Published online May 20, 2022. doi: 10.5662/wjm.v12.i3.164
Peer-review started: December 1, 2021
First decision: January 12, 2022
Revised: January 20, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 20, 2022
Processing time: 168 Days and 1.4 Hours
The role of vitamin D supplementation in gestational diabetes mellitus (GDM) patients is unclear.
To determine the burden and risk of post-randomization GDM patient attrition from vitamin D-supplemented arms of randomized controlled trials (RCTs). The auxiliary aim was to compare the effects of nutritional supplements on their fasting blood glucose (FPG) levels and perinatal outcomes.
RCTs were searched in the PubMed, Embase, and Scopus databases. Random-effect prevalence and pairwise meta-analysis were performed for the primary objective. The auxiliary aim was to compare the effects of nutritional supplements on their fasting blood glucose (FPG) levels and perinatal outcomes. Fixed-effect network meta-analyses were undertaken for the secondary goals. All analyses were performed using Stata software, and statistical significance was determined at P < 0.05.
Thirteen RCTs from Iran and China were reviewed. The participant attrition burden in vitamin D recipients was 6% [95% confidence interval (CI): 0.03, 0.10], and its risk did not vary from non-recipients. Vitamin D and calcium co-supplementation reduced the cesarean section incidence in GDM patients [risk ratio (RR): 0.37; 95%CI: 0.18, 0.74]. The hyperbilirubinemia or hospitalization risk in their newborns decreased with vitamin D supplementation (RR: 0.47; 95%CI: 0.27, 0.83) and co-supplementation with calcium (RR: 0.35; 95%CI: 0.16, 0.77) or omega-3 fatty acids (RR: 0.25; 95%CI: 0.08, 0.77). Vitamin D and probiotics co-supplementation decreased newborn hyperbilirubinemia risk (RR: 0.28; 95%CI: 0.09, 0.91). FPG levels and macrosomia risk did not vary across interventions.
In RCTs, vitamin D supplementation or co-supplementation in GDM patients showed a low participant attrition burden and low risk of cesarean section, newborn hyperbilirubinemia, and newborn hospitalization.
Core Tip: This meta-analysis was conducted on efficacy trials testing the effect of vitamin D in gestational diabetes mellitus (GDM) patients and/or their neonates. The post-randomization attrition burden of GDM patients from vitamin D-supplemented trial arms was low. The risk of hyperbilirubinemia and hospitalization in newborns was low with vitamin D and its omega-3 fatty acids and calcium co-supplemented forms. Vitamin D co-supplementation with calcium and probiotics reduced the risk of cesarean section and newborn hyperbilirubinemia, respectively. Compared to omega-3 fatty acids, the risk of hyperbilirubinemia and hospitalization among neonates was low when it was co-supplemented with vitamin D.
- Citation: Saha S, Saha S. Participant attrition and perinatal outcomes in prenatal vitamin D-supplemented gestational diabetes mellitus patients in Asia: A meta-analysis. World J Methodol 2022; 12(3): 164-178
- URL: https://www.wjgnet.com/2222-0682/full/v12/i3/164.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i3.164
Gestational diabetes mellitus (GDM) is a condition of glucose intolerance that is detected or diagnosed for the first time during pregnancy. The prevalence of GDM in pregnancy is between 4% and 18%, depending on the diagnostic criteria used[1]. The treatment of GDM is crucial as it can cause perinatal complications such as cesarean section (CS) in the mother and macrosomia in her newborn[2]. The benefits of standard GDM care with medical nutrition, lifestyle modification, and self-blood glucose monitoring are inconsistent across different treatment outcomes. For example, it decreases macrosomia risk but not CS occurrence compared to non-GDM care recipients[3]. Therefore, researchers have investigated the role of standard GDM care adjuncts for better perinatal outcomes. In this regard, vitamin D has drawn substantial attention due to the plausible association of its deficiency and GDM[4]. Although several randomized controlled trials (RCTs)[5] have assessed vitamin D efficacy in GDM patients, the burden and risk of post-randomization participant attrition from vitamin D-supplemented arms of these trials remain unclear. Notably, participant attrition happens even in adequately conducted RCTs[6]. Besides, the efficacy of vitamin D, its co-supplements, and other supplements included in these trials, remain unclear. Existing meta-analyses have compared how vitamin D affects the occurrence of perinatal outcomes and maternal fasting blood glucose (FPG) levels[7,8]. However, these did not distinguish how the effects of vitamin D can be differentiated from its co-supplemented forms (like with calcium) and other non-vitamin D supplements (e.g., omega-3 fatty acids) included in these trials. This meta-analysis article attempted to address these underexplored areas of perinatal medicine.
The fat-soluble vitamin D hormone is available from the diet and nutritional supplements in the inactive D2 (ergocalciferol) and D3 (cholecalciferol) forms[9,10]. Cholecalciferol is further synthesized in the skin from sunlight. The pre-vitamin D undergoes hydroxylation in the liver and forms the albumin-bound circulatory 25-hydroxyvitamin D[9,11,12]. This active form of vitamin D causes calcium absorption by its action on the intestine and kidneys[10]. The physiologic role of vitamin D in pregnancy occurs via its binding to its receptors in the uteroplacental tissue[9,12]. The dietary allowance and the tolerable upper limit of vitamin D in pregnancy are 600 and 4000 IU, respectively[9].
The vitamin D supplementation effects on GDM mothers and their neonates have been assessed in several RCTs. Commonly tested oral dosages of vitamin D are 200-500 IU daily[13,14] or 50000 IU 2-3 weekly[15-18]. While some RCTs supplemented vitamin D as a mono-supplement, others co-supplemented it with zinc, calcium, and magnesium[14,16].
This review aimed to determine the burden and risk of post-randomization GDM patient attrition from vitamin D-supplemented arms of RCTs. Additionally, it determined the changes in FPG levels and risk of different perinatal outcomes (neonatal hyperbilirubinemia, newborn hospitalization, microsomia, and CS) across nutritional supplements tested in these RCTs.
A pre-published protocol exists for this review, and it is registered in the PROSPERO (CRD42020180634)[19,20]. The preliminary findings of this review were presented at a conference[21]. This report adheres to The Preferred Reporting Items for Systematic Review and Meta-Analysis 2020 statement (Supplementary Table 1)[22].
Trial design: Parallel arm RCTs of any duration.
Trial population: GDM patients of any age irrespective of their gestational age and previous GDM history.
Intervention arm/s: Prenatal vitamin D or its co-supplemented form with other nutrients orally.
Comparator arm: No nutritional supplements or placebo and/or prenatal nutritional supplement/s that does not contain vitamin D.
Primary outcome: GDM patients leaving the trial post-randomization during the intervention period. The participants excluded from analysis by trialists were not the outcome of interest.
Secondary outcomes (post-nutrient supplementation outcomes): Mean FPG levels and its standard deviation and CS frequency. Other outcomes of interest included macrosomia, hyperbilirubinemia, and hospitalization of newborns.
The diagnosis and management of GDM and the dosages and regimen of the nutritional supplements were accepted as per the trialists.
