ependymoma (grades I–III) and glioblastoma (grade IV)[3,4]. Glioblastoma is aggressive in nature and the survival rate is low, with death within 2 years of diagnosis despite receiving maximal surgical removal of the tumor and medical therapies including chemotherapy and radiotherapy. Therefore, there is an urgent need to find comprehensive treatment strategies to enhance the survival rate[5].
Adapting the Virchow theory, various studies concluded that inflammation is one of the major hallmarks of cancer formation[6,7]. Within the cancerous microenvironment, inflammatory cells and cytokines have pleomorphic roles. On the one hand, these aid in tumor suppression, while on the other hand, they support malignant cell transformation, tumor growth, inhibition of apoptosis, invasion, angiogenesis, cell migration, tumor cells differentiation and immuno-suppression[8-11]. A number of studies showed varied circulating levels of cytokines in glioma. On the basis of The Cancer Genome Atlas database, interleukin (IL)-6 has a significant role in progression and apoptosis resistance of glioma[12-15].
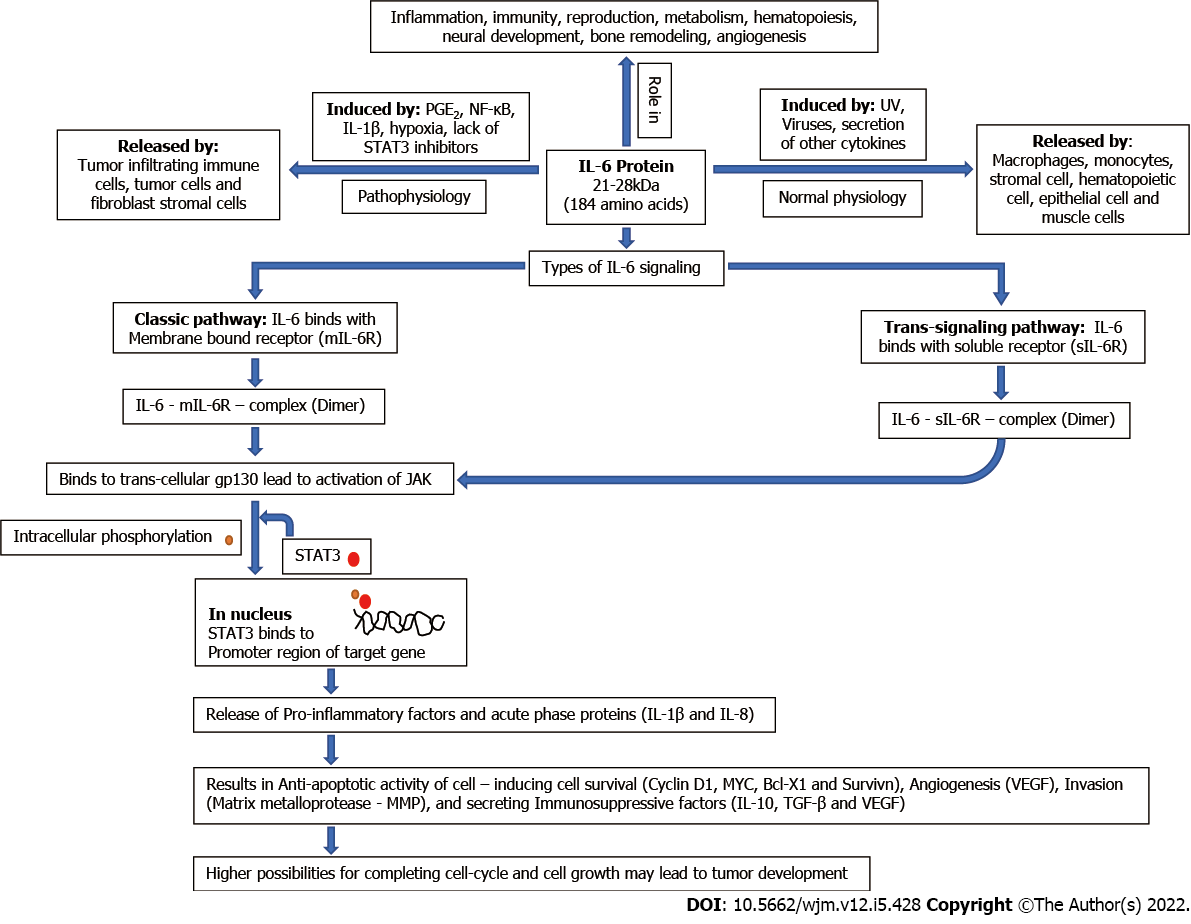
IL-6 is a pleiotropic proinflammatory cytokine with a 21–28-kDa four-helix bundled glycoprotein with 184 amino acids[16,17]. Under normal conditions, IL-6 secretion is initiated in response to stimuli such as viruses, UV and secretion of other cytokines, and it is released by a variety of cells including macrophages, monocytes, hematopoietic cells, stromal cells, muscles cells and epithelial cells. IL-6 has a significant role in the process of immunity, inflammation, angiogenesis, neural development, reproduction, metabolism hematopoiesis, and bone remodeling[18,19]. In tumor vasculature, IL-6 is released by tumor cells, tumor-infiltrating immune cells and fibroblast stromal cells, and induced by several factors such as prostaglandin E2, IL-1β, hypoxia, nuclear factor (NF)-κB, miRNAs and lack of signal transducer and activator of transcription (STAT)3 inhibitors[16,18,20-23]. IL-6 exerts its function by binding to its receptor either by membrane bound receptor (mIL-6R), the classical pathway or by soluble receptor (sIL-6R), the trans-signaling pathway. Binding of IL-6 to its receptor causes the activation of gp130, which subsequently activates cytoplasmic tyrosine kinases (Janus kinase, JAK) via its phosphorylation that is responsible for intracellular signaling by phosphorylation of STATs (especially the STAT3 pathway). Phosphorylated STAT3 dimer translocates to the nucleus, which leads to the transcription of targeted genes (Bcl-2, Bcl-xL, Cyclin D1, VEGF, etc.) and production of other proinflammatory cytokines and exerts an acute-phase response[16,18,24]. These activated genes may code for the proteins involved in cell survival (cyclin D1, survivin and MYC)[18], antiapoptotic condition (Bcl-x and MYC)[16,25], angiogenesis (vascular endothelial growth factor; VEGF)[16], invasion (MMP)[16], tumor growth and immunosuppressive factor secretion [transforming growth factor (TGF)-β, IL-10 and VEGF][26,27]. A systematic diagram showing the physiology of IL-6 is shown in Figure 1. The STAT3 signaling pathway is downregulated in different ways, such as suppressor of cytokine signaling (SOCS)3 inhibits phosphorylation of JAK proteins and protein inhibitor of activated STAT3 (PIAS3) inhibits dimerization of STAT3 monomers.
Besides these key roles, IL-6 also plays key roles in inflammation, proliferation and differentiation of B and T lymphocytes and natural killer cells[28]. IL-6 blocks MHC class II expression of Th1 cells and halts the secretion of IL-2 and interferon-γ and hence reduces cytotoxic T-lymphocyte activity[29]. Inhibition of the activity of T lymphocytes helps cancer cells to inhibit the immune response. Several miRNAs are involved in the production of IL-6 in a paracrine manner[30]
In various studies, a higher level of IL-6 was found to be associated with tumor progression and poor survival rate in several cancers including glioma. In glioma, IL-6 affects tumor formation and progression by triggering the JAK/STAT3 signaling pathway, which may further lead to continuous cell growth[31], tumor development, cell invasion and migration[32,33], angiogenesis[34] and inhibition of apoptosis[35,36]. The mRNA expression of IL-6 gene has been found to correlate with higher grade of glioma (glioblastoma)[37], in addition IL-6 gene amplification in tissues samples was 54% (15 of 36) on glioblastoma and none of 17 in lower grade of glioma[38]. Immunohistochemistry revealed that IL-6 receptors were totally absent in normal brain tissue and all the tissues of glioblastoma samples[39]. STAT3 promotes tumor growth by inhibiting apoptosis in glioma and increased level of phosphorylated STAT3 is found in recurrent glioblastoma as compared to primary glioma[40].
In this systematic review, we reviewed all the published case–control studies investigating the role of circulatory IL-6 in the development and progression of glioma and its utility as a diagnostic or prognostic biomarker.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines[41] were adapted to perform this systematic review.
