Published online May 20, 2022. doi: 10.5662/wjm.v12.i3.179
Peer-review started: December 9, 2021
First decision: February 15, 2022
Revised: March 3, 2022
Accepted: April 29, 2022
Article in press: April 29, 2022
Published online: May 20, 2022
Processing time: 160 Days and 11.9 Hours
Occult hepatitis C infection (OCI) is characterized by the presence of hepatitis C virus (HCV) RNA in the liver, peripheral blood mononuclear cells (PBMC) and/or ultracentrifuged serum in the absence of detectable HCV-RNA in serum. OCI has been described in several categories of populations including hemodialysis patients, patients with a sustained virological response, immunocompromised individuals, patients with abnormal hepatic function, and apparently healthy subjects.
To highlight the global prevalence of OCI.
We performed a systematic and comprehensive literature search in the following 4 electronic databases PubMed, EMBASE, Global Index Medicus, and Web of Science up to 6th May 2021 to retrieve relevant studies published in the field. Included studies were unrestricted population categories with known RNA status in serum, PBMC, liver tissue and/or ultracentrifuged serum. Data were extracted independently by each author and the Hoy et al tool was used to assess the quality of the included studies. We used the random-effect meta-analysis model to estimate the proportions of OCI and their 95% confidence intervals (95%CI). The Cochran's Q-test and the I2 test statistics were used to assess heterogeneity between studies. Funnel plot and Egger test were used to examine publication bias. R software version 4.1.0 was used for all analyses.
The electronic search resulted in 3950 articles. We obtained 102 prevalence data from 85 included studies. The pooled prevalence of seronegative OCI was estimated to be 9.61% (95%CI: 6.84-12.73) with substantial heterogeneity [I² = 94.7% (95%CI: 93.8%-95.4%), P < 0.0001]. Seropositive OCI prevalence was estimated to be 13.39% (95%CI: 7.85-19.99) with substantial heterogeneity [I2 = 93.0% (90.8%-94.7%)]. Higher seronegative OCI prevalence was found in Southern Europe and Northern Africa, and in patients with abnormal liver function, hematological disorders, and kidney diseases. Higher seropositive OCI prevalence was found in Southern Europe, Northern America, and Northern Africa.
In conclusion, in the present study, it appears that the burden of OCI is high and variable across the different regions and population categories. Further studies on OCI are needed to assess the transmissibility, clinical significance, long-term outcome, and need for treatment.
Core Tip: This study showed that the burden of seropositive and seronegative occult hepatitis C infections (OCIs) is high and variable in different regions and population categories. Patients with hematological disorders, kidney diseases, and abnormal liver function showed the highest OCI prevalence.
- Citation: Mbaga DS, Kenmoe S, Njiki Bikoï J, Takuissu GR, Amougou-Atsama M, Atenguena Okobalemba E, Ebogo-Belobo JT, Bowo-Ngandji A, Oyono MG, Magoudjou-Pekam JN, Kame-Ngasse GI, Nka AD, Feudjio AF, Zemnou-Tepap C, Adamou Velhima E, Ndzie Ondigui JL, Nayang-Mundo RA, Touangnou-Chamda SA, Kamtchueng Takeu Y, Taya-Fokou JB, Mbongue Mikangue CA, Kenfack-Momo R, Kengne-Ndé C, Sake CS, Esemu SN, Njouom R, Ndip L, Riwom Essama SH. Global prevalence of occult hepatitis C virus: A systematic review and meta-analysis. World J Methodol 2022; 12(3): 179-190
- URL: https://www.wjgnet.com/2222-0682/full/v12/i3/179.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i3.179
In 2019, the World Health Organization (WHO) estimated that 58 million people are living with hepatitis C virus (HCV)[1], making HCV infection a major global public health problem[2,3]. Each year, more than 1.5 million people around the world are newly infected with HCV[4] and more than 290000 people die from it[5]. HCV infection is increasingly affecting healthcare particularly in highly endemic areas[3]. The prevalence of HCV varies greatly between regions and ranges from 0.2% to 20% in the general population[6]. HCV infection can lead to liver cirrhosis (10%-20% of cases) and hepatocellular carcinoma (HCC) (1%-5% of cases)[7].
The principal multiplication site for HCV is hepatocytes, but evidence of HCV replication has been reported in peripheral blood mononuclear cells (PBMC) and other extrahepatic organs[8,9].