Study designs other than that stated above (e.g., crossover study, observational study). Non-GDM type of diabetes including type 1 and type 2 diabetes.
The title and abstract of the articles published in the English language were searched in the PubMed, Embase, and Scopus databases irrespective of the date of publication and geographic boundary. Additionally, the bibliographies of articles included in this review were searched. The search string used to search in the PubMed was composed of the following words and phrases: "vitamin D" OR calciferol OR "vitamin D2" OR ergocalciferol OR "vitamin D3" OR cholecalciferol AND gdm OR "gestational diabetes." Identical search strings were used in the remaining databases. The complete search string with their electronic links, when available, are presented in Supplementary Table 2.
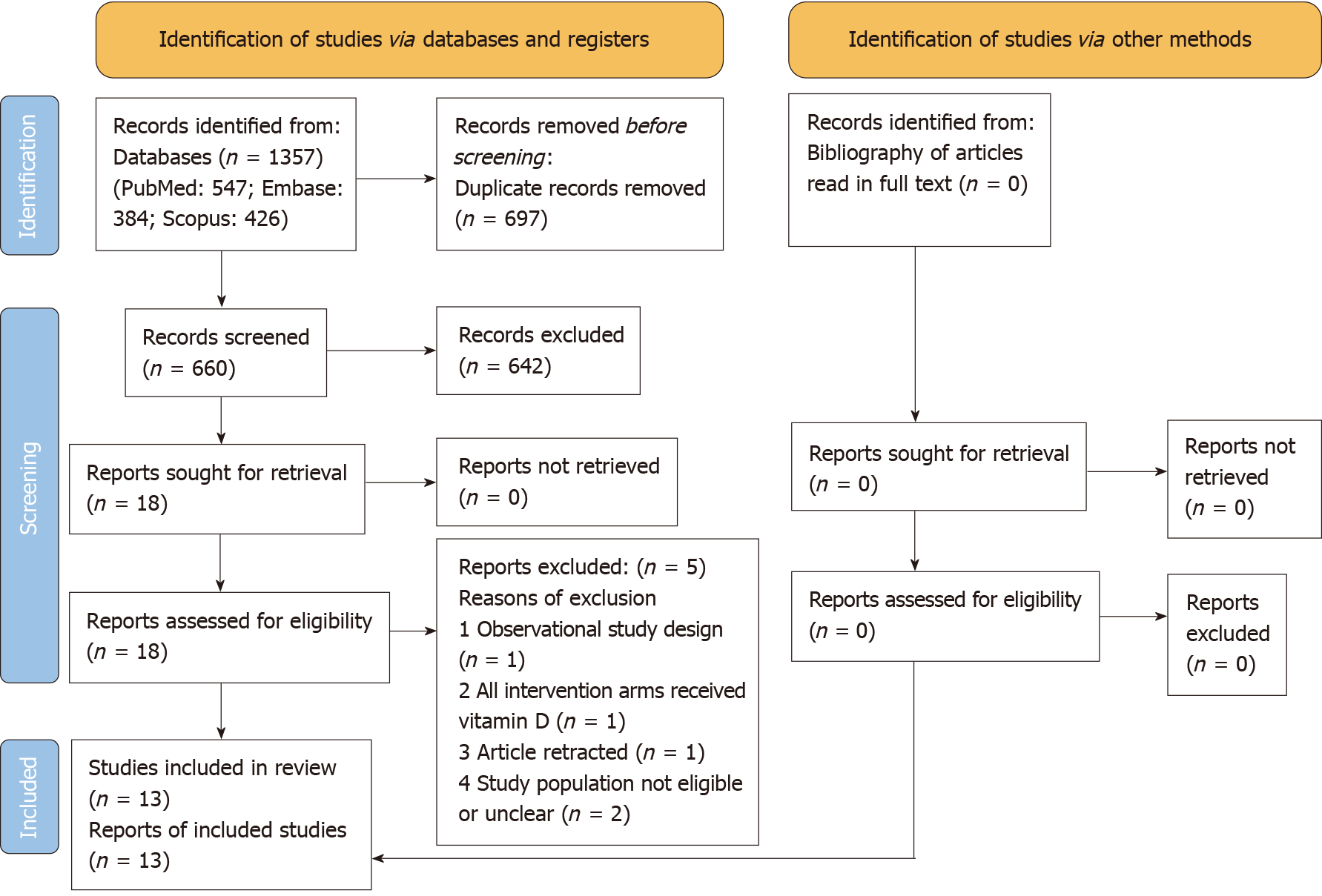
After uploading the retrieved citations to a reference handling software, the title and abstract of the articles were skimmed against the above eligibility criteria. Full-text reading transpired when articles appeared eligible or dubious for inclusion in this review. Figure 1 depicts the reasons for the elimination of articles read in full text. Salient detail abstraction about the trials (including its registration number and country of conduct), participants, interventions tested in respective treatment arms, and the outcomes of interest transpired.
Using the Cochrane risk of bias (RoB) tool for RCTs, the following RoB components of the reviewed trials were evaluated[23]. The randomization method and successive allocation concealment method of interventions to different treatment arm participants were used to judge the selection bias. Utilizing the blinding mechanism used for trial personnel and participants and that of outcome assessors, performance and detection bias evaluation occurred, respectively. The attrition bias risk evaluation was assessed by comparing the frequency and reason of missing outcome data across intervention arms. By comparing trial findings with the pre-stated intentions of trialists, the risk of reporting bias was assessed. Any other bias besides those mentioned above was classified as miscellaneous bias.
The review authors performed the database search, study selection, data abstraction, and RoB assessment independently and resolved any conflict in an opinion by discourse. A third-party opinion or contact with the trialists was not required.
The overall prevalence of post-randomization participant attrition from the vitamin D-supplemented arms was estimated using random-effect (DerSimonian and Laird) prevalence meta-analysis (exact binomial method). Trials with zero numerators, when all participants followed up until the end of the trial period, did not get included in the analysis.
A random effect pairwise meta-analysis model (DerSimonian and Laird) contrasted the participant attrition risk between vitamin D recipients and non-recipients and determined the summary effect in the risk ratio (RR). When any cell of the 2 × 2 table had no event, 0.5 got added to all cells. Forest plots were used to present the results of prevalence and pairwise meta-analysis.
Heterogeneity was determined using χ2 statistics (statistical significance determined at P < 0.1) and was successively quantified using I2 statistics (at values 25%, 50%, and 75% heterogeneity were classified as low, moderate, and high, respectively)[24].
A frequentist method network meta-analysis (NMA) ensued for each outcome to determine the relative efficacy across various supplements tested in the reviewed trials. For FPG, the weighted mean difference was estimated, and its values were included in mg/dL (FPG values in mmol/L got converted into mg/dL). A fixed-effect NMA ensued for categorical outcomes (effect size estimated in RR) due to the absence of freedom for heterogeneity in respective models. An augmentation method was used when these binomial outcomes had zero events.