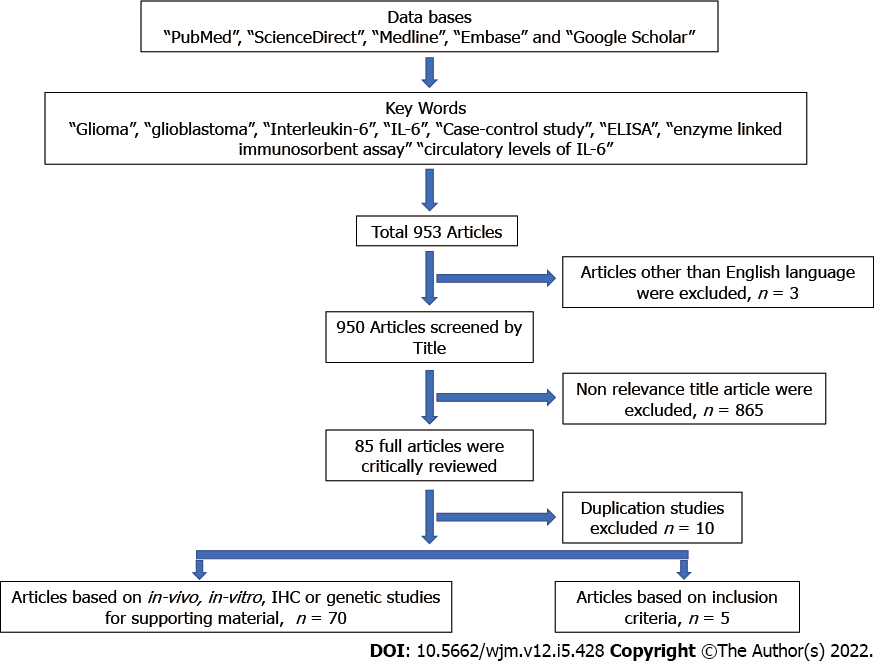
An exhausted literature search on March 1, 2021 was done by two research scientists independently using various combination of keywords “glioma”, “glioblastoma”, “interleukin-6”, “IL-6”, “case-control study”, “ELISA”, “enzyme linked immunosorbent assay” “circulatory levels of IL-6” using the Reference Citation AnalysisTM (RCA) tool, which is an artificial intelligence technology-based open multidisciplinary citation analysis database. On RCA window, keywords were entered in the designated area and after selecting the “Find an Article”, we obtained a list of the latest highlighted articles that was further filtered by selecting Impact Index Per Article. The systematic search was limited to articles published in English language. The relevant full-text articles were obtained. References were also evaluated to retrieve additional studies. The researchers thoroughly evaluated full-length original articles based on the inclusion and exclusion criteria for the inclusion in this systematic review [41].
All the retrieved studies were screened and filtered on the basis of PICO (patient/population, intervention, comparison and outcomes) strategy as follows: (1) Participants: histopathological confirmed cases of glioma; (2) intervention: conditions including progression and invasion of glioma; (3) comparison: controls free from any malignancy; (4) observation: IL-6 expression level by ELISA or multiplex assay; and (5) case–control studies. A flow chart (PRISMA) showing the search strategy is shown in Figure 2.
The studies were excluded based on the following criteria: (1) Studies with insufficient information regarding the level of IL-6; (2) review articles, meta-analyses, editorials, letters, and duplicate articles; (3) conference proceedings; and (4) not in English language.
Gathering of information from the relevant articles was carefully done on the basis of inclusion criteria. From each relevant study, the following information was collected and organized in Table 1: First author’s last name, year of publication, ethnicity of the study population, sample size, sample collected (serum or plasma), method of analysis (ELISA), IL-6 expression and glioma outcome (increased or decreased) in comparison to controls.
| No. | Author name | Year | Region | Sample | Case (glioma) | Control | IL-6 level (pg/mL) |
| 1 | Doroudchi et al[42] | 2013 | Iran | serum | 38 | 26 | Decreased as compared to controls |
| 2 | Shan et al[43] | 2015 | China | Serum | 86 | 18 | IL-6 level increased with the elevation of grade |
| 3 | Albulescu et al[8] | 2013 | Romania | Serum | 55 | 20 | 3-fold upregulated than control |
| 4 | Schwartzbaum et al[44] | 2017 | Norway | Serum | 487 | 487 | insignificant association with the disease |
| 5 | Zhenjiang et al[45] | 2018 | Sweden | Serum | GBM = 145 | Non-GBM = 60 | 45%–50% cases of GBM observed with detectable level of IL-6 & 55%–60% cases of Non-GBM malignant glioma observed with detected level of IL-6 |
A total of 953 studies were identified in the literature search and five studies have been included for full evaluation in this systematic review (Figure 2). The critically evaluated studies are summarized in Table 1.