Occult hepatitis C infection (OCI) was first described by Castillo et al[10] in 2004. This new form of hepatitis is defined as the absence of RNA in serum and its presence in hepatocytes, PBMC or ultracentrifuged serum[3,11-13]. OCI is further classified as seronegative OCI in subjects who are anti-HCV negative and seropositive OCI in those who are anti-HCV positive[14]. Seropositive OCI individuals represent those chronically infected with HCV who have recovered (absence of RNA in serum) either spontaneously or following treatment. There are asymptomatic carriers of OCI with normal liver enzyme levels and some with abnormal liver function[10,15-23]. OCI can also lead to hepatic attacks including cases of liver cirrhosis and even HCC in high-risk groups[24]. The first syntheses performed at the global level and in the Middle East and the Eastern Mediterranean showed highly variable OCI prevalence (ranging from 0%-89%) according to the population groups including apparently healthy individuals, patients with hematological disorders, chronic liver disease, HIV, patients who have achieved a sustained virological response (SVR), and transplant recipients[20,25,26]. The review conducted in the Middle East and Eastern Mediterranean revealed that high frequencies of OCI were recorded in patients with chronic liver disease, HIV, and injecting drug users[20]. In the review by Hedayati-Moghaddam et al[20], no statistically significant difference was observed in the variability of OCI prevalence across countries, patient anti-HCV status, and HCV detection method. OCI highlights multiple concerns including the potential for transmission of this form of infection through blood transfusion or hemodialysis[27]. To date, there is no global data synthesis on the prevalence of OCI in different population categories. To eradicate HCV infection by 2030 as recommended by WHO, making data available on the burden of OCI is crucial[28,29]. The objective of this systematic review and meta-analysis is to determine the global prevalence of OCI and evaluate the potential factors resulting in heterogeneity between the population groups and regions. Findings from this review may help prioritize population groups and regions most at risk for OCI screening and managing programs.
We used the preferred reporting items for systematic reviews and meta–analyses (PRISMA) checklist to design this systematic review (Supplementary Table 1)[30]. The systematic review was declared in the PROSPERO international database under the number CRD42021252763.
We included all studies without time restriction, published in peer-reviewed journals in English or in French and which fulfilled the following criteria: having a cross-sectional or case-control study design and for cohorts and clinical trials, only the baseline data were considered. We considered studies with patients of all ages tested for seropositive OCI (anti-HCV positive) and for seronegative OCI (anti-HCV negative). One study could contribute to several prevalence data that we called effect ratings. We included studies that detected HCV RNA by molecular methods in PBMCs, hepatocytes or ultracentrifuged serum[11,15,20,31]. To strengthen the robustness of our estimates, we considered only studies with at least 10 participants.
We excluded all studies that did not provide an opportunity to extract data on OCI prevalence, and studies with no baseline data for longitudinal study. Case reports, studies selecting participants with an already known OCI result, comments on an article, reviews, editorials, duplicates and studies for which the full article or abstract could not be found were also excluded.
We performed a systematic and comprehensive literature search in 4 electronic databases: PubMed, EMBASE, Global Index Medicus, and Web of Science from inception until 6th May 2021 to retrieve relevant studies published in the field. The electronic search strategy conducted in PubMed covered the key words of OCI (Occult Hepatitis C OR Occult Viral hepatitis C OR Occult Hepatitis C Virus OR Occult HCV) and was adapted to other databases. We also manually searched all included studies and previous systematic reviews on the topic to identify additional references. The references cited in this article were checked in the Reference Citation Analysis website (https://www.referencecitationanalysis.com/).
The duplicate articles found in the databases were removed using EndNote software. Two investigators (JTEB and SK) independently selected articles on the basis of title and abstract using Rayyan review platform. The full texts of selected articles were then read by 22 authors on the basis of the eligibility criteria. Disagreements were resolved through discussion and consensus.
Data were extracted independently by each author via the Google Forms for articles that met the inclusion criteria. The data extracted were as follows; the name of the first author, the date of publication, the period of recruitment of the participants, the design of the study, the sampling method, the number of study sites, the time of collection of the data, country, United Nations Statistics Division (UNSD) region, type of population studied, patient demographic details such as gender, age, and location of recruitment, OCI type (seronegative or seropositive), risk of bias assessment, detection test, target detected, type of sample used, number of samples tested, and number of samples positive for OCI. All disagreements regarding eligibility and data collected were resolved by discussion and consensus.
We used the Hoy et al[32] tool to assess the quality of the included studies (Supplementary Table 2). This tool takes into account 10 elements to assess the internal and external validity of prevalence studies. For each item, a score of 1 is assigned to a “yes” response and a score of 0 is assigned to the other responses (“no”, “not clear”, “not applicable”). Basically, a study was considered to be low risk, moderate risk, or high risk of bias if the total score was 0-3, 4-6, and 7-10, respectively.
To estimate proportions of OCI and their 95% confidence intervals (95%CI), we chose the random-effect meta-analysis model due to the heterogeneity expected for observational studies. The I2 statistics and Cochran's Q-test were used to assess heterogeneity between studies[33]. The I2 cut-offs > 50% indicate substantial heterogeneity. Potential sources of heterogeneity were explored by subgroup analyses and meta-regression including covariates: study design, sampling, setting, timing of samples collection, countries, WHO region, UNSD region, country income level, age range, population categories, OCI diagnostic method, and sample types. We used Funnel plot and Egger test to examine publication bias[34]. R software version 4.1.0 was used for all analyses[35,36].