The NMA models did not include open-label trials to minimize the intransitivity risk. Local and global inconsistency models were used to assess inconsistency.
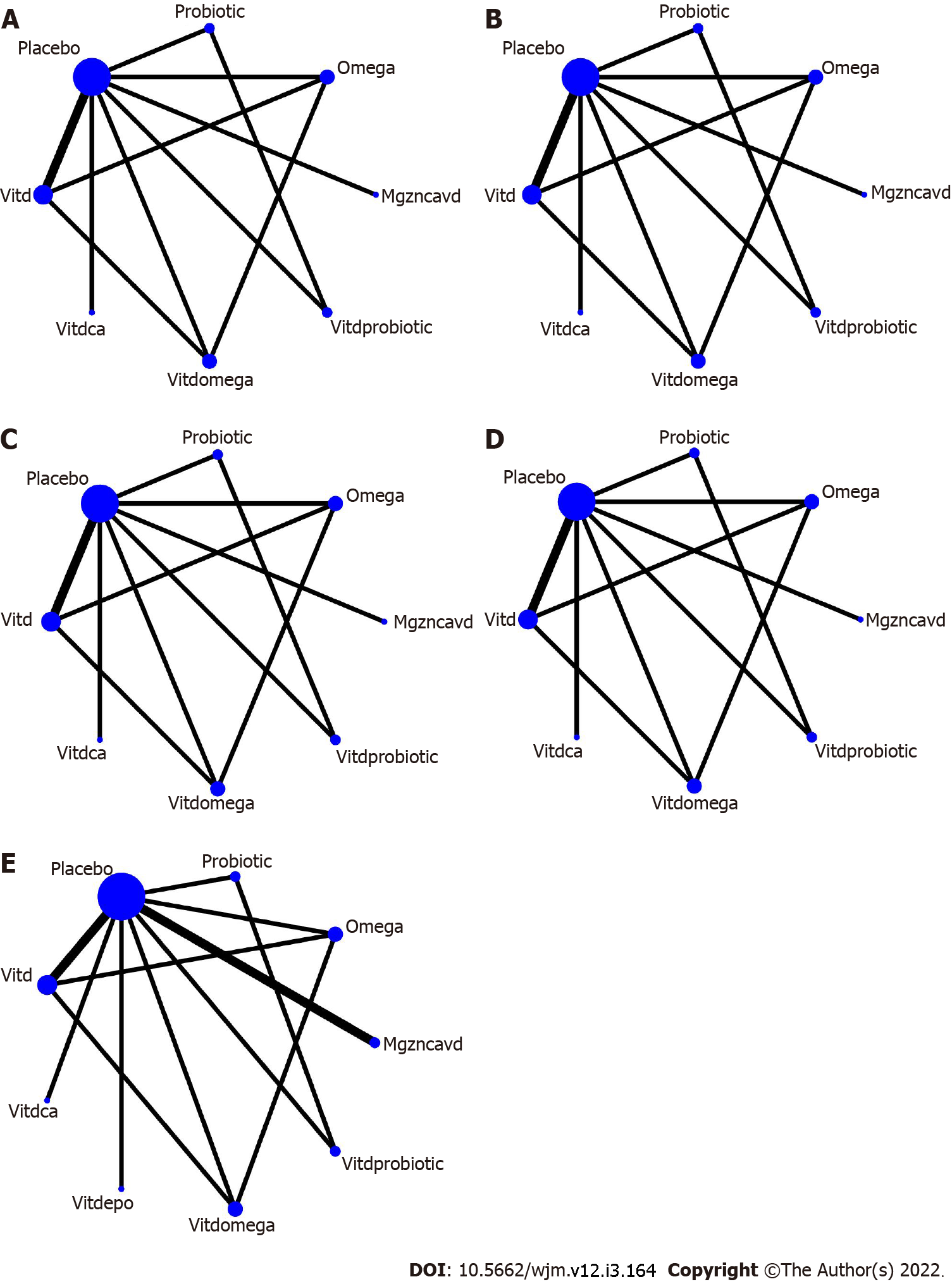
Utilizing network maps, a visual conceptualization of the relationship across various nutritional supplements tested in the trials transpired for each outcome. The nodes represent the intervention types received, and it enlarges with the increase in sample size receiving these. The node connectors represent the trials testing the interventions represented by the nodes, and it thickens as the no of trials increases.
The effect sizes and their corresponding confidence interval (CI) are presented in league tables. The diagonal cells of these tables represented the interventions compared. The surface under the cumulative ranking curve values got utilized to predict the best supplement for outcomes with statistically significant effect sizes.
Subgroup analysis and meta-regression were not applicable, as the heterogeneity was not high in the prevalence and pairwise meta-analysis.
Small study effect assessment for the pairwise meta-analysis ensued using funnel plot and Egger’s test. The RoB across studies included in the NMA models occurred by identifying any selective reporting that deviates from the pre-stated notions[25].
The prevalence and pairwise meta-analysis iteration happened by dropping a study (every time the analysis was repeated) and by a fixed-effect model, respectively.
For statistically significant meta-analysis results, the Grading of Recommendations Assessment, Development, and Evaluation approach[26] was used to determine the evidence quality.
The metaprop, meta, and network packages of Stata statistical software (version 16) were used for the prevalence, pairwise, and network meta-analysis, respectively. The statistical significance was determined at P < 0.05 and 95%CI.
The database search retrieved 1357 citations (PubMed: 547; Embase: 384; Scopus: 426) (Figure 1). The last date of the search was July 4, 2021. Five articles read in full text were excluded[18,27-30]. Additional searches did not produce new articles. The review included 13 publications with 1109 GDM patients’ data from Iran[14-17,31-37] and China[13,38]. The salient features of these trials are presented in Table 1.