The study of Doroudchi et al[42] comprising 38 cases and 26 controls found a significantly decreased level of IL-6 in the serum of glioma cases (2.34 ± 4.35 pg/mL) as compared to controls (4.67 ± 4.35 pg/mL), while some other studies observed a significantly increased level of IL-6 in cases as compared to controls[8,42,43]. A study including 55 cases of glioblastoma and 20 healthy controls found fourfold upregulation of IL-6 in the cases of glioblastoma as compared to controls[8]. In contrast, Schwartzbaum et al[44], with a large number of cases of glioma (n = 487) and healthy controls (n = 487), did not find any significant (OR = 0.77) association of case–control correlation in differentially expressed level of IL-6. Level of IL-6 in glioma patients aged > 30 years showed a lower value as compared to young patients; however, the investigators did not find a significant correlation[42].
Comparative level of IL-6 among the different grade of glioma cancer observed a higher level (4.02 ± 7.80 pg/mL) with low grade of cancer and lower levels (1.74 ± 1.55 pg/mL) with high grade of cancer[42]. In contrast, in a few studies, the serum levels of IL-6 increased with the progression of glioma grading[43]. Univariate analysis indicated that the increased level of IL-6 declined after surgical removal of the glioma[43]. This indicates that, along with immune cells including inflammatory cells, tumor cells can also release the IL-6. Zhenjiang et al[45] has compared the circulating level of IL-6 along with other cytokines between glioblastoma multiforme (GBM) and non-GBM malignant glioma. They observed a detectable concentration of IL-6 in 45%–50% of cases, along with IL-4 and IL-5 in GBM patients, while 55%–60% cases with non-GBM glioma expressed IL-6 along with IL-4 and IL-5[45]. The investigators also analyzed the combination effects of selected cytokines (IL-4/IL-5/IL-6) on patients’ survival and found that if all were present or all absent, it was associated with better survival rate.
Many biomarkers are differentially expressed in cases versus controls using tissue samples; however, the current need is based and focused on circulatory biomarkers. Recently, liquid biopsy has been used to investigate disease development and progression using easily accessible samples like blood or urine or saliva. The published literature shows that there has been a scarcity of studies on the association between human brain cancer and IL-6, and published results are contradictory. However, in vivo studies have shown a strong relationship between IL-6 and disease initiation and progression. This indicates an urgent need to design studies to establish how IL-6 can be exploited as diagnostic or prognostic marker.
Glioma is a fatal disease with a reported survival rate of 5% despite surgical resection along with radiotherapy and/or chemotherapy. In spite of extensive research, the overall survival has not much improved[46]. Several experimental studies have shown that IL-6 can be produced by tumor cells, and glioma is characterized by systemic immunosuppression that hinders the response to immunotherapy and helps with tumor progression. Immunotherapy is currently the most explored area of cancer biology and has been shown to increase survival rate in patients with malignancies; however, for glioma its efficacy is currently still being revealed[47]. In glioblastoma, programmed death-ligand 1 (PD-L1) is the critical mediator of immunosuppression and myeloid cells (noncancerous cells) in the tumor microenvironment and circulation express an elevated level of PD-L1[48,49]. Experimental studies have shown that glioblastoma-derived IL-6 is mandatory and sufficient for the induction of PD-L1, and the correlation between IL-6 and immunosuppression has been recognized in vitro and in vivo[50,51].