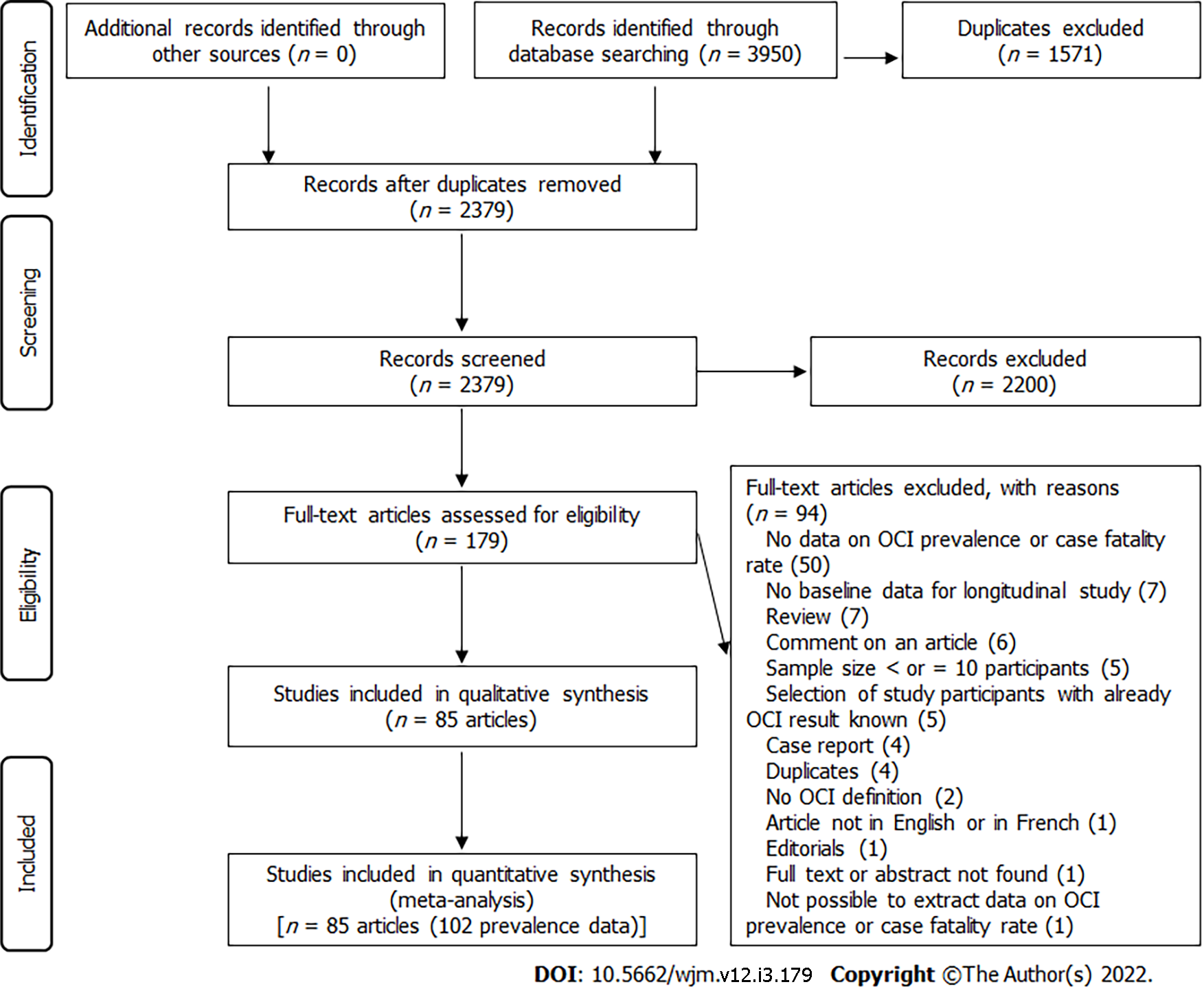
The electronic search identified 3950 articles (EMBASE (2025), Web of Science (1183), PubMed (706), and Global Index Medicus (36) (Figure 1). The eligibility review of 179 articles resulted in the exclusion of 94 and the inclusion of 85. The excluded articles and the individual reasons for exclusion are presented in Supplementary Table 3, while the included articles are indicated in Supplementary Text 1.
Overall, we obtained 102 prevalence data from the 85 included studies (75 seronegative OCI, 24 seropositive OCI, and 3 seropositive OCI and/or seronegative OCI (Supplementary Tables 4 and 5). The prevalence data were published from 1995 to 2021 and for studies with data reported, the participants were recruited from 2002 to 2019. The majority of the prevalence data were cross-sectional design (94 out of 102) with non-random sampling (97 out of 102) and consecutive sampling methods (95 out of 102). The setting of the study was hospital-based (98 out of 102) and monocentric (83 out of 102). Prevalence data were reported predominantly in the Eastern Mediterranean (51 out of 102) and in European (41 out of 102) WHO regions. The highest numbers of prevalence data were from high-income countries (40 out of 102) and upper-middle-income countries (35 out of 102). Prevalence data predominantly involved adults (33 out of 102), patients on hemodialysis (25 out of 102), and patients who achieved a SVR (15 out of 102). The most used sample type was PBMC (86 out of 102). The OCI diagnosis was performed using classical RT-PCR (49 out of 102) or real-time RT-PCR (44 out of 102). In most prevalence data, the risk of bias was moderate (64 out of 102) (Supplementary Table 6).