| Ref. | Design | Participants | Interventions | Outcomes |
| Jamilian et al[37], 2016 | Randomized, double-blind, placebo-controlled clinical trial; Intervention arms: Two; Single-centered trialTrial duration: 6 wk. Trial conducted in: Iran; Obtained ethical clearance and participant consent. Funding information provided. Clinical trial registration number: IRCT201509115623N52 | Participants diagnosed with GDM (used ADA criteria); 60 participants randomized into different treatment arms (vitamin D3 and evening primrose oil: n = 30, placebo: n = 30); Mean age of participants: -Vitamin D3 and evening primrose oil receiving group: 28.4 ± 6.2 yr; -Placebo receiving group: 29.6 ± 4.3 yr | Two intervention arms: (1) 1000 IU of vitamin D and 1000 mg of evening primrose oil daily for 6 wk; and (2) Placebo | Attrition from vitamin D supplemented arm: n = 3; Other outcomes reported: Fasting plasma glucose |
| Jamilian et al[17], 2017 | Randomized, double blinded, placebo-controlled clinical trial; Intervention arms: four; Single centered trial; Trial duration: 6 wk. Trial conducted in: Iran; Obtained ethical clearance and participant consent. Funding information provided. Clinical trial registration number: IRCT201605135623N78 | Participants diagnosed with GDM (used ADA criteria); 140 participants randomized into different treatment arms (vitamin D and omega-3 fatty acid receiving group: n = 35, vitamin D receiving arm: n = 35, omeag-3 fatty acid receiving arm: n = 35, placebo receiving arm: n = 35); Mean age of participants: -Vitamin D and omega-3 fatty acid receiving group: 31.2 ± 4.3 yr; -Vitamin D receiving group: 31.5 ± 7.0 yr; -Omega-3 receiving group: 30.7 ± 3.5 yr; -Placebo receiving group: 30.7 ± 4.1 yr | Four intervention arms: (1) Vitamin D and omega-3 fatty acid: 50000 IU of vitamin D two weekly and 1000 mg omega-3 fatty acid twice daily; (2) Vitamin D: 50000 IU vitamin D every 2 wk; (3) Omega-3 fatty acid: 1000 mg omega-3 fatty acids two times a day; and (4) Placebo | No attrition from vitamin D supplemented arm; Other outcomes reported: Fasting plasma glucose |
| Jamilian et al[33], 2019a | Randomized, double-blind, placebo-controlled; Intervention arms: 3; Trial conducted in: Iran; Single centered trial; Trial duration: 6 wk; Obtained ethical clearance and participant consent. Funding information provided. Trial ID: IRCT201706075623N119 | Participants diagnosed with GDM (used ADA criteria); 90 participants randomized into different treatment arms (probiotic arm: n = 30, vitamin D and probiotic arm: n = 30, placebo arm: n = 30); Mean age of participants: -Probiotic arm: 31.2 ± 5.9 yr; -Vitamin D and probiotic arm: 28.9 ± 6.1 yr; -Placebo arm: 29.9 ± 3.7 yr | Three intervention arms: (1) Probiotic: 8 × 109 CFU/g; (2) Vitamin D3 (50,000 IU) every 2 wk plus 8 × 109 CFU/g probiotic; Placebo | No attrition from vitamin D supplemented arm; Other outcomes reported: (1) Newborn hyperbilirubinemia; (2) Newborn hospitalization; (3) Macrosomia; and (4) Cesarean section. Fasting plasma glucose |
| Jamilian et al[36], 2019b | Randomized, double-blind, placebo-controlled. Intervention arms: 2; Trial conducted in: IranSingle centered trialTrial duration: 6 wkObtained ethical clearance and participant consent. Funding information provided.Trial ID: IRCT201704225623N109 | Participants diagnosed with GDM (used ADA criteria); 60 participants randomized into different treatment arms (vitamin D-magnesium-zinc-calcium arm: n = 30, placebo arm: n = 30). Mean age of participants: -Vitamin D-magnesium-zinc-calcium arm: 27.7 ± 4.0 yr; -Placebo arm: 29.1 ± 4.1 yr | Two intervention arms: (1) Vitamin D (200 IU) along with 100 mg magnesium, 4 mg zinc, 400 mg calcium twice daily; and (2) Placebo | No attrition from vitamin D supplemented armOther outcomes reported: (1) Newborn hyperbilirubinemia; (2) Newborn hospitalization; (3) Macrosomia; and (4) Cesarean section. Fasting plasma glucose |
| Asemi et al[31], 2014a | Randomized, double-blind, placebo-controlled trial. Intervention arms: 2; Trial conducted in: Iran; Single centered trial; Trial duration: 6 wk; Obtained ethical clearance and participant consent. Funding information provided.Trial ID: IRCT201305115623N7 | Participants diagnosed with GDM (used ADA criteria); 50 participants randomized into different treatment arms (vitamin D arm: n = 25, placebo arm: n = 25). Mean age of participants: -Vitamin D arm: 31.1 ± 5.5 yr; -Placebo arm: 30.8 ± 6.2 yr | Two intervention arms: (1) Vitamin D: 50,000 IU vitamin D3 pearl two times during the trial period (at baseline and day 21); and (2) Placebo | Attrition from vitamin D supplemented arm: n = 3; Other outcomes reported: (1) Newborn hyperbilirubinemia; (2) Newborn hospitalization; (3) Macrosomia; and (4) Cesarean section |
| Asemi et al[16], 2014b | Randomized, placebo-controlled clinical trial. Intervention arms: TwoMulti-centric trial. Trial duration: 6 wk. Trial conducted in: IranObtained ethical clearance and participant consent. Funding information provided. Clinical trial registration number: IRCT201311205623N11 | Participants diagnosed with GDM (used ADA criteria); 56 participants randomized into different treatment arms (vitamin D and calcium: n = 28, placebo receiving group: n = 28). Mean age of participants: -Vitamin D and calcium receiving arm: 28.7 ± 6.0 yr; -Placebo receiving arm: 30.8 ± 6.6 yr | Two intervention arms: (1) 1000 mg calcium carbonate daily and 50000 U vitamin D3 at the baseline and day 21 of the study; and (2) Placebo | Attrition from vitamin D supplemented arm: n = 3. Other outcomes reported: Fasting plasma glucose |