In this systematic review, the overall result was inconclusive. However, we found that most studies observed an elevated level of IL-6 in serum of glioma patients as compared to controls, which indicate the immunosuppressive role of IL-6 in tumor development[3,43]. IL-6, IL-8 and IL-1β are the proinflammatory cytokines and their circulatory expression is upregulated along with downregulated level of anti-inflammatory cytokine IL-4 in glioma, and higher secretion of proinflammatory cytokines is related to the progression of glioblastoma and poor survival rate[8,52,53]. In addition, studies based on expression analysis have shown that expression of IL-6 in glioma cases is significantly different from that in controls. Among grading of glioma, the intensity of IL-6 staining increases with increasing grading, which shows that patients with poorly differentiated tumor have a higher level of IL-6[43]. Therefore, measuring the circulatory levels of IL-6 before and after surgery can be standardized for the prediction of clinical prognosis of glioma.
The uptake and role of IL-6 in glioma invasion has been demonstrated by trans well invasion assay using glioma cell lines (U251 cells, U87 cells T98G cells and A172 cells) incubated with exogenous IL-6[43]. These studies observed IL-6 in the supernatant of the glioma cell lines[43]. STAT3 gene is considered to have a conserved sequence and mutation is rare; therefore, it is believed that its consti
The exact regulatory network of IL-6 in the tumor microenvironment is complex; therefore, targeting the underlying mechanism of IL-6 regulation should be undertaken to understand how its upregulation or over-active signaling pathways (especially IL-6/JAK/STAT3 signaling pathway) can help in tumor development, progression or recurrence[56]. Tumor formation is not a consequence of an adverse effect of a single risk factor or cytokine, but rather a group of cytokines, including chemokines, angiogenesis factors and growth factors. Therefore, combinational effects of cytokines can be used to assess their role in glioma and the results may be applied for future tailored immunotherapy and immune-monitoring procedures. Targeting and reducing the molecules hindering the activity of specific therapy may lead to re-sensitization to delivered therapy. Few clinical trials are investigating this idea[57].
This systematic review found five published research articles investigating the role of IL-6 as a potential biomarker of glioma in case–controls studies. The overall results are inconsistent; however, most studies found an elevated level of IL-6 in cases of glioma as compared to controls. The level of IL-6 was more than twofold in cases, which means that IL-6 can be considered as potential diagnostic biomarker. In tumors with progressive growth (advanced grade), the circulating level of IL-6 is also increased and hence can be used as a prognostic marker for glioma. Immunotherapy that can produce a durable and tumor-specific immune response can be implemented by disrupting IL-6 signaling and re-sensitizing the immune response to halt or reduce tumor growth and enhance survival rate based on REMARK (reporting recommendation for tumor biomarker prognostics studies) guidelines[58,59].
Interleukin (IL)-6 is a proinflammatory cytokine that is involved in immunity, inflammation, angiogenesis, neural development and reproduction. The tumor microenvironment containing tumor cells, tumor-infiltrating immune cells and fibroblast stromal cells releases IL-6. IL-6 acts on the Janus kinase and signal transducer and activator of transcription factor pathway. These pathways release or associate with proteins that are responsible for major cellular functions.
This systematic review was motivated by a number of research studies that investigated the association between IL-6 and glioma.
In this systematic-review, case-control studies investigating the role of IL-6 with glioma development and progression have been discussed to review the utility of IL-6 as a biomarker.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were applied to filter the relevant studies based on inclusion and exclusion criteria. We used a combination of keywords and Reference Citation Analysis (RCA) tool to search for potential studies and performed data extraction from selected studies.
Five case–control studies were included for full evaluation. Most studies found a significantly higher level of IL-6 in cases as compared to controls although a study with contradictory results and a study with no difference in IL-6 level was also observed. Il-6 level varies with glioma stage, and some studies have reported lower levels in high-stage of cancer, whereas others have reported higher levels of IL-6 in early-stage glioma. Age at the time of diagnosis of glioma and IL-6 level could also have a significant relationship with glioma.
IL-6 could be a potential biomarker for the diagnosis and prognosis of glioma as it was increased twofold in cases of glioma as compared to controls.