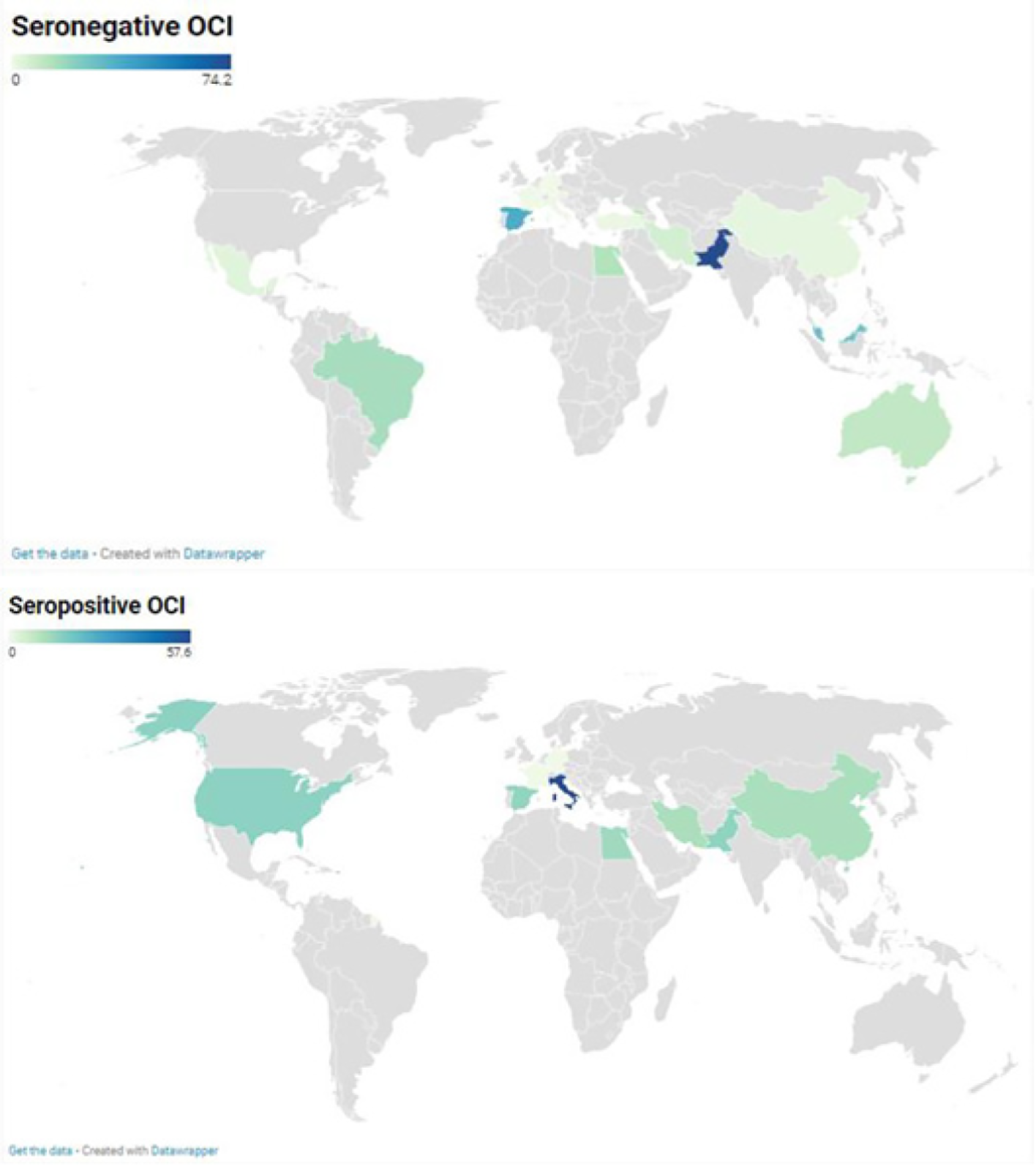
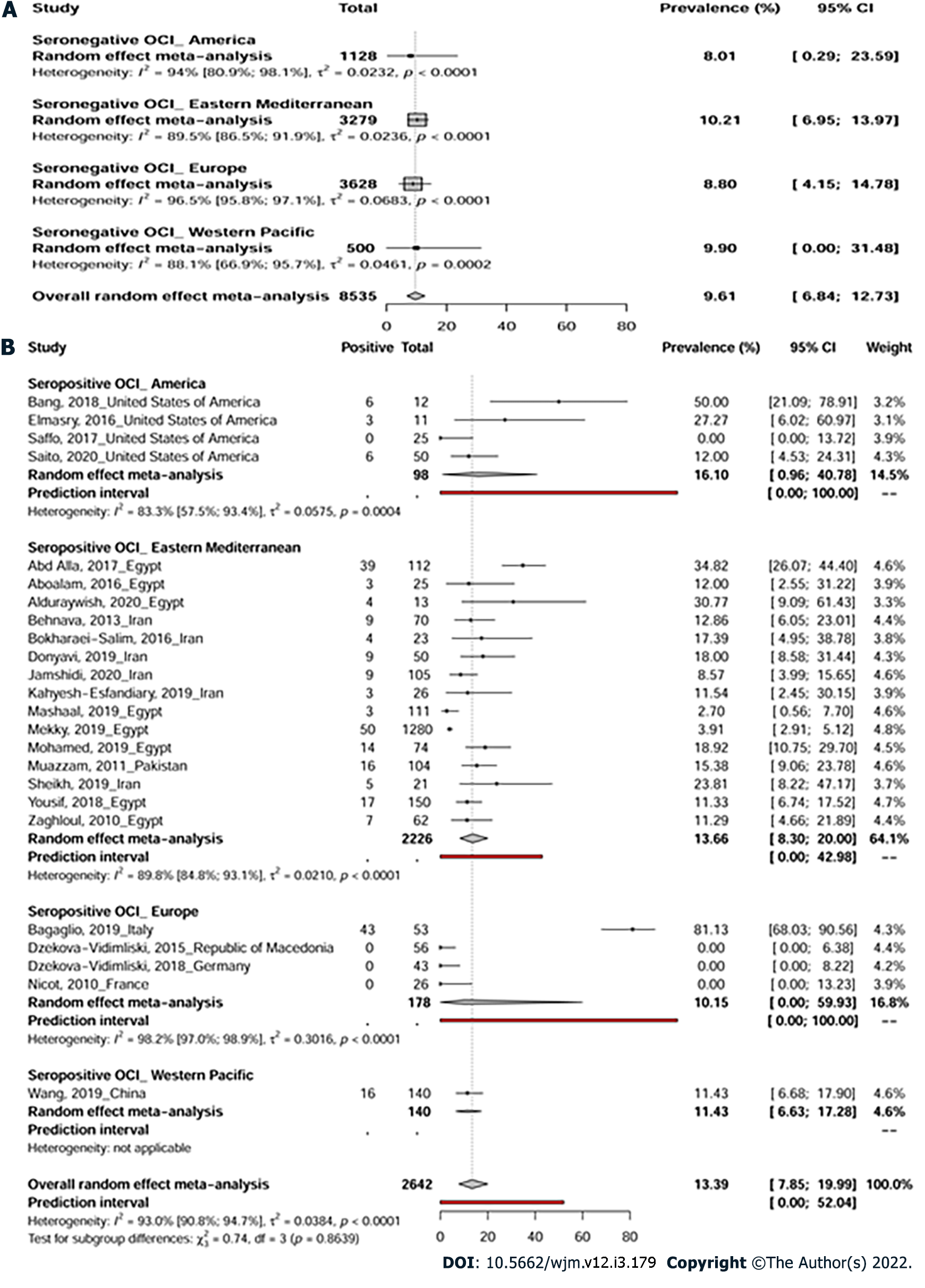
A total of 75 prevalence data reporting seronegative OCI were conducted across 4 WHO regions: America, Eastern Mediterranean, Europe, and Western Pacific (Figure 2). The pooled OCI prevalence was estimated to be 9.6% (95%CI: 6.8-12.7) in samples of 8535 participants with high heterogeneity [I² = 94.7% (95%CI: 93.8%-95.4%), P < 0.0001] (Figure 3A, Table 1, and Supplementary Figure 1). There was significant publication bias (Supplementary Figure 2, P = 0.006). Trim-and-fill adjusted analysis indicated a lower prevalence of 5.3% (95%CI: 2.9-8.2) with an addition of 10 studies.
| Prevalence % (95%CI) | 95% prediction interval | Studies (n) | Participants (n) | 1H (95%CI) | 2I² (95%CI) | P heterogeneity | |
| Seronegative OCI | |||||||
| Overall | 9.6 (6.8-12.7) | (0-44.1) | 75 | 8535 | 4.3 (4-4.6) | 94.7 (93.8-95.4) | < 0.001 |
| Trim-and-fill adjusted analysis | 5.3 (2.9-8.2) | (0.0-45.1) | 85 | NA | 5.1 (4.8-5.4) | 96.2 (95.7-96.6) | < 0.001 |
| Cross-sectional | 9.3 (6.5-12.6) | (0-43.6) | 68 | 8250 | 4.4 (4.1-4.8) | 94.9 (94.1-95.6) | < 0.001 |
| Low risk of bias | 10.2 (5.9-15.5) | (0-45.9) | 28 | 3372 | 4.3 (3.8-4.8) | 94.6 (93.1-95.7) | < 0.001 |
| Seropositive OCI | |||||||