| Karamali et al[32], 2016 | Randomized, double-blind, placebo-controlled trial; Intervention arms: 2; Trial conducted in: Iran; Multicentric trialTrial duration: 6 wk; Obtained ethical clearance and participant consent. Funding information provided. Trial ID: IRCT201407115623N23 | Participants diagnosed with GDM (used ADA criteria); 60 participants randomized into different treatment arms (vitamin D and calcium arm: n = 30; placebo arm: n = 30). Mean age of participants: -Vitamin D and calcium arm: 28·7 ± 6·1 yr; -Placebo arm: 31·6 ± 6·3 yr | Two intervention arms: (1) Vitamin D3 (50000 IU) at baseline and day 21 along with 1000 mg calcium carbonate daily; and (2) Placebo | No attrition from vitamin D supplemented arm; Other outcomes reported: (1) Newborn hyperbilirubinemia; (2) Newborn hospitalization; (3) Macrosomia; and (4) Cesarean section |
| Karamali et al[14], 2018 | Randomized, double-blind, placebo-controlled trial; Intervention arms: 2; Single centered trial. Trial duration: 6 wk; Trial conducted in: Iran; Obtained ethical clearance (participant consent information unclear). Funding information provided. Trial registration details: Unclear | Participants diagnosed with GDM (used ADA criteria); 60 participants randomized into different treatment arms; (Magnesium, zinc, calcium and vitamin D supplements arm: n = 30; Placebo arm: n = 30); Mean age of participants: -Magnesium, zinc, calcium and vitamin D: 30.0 ± 4.5 yr; -Placebo arm: 31.1 ± 4.2 yr | Two intervention arms: (1) 100 mg magnesium, 4 mg zinc, 400 mg calcium and 200 IU vitamin D two times a day for 6 wk; and (2) Placebo | No attrition from vitamin D supplemented arm; Other outcomes reported: Fasting plasma glucose |
| Razavi et al[35], 2017 | Randomized, double-blind, placebo-controlled, Intervention arms: 4; Trial conducted in: Iran. Single centered trial. Trial duration: 6 wk; Obtained ethical clearance and participant consent. Funding information provided. Trial ID: IRCT201701305623N106 | Participants diagnosed with GDM (used ADA criteria); 120 participants randomized into different treatment arms (vitamin D and omega-3 arm: n = 30; omega-3 arm: n = 30; vitamin D arm: n = 30; placebo: n = 30); Mean age of participants: -Vitamin D and omega-3 arm: 29.9 ± 4.0 yr; -Omega-3 arm: 29.7 ± 3.6 yr; -Vitamin D arm: 29.9 ± 5.0 yr; -Placebo: 29.2 ± 3.4 yr | Four intervention arms: (1) Vitamin D (50000 IU): Two weekly two times a day; (2) Vitamin D (50000 IU) two weekly plus 1000 mg omega-3 fatty acids two times a day; (3) 1000 mg omega-3 fatty acids two times a day; and (4) Placebo | No attrition from vitamin D supplemented arm; Other outcomes reported: (1) Newborn hyperbilirubinemia; (2) Newborn hospitalization; (3) Macrosomia; and (4) Cesarean section |
| Valizadeh et al[34], 2016 | Randomized controlled trial. Investigators and patients were not blinded. Intervention arms: 2; Single centered trial; Trial conducted in: Iran; Trial duration: Until delivery; Obtained ethical clearance and participant consent. Funding information provided. Trial ID: IRCT2012101611144N1 | Participants diagnosed with GDM (used ADA criteria); 96 participants randomized into different treatment arms (vitamin D arm: n = 48; no supplement arm: n = 48); Mean age of participants: -Vitamin D arm: 32.0 ± 5.5 yr; -No supplement arm: 32.4 ± 4.7 yr | Two intervention arms: (1) 700000 IU vitamin D3 in total (regimen differed by gestational age of GDM patients); and (2) Comparison group did not receive any supplementation | Attrition from vitamin D supplemented arm: n = 4; Other outcomes reported: (1) Newborn hyperbilirubinemia; (2) Macrosomia; (3) Cesarean section; and (4) Fasting plasma glucose |
| Yazdchi et al[15], 2016 | Randomized, double-blinded placebo-controlled clinical trial; Intervention arms: 2; Multi-center trial; Trial duration: 8 wk. Trial conducted in: Iran; Obtained ethical clearance and participant consent. Funding information provided. Clinical trial registration number: IRCT201306253140N11 | Participants diagnosed with GDM (used International Association of Diabetes and Pregnancy Study Groups criteria); 76 participants randomized into different treatment arms: Vitamin D arm: n = 38; placebo arm: n = 38; Mean age of participants: -Vitamin D arm: 31.64 ± 4.40 yr; -Placebo arm: 32.11 ± 3.61 yr | Two intervention arms:(1) 50000 IU vitamin D3 oral capsules two weekly for 8 wk; and (2) Placebo | Attrition from vitamin D supplemented arm: n = 4; Other outcomes reported: Fasting plasma glucose |
| Zhang et al[38], 2016 | Randomized, double-blind, placebo-controlled trial. Intervention arms: 4; Single centered trial. Trial duration: 24-28 wk of pregnancy to delivery; Trial conducted in: China; Obtained ethical clearance and participant consent. Funding information provided. Clinical trial registration details: Unclear | Participants diagnosed with GDM (criteria unclear). 133 participants randomized into different treatment arms (low dose vitamin D: n = 38; medium dose vitamin D: n = 38; high dose vitamin D: n = 37; placebo: n = 23); Mean age of participants: -Placebo arm: 29.8 ± 4.7; -Low dose vitamin D arm: 30.3 ± 5.1; -Medium dose vitamin D arm: 29.4 ± 4.9; -High dose vitamin D arm: 30.1 ± 4.5 | Four intervention arms: (1) Low dose vitamin D: 200 IU daily; (2) Medium dose vitamin D: 2000 IU monthly; and (3) High dose vitamin D: 50000 IU every 2 wk. Placebo | Attrition from vitamin D supplemented arm: n = 4 |
| Li and Xing[13], 2016 | Randomized, double-blinded clinical trial. Intervention arms: 2. Multi-centric trial. Trial duration: 16 wk. Trial conducted in: China; Obtained ethical clearance and participant consent. Funding information provided. Clinical trial registration details: Unclear | Participants diagnosed with GDM (used ADA criteria)103 participants randomized into different treatment arms (yoghurt with vitamin D: n = 52, plain yoghurt: n = 51); Mean age of participants: -Yoghurt supplemented with vitamin D receiving arm: 29.0 ± 5.3 yr; -Plain yoghurt arm: 28.3 ± 4.1 yr | Two intervention arms: (1) Yoghurt was supplemented with 500 IU of vitamin D3 twice daily for 16 wk; and (2) plain yoghurt: Twice daily for 16 wk | Attrition from vitamin D supplemented arm: n = 4. Other outcomes reported: Fasting plasma glucose |
The trials were primarily at low RoB except one at high RoB (due to lack of blinding of study personnel and participants) (Table 2)[34].