Immunotherapy based treatment can be implemented by trigging IL-6 protein associated pathways and re-sensitizing the immune response to inhibit tumor growth and enhance survival rate.
| 1. | Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19:v1-v88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1204] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 2. | Maile EJ, Barnes I, Finlayson AE, Sayeed S, Ali R. Nervous System and Intracranial Tumour Incidence by Ethnicity in England, 2001-2007: A Descriptive Epidemiological Study. PLoS One. 2016;11:e0154347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003-2007. Neuro Oncol. 2017;19:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 11212] [Article Influence: 1121.2] [Reference Citation Analysis (0)] |
| 5. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 16429] [Article Influence: 782.3] [Reference Citation Analysis (0)] |
| 6. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5856] [Article Influence: 234.2] [Reference Citation Analysis (1)] |
| 7. | Westermark B. Glioblastoma--a moving target. Ups J Med Sci. 2012;117:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, Teodoru M, Tanase CP. Cytokine patterns in brain tumour progression. Mediators Inflamm. 2013;2013:979748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2125] [Article Influence: 125.0] [Reference Citation Analysis (2)] |
| 10. | Magaña-Maldonado R, Chávez-Cortez EG, Olascoaga-Arellano NK, López-Mejía M, Maldonado-Leal FM, Sotelo J, Pineda B. Immunological Evasion in Glioblastoma. Biomed Res Int. 2016;2016:7487313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Placone AL, Quiñones-Hinojosa A, Searson PC. The role of astrocytes in the progression of brain cancer: complicating the picture of the tumor microenvironment. Tumour Biol. 2016;37:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Cheng W, Ren X, Zhang C, Cai J, Liu Y, Han S, Wu A. Bioinformatic profiling identifies an immune-related risk signature for glioblastoma. Neurology. 2016;86:2226-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J Pharm Pharmacol. 2008;60:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | McFarland BC, Hong SW, Rajbhandari R, Twitty GB Jr, Gray GK, Yu H, Benveniste EN, Nozell SE. NF-κB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One. 2013;8:e78728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with α5β1 integrin in glioma. Oncogene. 2013;32:327-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 2322] [Article Influence: 290.3] [Reference Citation Analysis (0)] |
| 17. | Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553-11572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 790] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 18. | Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 552] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Lippitz BE, Harris RA. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 2016;5:e1093722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Huynh PT, Beswick EJ, Coronado YA, Johnson P, O'Connell MR, Watts T, Singh P, Qiu S, Morris K, Powell DW, Pinchuk IV. CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int J Cancer. 2016;138:1971-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Lesina M, Wörmann SM, Neuhöfer P, Song L, Algül H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol. 2014;26:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Pop VV, Seicean A, Lupan I, Samasca G, Burz CC. IL-6 roles - Molecular pathway and clinical implication in pancreatic cancer - A systemic review. Immunol Lett. 2017;181:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 24. | Yeung YT, McDonald KL, Grewal T, Munoz L. Interleukins in glioblastoma pathophysiology: implications for therapy. Br J Pharmacol. 2013;168:591-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Wang ZY, Zhang JA, Wu XJ, Liang YF, Lu YB, Gao YC, Dai YC, Yu SY, Jia Y, Fu XX, Rao X, Xu JF, Zhong J. IL-6 Inhibition Reduces STAT3 Activation and Enhances the Antitumor Effect of Carboplatin. Mediators Inflamm. 2016;2016:8026494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1211] [Article Influence: 110.1] [Reference Citation Analysis (8)] |
| 27. | Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 28. | De Vita F, Romano C, Orditura M, Galizia G, Martinelli E, Lieto E, Catalano G. Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J Interferon Cytokine Res. 2001;21:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Kitamura H, Ohno Y, Toyoshima Y, Ohtake J, Homma S, Kawamura H, Takahashi N, Taketomi A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1947-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 30. | Patel SA, Gooderham NJ. IL6 Mediates Immune and Colorectal Cancer Cell Cross-talk via miR-21 and miR-29b. Mol Cancer Res. 2015;13:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Waxman AB, Kolliputi N. IL-6 protects against hyperoxia-induced mitochondrial damage via Bcl-2-induced Bak interactions with mitofusins. Am J Respir Cell Mol Biol. 2009;41:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J. IL 6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up regulation of MMP 2 and fascin 1. Oncol Rep. 2010;23:1553-1559. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Liu Q, Li G, Li R, Shen J, He Q, Deng L, Zhang C, Zhang J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol. 2010;100:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 618] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 35. | Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 447] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 36. | Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 958] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 37. | Rolhion C, Penault-Llorca F, Kémény JL, Lemaire JJ, Jullien C, Labit-Bouvier C, Finat-Duclos F, Verrelle P. Interleukin-6 overexpression as a marker of malignancy in human gliomas. J Neurosurg. 2001;94:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Tchirkov A, Khalil T, Chautard E, Mokhtari K, Véronèse L, Irthum B, Vago P, Kémény JL, Verrelle P. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007;96:474-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Kudo M, Jono H, Shinriki S, Yano S, Nakamura H, Makino K, Hide T, Muta D, Ueda M, Ota K, Ando Y, Kuratsu J. Antitumor effect of humanized anti-interleukin-6 receptor antibody (tocilizumab) on glioma cell proliferation. Laboratory investigation. J Neurosurg. 2009;111:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, Tanino M, Kimura T, Nishihara H, Tanaka S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 41. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48684] [Article Influence: 2863.8] [Reference Citation Analysis (3)] |
| 42. | Doroudchi M, Pishe ZG, Malekzadeh M, Golmoghaddam H, Taghipour M, Ghaderi A. Elevated serum IL-17A but not IL-6 in glioma versus meningioma and schwannoma. Asian Pac J Cancer Prev. 2013;14:5225-5230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Shan Y, He X, Song W, Han D, Niu J. Role of IL-6 in the invasiveness and prognosis of glioma. Int J Clin Exp Med. 2015;8:9114-9120. |
| 44. | Schwartzbaum J, Wang M, Root E, Pietrzak M, Rempala GA, Huang RP, Johannesen TB, Grimsrud TK. A nested case-control study of 277 prediagnostic serum cytokines and glioma. PLoS One. 2017;12:e0178705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Zhenjiang L, Rao M, Luo X, Valentini D, von Landenberg A, Meng Q, Sinclair G, Hoffmann N, Karbach J, Altmannsberger HM, Jäger E, Peredo IH, Dodoo E, Maeurer M. Cytokine Networks and Survivin Peptide-Specific Cellular Immune Responses Predict Improved Survival in Patients With Glioblastoma Multiforme. EBioMedicine. 2018;33:49-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1927] [Article Influence: 214.1] [Reference Citation Analysis (0)] |
| 47. | Sampson JH, Maus MV, June CH. Immunotherapy for Brain Tumors. J Clin Oncol. 2017;35:2450-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 48. | Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462-7467. |
| 49. | Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, Sedighim S, Treger J, Odesa S, Tucker A, Yong WH, Li G, Cloughesy TF, Liau LM, Prins RM. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 50. | Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, Dziurzynski K, Gilbert M, Heimberger AB. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 51. | Lamano JB, Lamano JB, Li YD, DiDomenico JD, Choy W, Veliceasa D, Oyon DE, Fakurnejad S, Ampie L, Kesavabhotla K, Kaur R, Kaur G, Biyashev D, Unruh DJ, Horbinski CM, James CD, Parsa AT, Bloch O. Glioblastoma-Derived IL6 Induces Immunosuppressive Peripheral Myeloid Cell PD-L1 and Promotes Tumor Growth. Clin Cancer Res. 2019;25:3643-3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 52. | Bunevicius A, Radziunas A, Tamasauskas S, Tamasauskas A, Laws ER, Iervasi G, Bunevicius R, Deltuva V. Prognostic role of high sensitivity C-reactive protein and interleukin-6 in glioma and meningioma patients. J Neurooncol. 2018;138:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H, Duan H, Ma P, Zhang L, Zamvil SS, Hidalgo J, Zhang Z, O'Rourke DM, Dahmane N, Brem S, Mou Y, Gong Y, Fan Y. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat Commun. 2018;9:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 54. | Ouédraogo ZG, Biau J, Kemeny JL, Morel L, Verrelle P, Chautard E. Role of STAT3 in Genesis and Progression of Human Malignant Gliomas. Mol Neurobiol. 2017;54:5780-5797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308-3316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | West AJ, Tsui V, Stylli SS, Nguyen HPT, Morokoff AP, Kaye AH, Luwor RB. The role of interleukin-6-STAT3 signalling in glioblastoma. Oncol Lett. 2018;16:4095-4104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Heimberger AB. The therapeutic potential of inhibitors of the signal transducer and activator of transcription 3 for central nervous system malignancies. Surg Neurol Int. 2011;2:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | McShane LM, Hayes DF. Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol. 2012;30:4223-4232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tantau AI, Romania; Zhang GL, China S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wu YXJ