| Overall | 13.4 (7.9-20) | (0-52) | 24 | 2642 | 3.8 (3.3-4.3) | 93 (90.8-94.7) | < 0.001 |
| Trim-and-fill adjusted analysis | 5.3 (1.4-10.7) | (0.0-49.9) | 32 | NA | 4.5 (4.0-5.0) | 95.1 (93.9-96.0) | < 0.001 |
| Cross-sectional | 12.5 (7.2-18.7) | (0-48.5) | 23 | 2530 | 3.5 (3.1-4.1) | 92 (89.3-94) | < 0.001 |
| Low risk of bias | 12.8 (4.6-23.6) | (0-57.6) | 9 | 1659 | 3.6 (2.8-4.6) | 92.3 (87.5-95.2) | < 0.001 |
Prevalence data reporting seropositive OCI were conducted in 4 WHO regions including America, Eastern Mediterranean, Europe, and Western Pacific (Figure 2). Overall, seropositive OCI prevalence was estimated to be 13.3% (95%CI: 7.8-19.9) with a total of 2642 participants from 24 prevalence data (Figure 3B). High heterogeneity was observed in the overall estimate of the prevalence of seropositive OCI [I2 = 93.0% (90.8%-94.7%), P < 0.0001]. There was a significant publication bias (Supplemen
Prevalence data reporting seronegative and/or seropositive OCI were conducted in 2 WHO regions (Eastern Mediterranean and Europe). Overall, seronegative and/or seropositive OCI prevalence was estimated to be 12.6% (95%CI: 1.2-32.2) with a total of 285 participants from 3 prevalence data. High heterogeneity was observed in the overall estimate of the prevalence of seronegative and/or seropositive OCI [I2 = 93.0% (83.0%-97.1%), P < 0.0001].