| Ref. | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias); All outcomes | Blinding of outcome assessment (detection bias); All outcomes | Incomplete outcome data (attrition bias); All outcomes | Selective reporting (reporting bias) | Other bias |
| Jamilian et al[37], 2016 | Low | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Low | Low | Low | Low |
| Jamilian et al[17], 2017 | Low | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Unclear | Low | Low | Low |
| Jamilian et al[33], 2019a | Low risk | Unclear risk; Comment: Precise mechanism unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Jamilian et al[36], 2019b | Low risk | Unclear risk; Comment: Precise mechanism unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Asemi et al[31], 2014a | Low risk | Unclear risk; Comment: Precise mechanism unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Asemi et al[16], 2014b | Low | Low | Low | Low | Low | Low | Low |
| Karamali et al[32], 2016 | Low risk | Unclear risk; Comment: Precise mechanism unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Karamali et al[14], 2018 | Low | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Low | Low | Low | Low |
| Razavi et al[35], 2017 | Low risk | Unclear risk; Comment: It’s unclear if the bottles were sequentially numbered and identical in appearance | Low risk | Low risk | Low risk | Low risk | Low risk |
| Valizadeh et al[34], 2016 | Low risk | Unclear risk | High risk; Comment: Both investigators and participants were not blinded | Low risk | Low risk | Low risk | |
| Yazdchi et al[15], 2016 | Low | Unclear | Unclear | Low | Low | Low | Low |
| Zhang et al[38], 2016 | Low | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Low | Low | Low |
| Li and Xing[13], 2016 | Low | Unclear; Comment: Precise mechanism unclear | Unclear; Comment: Precise mechanism unclear | Low | Low | Low | Low |
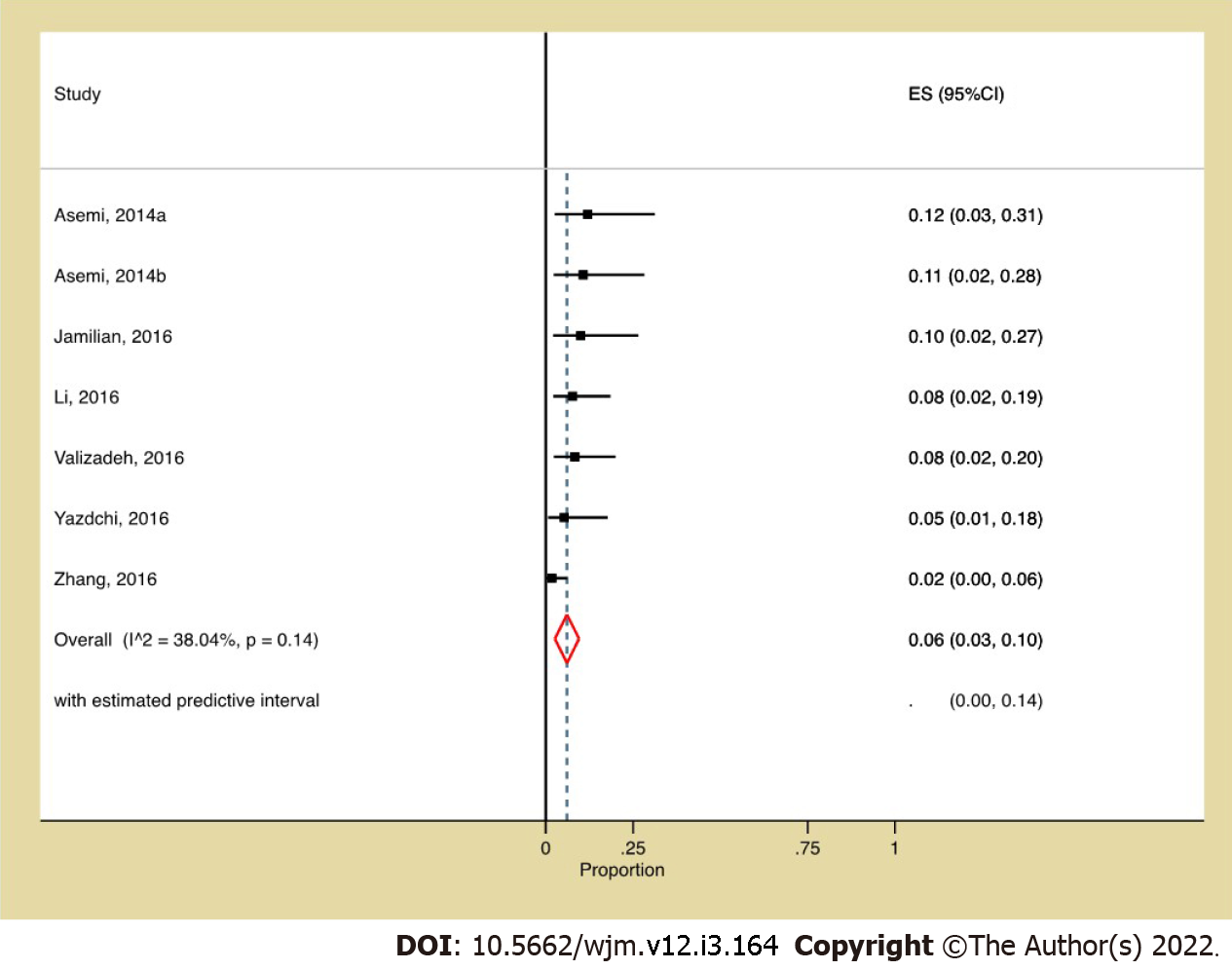
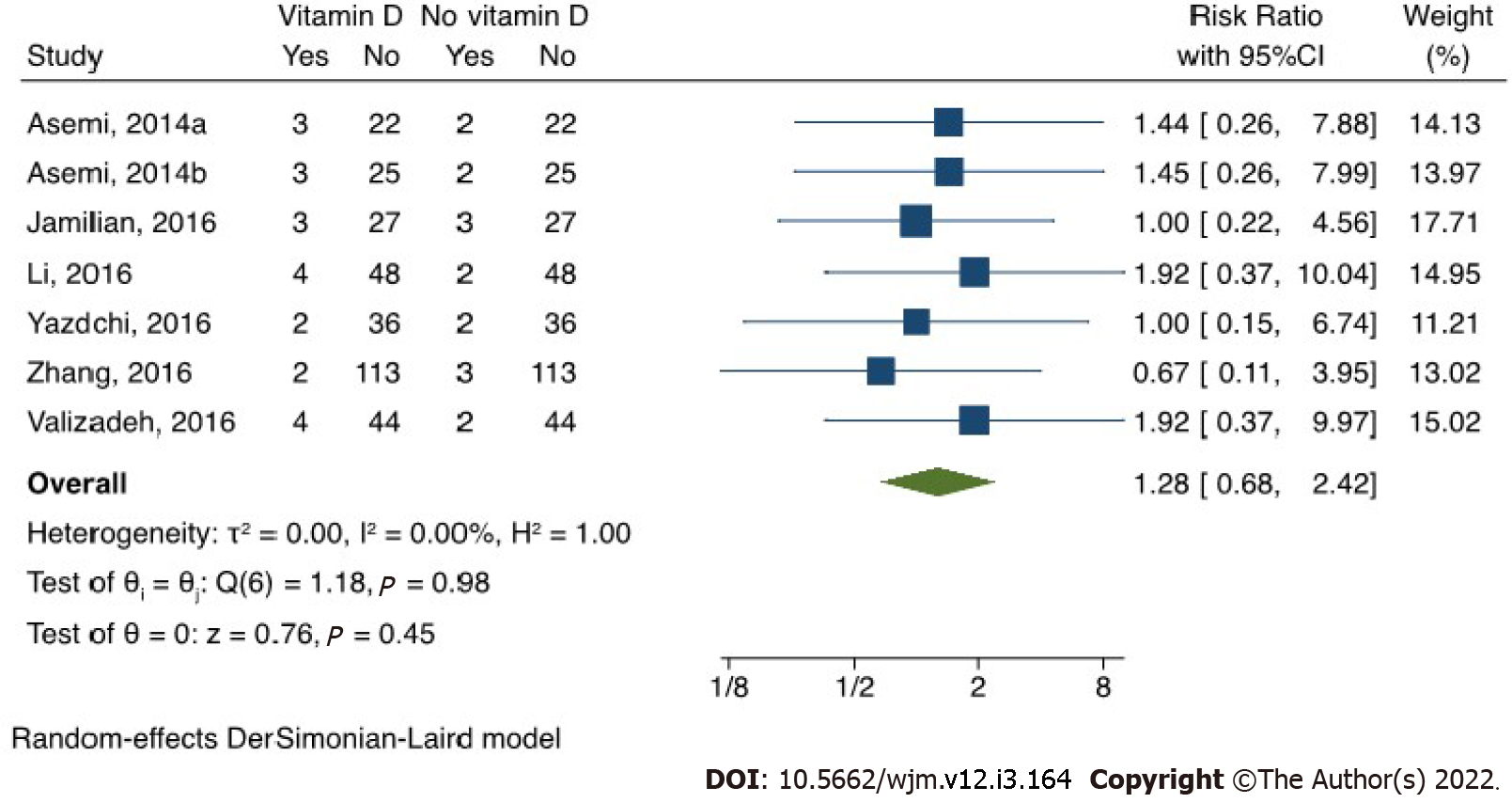
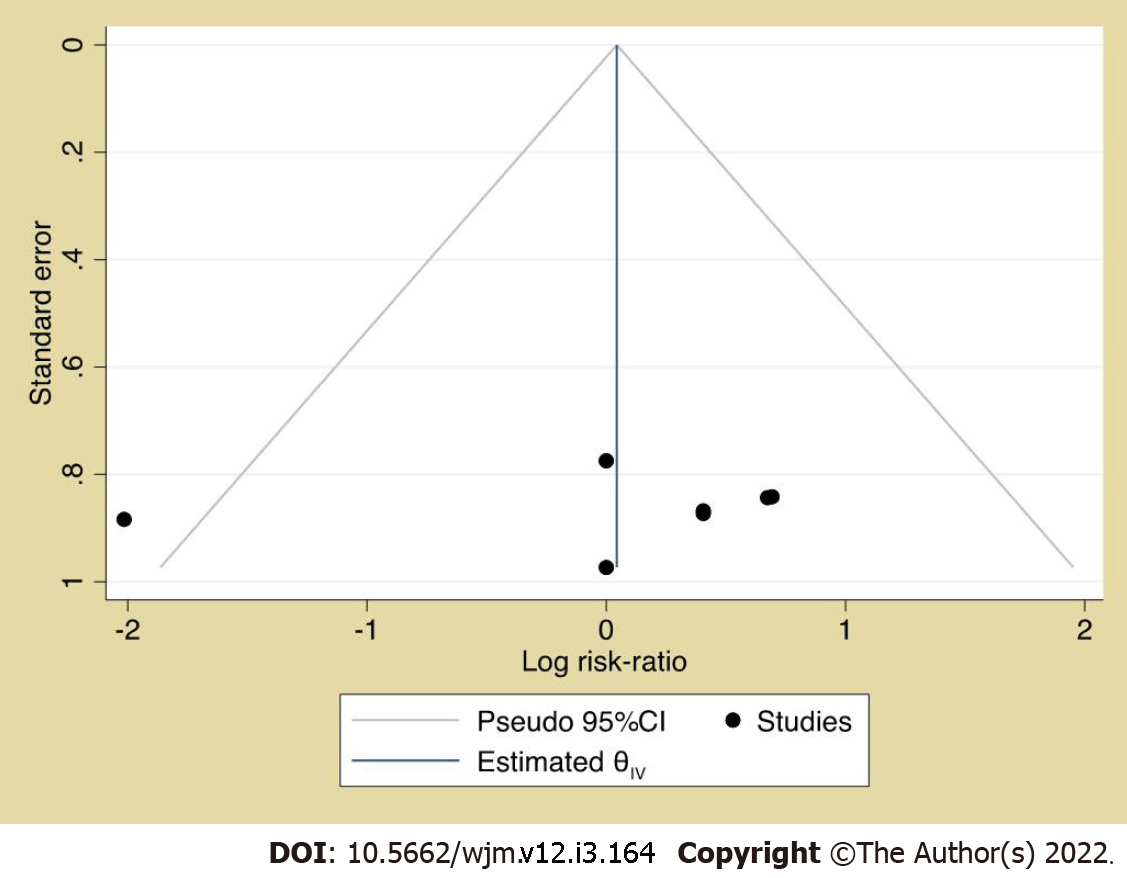
Prevalence and pairwise meta-analysis: The pooled prevalence of participant attrition among vitamin D recipients was 6% (95%CI: 0.03, 0.10, I2: 38.04%) (Figure 2), and its risk did not vary from non-vitamin D recipients (Figure 3). Although the funnel plot (Figure 4) appeared somewhat asymmetrical, Egger’s test did not suggest any small study effect (P = 0.6602).
NMA: Figure 5 depicts the network maps. The maps revealed a lack of direct comparison between any supplement and following nutrients co-supplemented with vitamin D- calcium or magnesium-zinc-calcium combination or evening prime rose oil. The global and local inconsistency tests for any of the outcomes were not suggestive of any inconsistency. The league tables are shown in Tables 3 and 4. Vitamin D (RR: 0.47; 95%CI: 0.27, 0.83) and its co-supplementation with probiotic (RR: 0.28; 95%CI: 0.09, 0.91), omega-3 fatty acids (RR: 0.25; 95%CI: 0.08, 0.77), and calcium (RR: 0.35; 95%CI: 0.16, 0.77) decreased the risk of newborn hyperbilirubinemia. Vitamin D (RR: 0.47; 95%CI: 0.27, 0.83) and its co-supplementation with omega-3 fatty acids (RR: 0.25; 95%CI: 0.08, 0.77) and calcium (RR: 0.35; 95%CI: 0.16, 0.77) reduced the risk of newborn hospitalization. The incidence of CS in GDM patients was lower with vitamin D and calcium co-supplementation (RR: 0.37; 95%CI: 0.18, 0.74). Vitamin D and omega-3 fatty acid co-supplementation in GDM patients decreased the risk of hyperbilirubinemia (RR: 0.30; 95%CI: 0.09, 0.98) and hospitalization (RR: 0.30; 95%CI: 0.09, 0.98) in their newborns compared to omega-3 supplementation alone.