Seronegative OCI: Higher proportions of seronegative OCI were estimated for studies which selected participants by non-probabilistic sampling (P = 0.001), conducted in Spain and Egypt (P < 0.001), in Southern Europe and Northern Africa (P < 0.001), or in countries with lower-middle income economies (P = 0.045), investigated children (P = 0.01) or patients with abnormal liver function, hematological disorders, and kidney diseases (P < 0.001), and detected OCI cases by real-time RT-PCR (P < 0.001) or by examining liver tissue (P < 0.001) (Supplementary Table 7). The heterogeneity of the prevalence of seronegative OCI was explained at 84.0% (R2 = 84.0%) (Supplementary Table 8).
Seropositive OCI: Higher proportions of seropositive OCI were estimated for studies performed as case controls (P < 0.001), conducted in Italy, United States of America, and Egypt (P < 0.001), in Southern Europe, Northern America, and Northern Africa (P = 0.001), or by examining liver tissue and PBMC (P = 0.023). The heterogeneity of the prevalence of seropositive OCI was explained at 46.2% (R2 = 46.2%).
This systematic review summarized the prevalence of seronegative and seropositive OCI in relevant articles published between 1995 and 2021 in 17 countries across 4 WHO regions: America, Europe, Eastern Mediterranean, and Western Pacific. Overall, we found a high prevalence of seronegative OCI (9.61%) and seropositive OCI (13.39%), respectively. Higher seronegative OCI prevalence was found in Southern Europe and Northern Africa and in patients with abnormal liver function, hematological disorders, and kidney diseases. Higher seropositive OCI prevalence was found in Southern Europe, Northern America, and Northern Africa.
Many studies have previously shown that multiple transfused subjects are at high risk of HCV infection[20,25,37-39]. Seronegative OCIs aligned well with classical HCVs and were very predominant in subjects with hematological disorders and renal diseases in this study. It is therefore important to implement screening measures for OCI in blood transfusion banks, dialysis and/or transplant units[40]. As in the present review, it has also been shown previously that patients with abnormal liver functions are at high risk of OCI[26]. There is, however, a significant residual heterogeneity in our estimates that could be related to the different types of chronic liver disease that we did not take into account. North Africa and particularly Egypt is the country with the highest prevalence of HCV in the world[41,42]. The findings of the present study corroborate this fact and show higher seronegative and seropositive OCI prevalence in North Africa (Egypt). However, it should be noted that Southern Europe and North America also showed high prevalence of OCI in this study, while most other regions were absent or poorly represented in the estimates. HIV patients, people who inject drugs and men who have sex with men are groups known to be at high risk of HCV infection and were poorly represented in this review. Additional studies characterizing the epidemiology of HCV in these groups are awaited to fully explain the global epidemiology of OCI and more specifically in the WHO regions of Africa and South-East Asia. In a first global review without meta-analysis conducted in 2017, Dolatimehr et al[25] reported OCI prevalence of 0%-45% and 0%-2% in hemodialysis patients (10 studies) and kidney transplant recipients (2 studies), respectively. In a conference abstract, Fu et al[26] reported a highly variable prevalence of OCI in different population groups ranging from 0% in patients with autoimmune hepatitis, 9% in patients with cryptogenic liver disease, 22% in patients with chronic liver disease who achieved a SVR, 33% in patients with long-standing abnormal liver-enzyme levels to 89% in patients with abnormal levels of serum aminotransferases. More recently, Hedayati-Moghaddam et al[20] reported the prevalence of OCI in the Middle East and Eastern Mediterranean in several population categories. This last systematic review also revealed a significant variability in OCI prevalence according to the category of the population with 19% for patients with hematological disorders, 12% for HIV-infected patients, 12% for patients with chronic liver diseases, 9% for hemodialysis patients, 8% for multitransfused patients, and 4% for apparently healthy populations. Similar to the reviews mentioned above, our study also noted a statistically significant difference in the prevalence of seronegative OCI according to population categories. However, it should be mentioned that the above reviews only included participants tested for PBMC unlike our work which also considered liver biopsies and ultracentrifuged serum. The strong heterogeneity recorded in our work could also be explained by the differences in HCV prevalence according to the regions with the areas of high HCV endemicity which should also be the areas of high prevalence of OCI[11]. As the different risk factors and the different approaches for controlling HCV infection also vary widely between studies, regions and populations, this could also potentially represent a considerable source of the variability observed in our work. We should also cite the examples of a history of accidental exposure to infected needle sticks, history of blood transfusion, history of surgery, history of endoscopy, history of unsafe sexual intercourse, history of liver disease, the length and frequency of dialysis sessions, immunodepression, injecting drugs, tattoos or imprisonment. Other potential sources of heterogeneity in our estimates may also include gender of participants, sample size, and year of participant recruitment. Our study revealed that although PBMC are an excellent non-invasive sampling approach for diagnosing OCI, liver tissue exhibits superior sensitivity for seronegative OCI. It was also found that the ultracentrifuged serum obtained was not an insignificant fraction in patients positive for OCI. These results suggest that it is potentially insufficient to test for OCI in one type of sample. We also observed that real-time RT-PCR was significantly more sensitive for the detection of seronegative OCI. This suggests a further improvement in the sensitivity of molecular techniques for OCI detection.
Our study is limited due to the included studies where the WHO Africa and South-East Asia regions are not represented. Our OCI prevalence could therefore be over- or underestimated. Substantial residual statistical heterogeneity in prevalence measures was identified in all aggregate and subgroup meta-analyses. Despite these limitations, the main strength of our study is that we identified a very large number of studies, which covered multiple categories of symptomatic, apparently healthy populations and those at high risk of HCV infection. We also accounted for the variability of the prevalence according to the anti-HCV serostatus.
Our results suggest that the implementation of screening programs for OCI in high-risk populations, especially patients with hematologic complications, hemodialysis patients, and patients with chronic liver disease should be initiated. More studies are needed to assess the transmissibility, clinical significance, long-term outcome, and need for OCI treatment.
In conclusion, it appears that the burden of seronegative and seropositive OCI is high and very variable according to regions and categories of populations.
In 2004, Castillo et al first described an unknown form of hepatitis C in humans that was different from the common chronic hepatitis C and was called occult hepatitis C Infection (OCI).
To eradicate hepatitis C virus (HCV) by 2030, as recommended by the WHO, it is crucial to determine the burden of OCI across the different regions of the world and in different population categories.
Highlight the global prevalence of seronegative and seropositive OCI according to population categories and regions of the world.
The authors searched PubMed, EMBASE, Global Index Medicus, and Web of Science databases from inception to May 6, 2021. Data were extracted independently by each author and the Hoy et al tool was used to assess the quality of included studies. Prevalence and 95% confidence intervals were determined using random-effect meta-analysis.
The authors included 85 articles out of the 3950 identified by the electronic search. The combined prevalence of seronegative OCI was 9.61% (95%CI: 6.84-12.73) and the prevalence of seropositive OCI was 13.39% (95%CI: 7.85-19.99). For variations by region, seropositive OCI prevalence was higher in Southern Europe, Northern America, and Northern Africa, and seronegative OCI prevalence was higher in Southern Europe and Northern Africa. For variations by population categories, seronegative OCI prevalence was higher in patients with abnormal liver function, hematological disorders, and kidney diseases.
The burden of OCI is high and greatly variable according to world regions and population categories.
Consideration should be given to the implementation of screening programs for OCI in high-risk populations such as patients with hematologic disorders, kidney disease, and those with abnormal liver function.
| 1. | Centers for Disease Control and Prevention. Global Viral Hepatitis: Millions of People are Affected. 2021. Available from: https://www.cdc.gov/hepatitis/global/index.htm. |
| 2. | Blanco RY, Loureiro CL, Villalba JA, Sulbarán YF, Maes M, de Waard JH, Rangel HR, Jaspe RC, Pujol FH. Decreasing prevalence of Hepatitis B and absence of Hepatitis C Virus infection in the Warao indigenous population of Venezuela. PLoS One. 2018;13:e0197662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Abdelmoemen G, Khodeir SA, Abou-Saif S, Kobtan A, Abd-Elsalam S. Prevalence of occult hepatitis C virus among hemodialysis patients in Tanta university hospitals: a single-center study. Environ Sci Pollut Res Int. 2018;25:5459-5464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Hepatitis C. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 5. | Sheikh M, Bokharaei-Salim F, Monavari SH, Ataei-Pirkooh A, Esghaei M, Moradi N, Babaei R, Fakhim A, Keyvani H. Molecular diagnosis of occult hepatitis C virus infection in Iranian injection drug users. Arch Virol. 2019;164:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 838] [Cited by in RCA: 834] [Article Influence: 43.9] [Reference Citation Analysis (9)] |
| 7. | Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, Iio E, Lubel J, Wang W, Wei B, Ide T, Preda CM, Conti F, Minami T, Bielen R, Sezaki H, Barone M, Kolly P, Chu PS, Virlogeux V, Eurich D, Henry L, Bass MB, Kanai T, Dang S, Li Z, Dufour JF, Zoulim F, Andreone P, Cheung RC, Tanaka Y, Furusyo N, Toyoda H, Tamori A, Nguyen MH. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J Hepatol. 2019;71:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Cacoub P, Comarmond C. Considering hepatitis C virus infection as a systemic disease. Semin Dial. 2019;32:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Forton DM, Karayiannis P, Mahmud N, Taylor-Robinson SD, Thomas HC. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol. 2004;78:5170-5183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Castillo I, Pardo M, Bartolomé J, Ortiz-Movilla N, Rodríguez-Iñigo E, de Lucas S, Salas C, Jiménez-Heffernan JA, Pérez-Mota A, Graus J, López-Alcorocho JM, Carreño V. Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J Infect Dis. 2004;189:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Austria A, Wu GY. Occult Hepatitis C Virus Infection: A Review. J Clin Transl Hepatol. 2018;6:155-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Frías M, Rivero-Juárez A, Téllez F, Palacios R, Jiménez-Arranz Á, Pineda JA, Merino D, Gómez-Vidal MA, Pérez-Camacho I, Camacho Á, Rivero A. Evaluation of hepatitis C viral RNA persistence in HIV-infected patients with long-term sustained virological response by droplet digital PCR. Sci Rep. 2019;9:12507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Mekky MA, Sayed HI, Abdelmalek MO, Saleh MA, Osman OA, Osman HA, Morsy KH, Hetta HF. Prevalence and predictors of occult hepatitis C virus infection among Egyptian patients who achieved sustained virologic response to sofosbuvir/daclatasvir therapy: a multi-center study. Infect Drug Resist. 2019;12:273-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Vidimliski PD, Nikolov I, Geshkovska NM, Dimovski A, Rostaing L, Sikole A. Review: Occult hepatitis C virus infection: still remains a controversy. J Med Virol. 2014;86:1491-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Bartolomé J, López-Alcorocho JM, Castillo I, Rodríguez-Iñigo E, Quiroga JA, Palacios R, Carreño V. Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C. J Virol. 2007;81:7710-7715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78:5867-5874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, Wilkinson J, Adair D, Rakela J, Laskus T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology. 2005;41:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Rao H, Chi X, Li B, Liu H, Wu L, Zhang H, Liu S, Zhou G, Li N, Niu J, Wei L, Zhao J. Detection of residual HCV-RNA in patients who have achieved sustained virological response is associated with persistent histological abnormality. EBioMedicine. 2019;46:227-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Abdel-Moneim AS. Occult hepatitis C infections: time to change the defined groups. Microbiol Immunol. 2019;63:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Hedayati-Moghaddam MR, Soltanian H, Ahmadi-Ghezeldasht S. Occult hepatitis C virus infection in the Middle East and Eastern Mediterranean countries: A systematic review and meta-analysis. World J Hepatol. 2021;13:242-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Martínez-Rodríguez ML, Uribe-Noguez LA, Arroyo-Anduiza CI, Mata-Marin JA, Benitez-Arvizu G, Portillo-López ML, Ocaña-Mondragón A. Prevalence and risk factors of Occult Hepatitis C infections in blood donors from Mexico City. PLoS One. 2018;13:e0205659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | De Marco L, Gillio-Tos A, Fiano V, Ronco G, Krogh V, Palli D, Panico S, Tumino R, Vineis P, Merletti F, Richiardi L, Sacerdote C. Occult HCV infection: an unexpected finding in a population unselected for hepatic disease. PLoS One. 2009;4:e8128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | De Marco L, Manzini P, Trevisan M, Gillio-Tos A, Danielle F, Balloco C, Pizzi A, De Filippo E, D'Antico S, Violante B, Valfrè A, Curti F, Merletti F, Richiardi L. Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease. PLoS One. 2012;7:e43541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Rezaee-Zavareh MS, Hadi R, Karimi-Sari H, Hossein Khosravi M, Ajudani R, Dolatimehr F, Ramezani-Binabaj M, Miri SM, Alavian SM. Occult HCV Infection: The Current State of Knowledge. Iran Red Crescent Med J. 2015;17:e34181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Dolatimehr F, Khosravi MH, Rezaee-Zavareh MS, Alavian SM. Prevalence of occult HCV infection in hemodialysis and kidney-transplanted patients: a systematic review. Future Virol. 2017;12:315-322. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Fu S, Zhang MM, Yao NJ, Feng YL, Zhao Y, Liu J. Prevalence of occult hepatitis C virus infection in patients with chronic liver disease: A systematic review and meta-analysis. Abstracts. Hepatol Inter. 2020;14:1-470. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Carreño V, Bartolomé J, Castillo I, Quiroga JA. New perspectives in occult hepatitis C virus infection. World J Gastroenterol. 2012;18:2887-2894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | World Health Organization. Combating hepatitis B and C to reach elimination by 2030. 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf;jsessionid=7DF15B71373378C993210C993495CB0F?sequence=1. |
| 29. | World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. 2016. Available from: https://www.who.int/publications/i/item/WHO-HIV-2016.06. |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48594] [Article Influence: 2858.5] [Reference Citation Analysis (3)] |
| 31. | Donyavi T, Bokharaei-Salim F, Khanaliha K, Sheikh M, Bastani MN, Moradi N, Babaei R, Habib Z, Fakhim A, Esghaei M. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Arch Virol. 2019;164:2493-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1949] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 33. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48538] [Article Influence: 2110.3] [Reference Citation Analysis (4)] |
| 34. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 42529] [Article Influence: 1466.5] [Reference Citation Analysis (5)] |
| 35. | Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. 1 ed: John Wiley & Sons, Ltd, 2009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6410] [Cited by in RCA: 5820] [Article Influence: 727.5] [Reference Citation Analysis (0)] |
| 37. | Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: a systematic review. Hemodial Int. 2010;14:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 38. | Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of Hepatitis C virus infection on kidney transplantation outcomes: A systematic review of 18 observational studies: The impact of HCV on renal transplantation. Hepat Mon. 2011;11:247-254. [PubMed] |
| 39. | Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Rezaee-Zavareh MS, Ramezani-Binabaj M, Moayed Alavian S. Screening for occult hepatitis C virus infection: Does it need special attention? Hepatology. 2015;62:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Alavian SM, Rezaee-Zavareh MS. The Middle East and hepatitis C virus infection: does it need special attention? Lancet Infect Dis. 2016;16:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep. 2018;8:1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Society of Global Health, No. 00768.
Specialty type: Virology
Country/Territory of origin: Cameroon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Biswas S, India; Hedayati-Moghaddam MR, Iran S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