| Interventions and effect sizes | |||||||
| Vitamin D and probiotic | 1.14 (0.22, 5.92)1 | 0.79 (0.19, 3.27) | 0.59 (0.16, 2.20) | 0.73 (0.18, 2.96) | 0.28 (0.09, 0.91)2 | 0.34 (0.09, 1.32) | 0.42 (0.10, 1.69) |
| 0.76 (0.27, 2.19) | Vitamin D and omega-3 fatty acid | 0.70 (0.17, 2.79) | 0.52 (0.16, 1.75) | 0.64 (0.14, 2.99) | 0.25 (0.08, 0.77) | 0.30 (0.09, 0.98) | 0.37 (0.09, 1.44) |
| 1.48 (0.52, 4.21) | 1.94 (0.71, 5.28) | Vitamin D and calcium | 0.75 (0.29, 1.96) | 0.91 (0.25, 3.35) | 0.35 (0.16, 0.77) | 0.43 (0.16, 1.19) | 0.53 (0.18, 1.55) |
| 0.69 (0.29, 1.68) | 0.91 (0.44, 1.90) | 0.47 (0.21, 1.07) | Vitamin D | 1.22 (0.37, 3.96) | 0.47 (0.27, 0.83) | 0.57 (0.27, 1.22) | 0.71 (0.28, 1.78) |
| 0.68 (0.30, 1.54) | 0.89 (0.34, 2.35) | 0.46 (0.17, 1.20) | 0.97 (0.45, 2.13) | Probiotic | 0.39 (0.14, 1.09) | 0.47 (0.14, 1.60) | 0.58 (0.16, 2.07) |
| 0.54 (0.25, 1.18) | 0.71 (0.35, 1.46) | 0.37 (0.18, 0.74) | 0.78 (0.52, 1.19) | 0.80 (0.42, 1.56) | Placebo | 1.22 (0.64, 2.33) | 1.50 (0.72, 3.14) |
| 0.68 (0.24, 1.90) | 0.89 (0.40, 1.99) | 0.46 (0.17, 1.22) | 0.98 (0.49, 1.96) | 1.00 (0.39, 2.58) | 1.24 (0.63, 2.45) | Omega-3 fatty acid | 1.23 (0.46, 3.28) |
| 1.23 (0.33, 4.57) | 1.61 (0.45, 5.79) | 0.83 (0.23, 2.97) | 1.76 (0.56, 5.53) | 1.81 (0.52, 6.33) | 2.25 (0.78, 6.52) | 1.81 (0.51, 6.37) | Magnesium, zinc, calcium, and vitamin D |
| Interventions and effect sizes | |||||||
| Vitamin D and probiotic | 1.27 (0.24, 6.66)1 | 0.88 (0.21, 3.69) | 0.66 (0.18, 2.49) | 0.97 (0.21, 4.41) | 0.31 (0.09, 1.03) | 0.38 (0.10, 1.49) | 0.47 (0.11, 1.91) |
| 0.94 (0.11, 8.45) | Vitamin D and omega-3 fatty acid | 0.70 (0.17, 2.79) | 0.52 (0.16, 1.75) | 0.76 (0.15, 4.02) | 0.25 (0.08, 0.77)2 | 0.30 (0.09, 0.98) | 0.37 (0.09, 1.44) |
| 3.36 (0.13, 88.67) | 3.56 (0.14, 93.17) | Vitamin D and calcium | 0.75 (0.29, 1.96) | 1.10 (0.26, 4.59) | 0.35 (0.16, 0.77) | 0.43 (0.16, 1.19) | 0.53 (0.18, 1.55) |
| 0.98 (0.13, 7.29) | 1.03 (0.18, 6.09) | 0.29 (0.01, 6.77) | Vitamin D | 1.46 (0.39, 5.50) | 0.47 (0.27, 0.83) | 0.57 (0.27, 1.22) | 0.71 (0.28, 1.78) |
| 1.93 (0.19, 20.18) | 2.05 (0.15, 27.32) | 0.58 (0.02, 20.11) | 1.98 (0.17, 22.68) | Probiotic | 0.32 (0.10, 1.07) | 0.39 (0.10, 1.53) | 0.48 (0.12, 1.97) |
| 0.37 (0.08, 1.77) | 0.40 (0.08, 1.85) | 0.11 (0.01, 1.98) | 0.38 (0.11, 1.36) | 0.19 (0.02, 1.55) | Placebo | 1.22 (0.64, 2.33) | 1.50 (0.72, 3.14) |
| 0.63 (0.08, 4.84) | 0.67 (0.12, 3.71) | 0.19 (0.01, 4.45) | 0.64 (0.13, 3.13) | 0.33 (0.03, 3.83) | 1.69 (0.45, 6.30) | Omega-3 fatty acid | 1.23 (0.46, 3.28) |
| 1.87 (0.14, 25.22) | 1.98 (0.15, 26.45) | 0.56 (0.02, 19.45) | 1.91 (0.17, 21.96) | 0.97 (0.05, 18.42) | 5.00 (0.62, 40.28) | 2.96 (0.25, 34.96) | Magnesium, zinc, calcium, and vitamin D |
The surface under the cumulative ranking curve values suggested vitamin D and calcium co-supplementation in GDM patients as the best supplement for reducing the CS requirement, and vitamin D and omega-3 fatty acid co-supplementation as the best supplement for reducing the risk of hospitalization and hyperbilirubinemia in their newborns (Table 5). The macrosomia risk and FPG levels (league table not shown) did not vary among the interventions.
| Intervention | Outcomes | |||||
| Newborn hyperbilirubinemia | Newborn hospitalization | Cesarean section | ||||
| SUCRA | Mean rank | SUCRA | Mean rank | SUCRA | Mean Rank | |
| Vitamin D and omega-3 fatty acid | 81.8 | 2.31 | 81.1 | 2.31 | 46.4 | 4.8 |
| Vitamin D and probiotic | 76.2 | 2.7 | 70.7 | 3.0 | 66.3 | 3.4 |
| Probiotic | 62.2 | 3.6 | 69.5 | 3.1 | 36.6 | 5.4 |
| Vitamin D and calcium | 67.9 | 3.3 | 67.2 | 3.3 | 87.6 | 1.91 |
| Vitamin D | 52.8 | 4.3 | 52.4 | 4.3 | 39.0 | 5.3 |
| Magnesium, zinc, calcium, and vitamin D | 32.4 | 5.7 | 32.2 | 5.7 | 73.8 | 2.8 |
RoB across studies: Evaluation of RoB across studies suggests that the trials primarily adhered to their pre-stated analytic notions.
Sensitivity analysis: On repeating the prevalence meta-analysis by dropping one study each time, the prevalence ranged between 5% and 8%. The pairwise meta-analysis findings were identical to the preliminary model when a fixed-effect model-based iteration occurred.
Overall, this review included 13 publications sourcing data from 1109 GDM patients from Iran and China. The RoB across the trials was primarily low except for one with a high RoB component. The burden of attrition of GDM patients from the vitamin D supplemented arms post-randomization was 6%, and this risk did not vary from GDM patients who did not receive the supplement. Vitamin D and calcium co-supplementation benefited the GDM patients (decreased the CS incidence) and their neonates (decreased hyperbilirubinemia and hospitalization risk). Vitamin D alone and its omega-3 fatty acid added form both reduced the newborn’s risk of hyperbilirubinemia and hospitalization. For these outcomes, co-supplementation of vitamin D and omega-3 fatty acids was superior to omega-3 fatty acids alone. Combining vitamin D with probiotics was effective in reducing the risk of newborn hyperbilirubinemia.
Using the Grading of Recommendations Assessment, Development, and Evaluation approach[26], the NMA-generated evidence was double downgraded to low quality. This decision stood on the fact that the statistically significant findings were unlikely to be generalizable as study participants were mostly from Iran; thus, a fixed-effect model NMA was used for the categorical outcomes, and the trials had few unclear RoB components.
Regarding the prevalence of participant attrition, to the best of our knowledge, no literature is available to contrast with the findings of this review, perhaps due to its conceptual novelty. Concerning the perinatal outcomes, existing reviews suggested that vitamin D supplementation decreases the risk of CS, macrosomia, neonatal hyperbilirubinemia, and newborn hospitalization[8,39]. However, unlike this paper’s findings, these reviews[8,39] did not sort out how perinatal outcomes vary across vitamin D, its co-supplemented forms, and other (non-vitamin D) supplements tested in these trials.
The key strength of this review is its incorporation of RCTs only, the highest level of epidemiological evidence. The intransitivity risk in the NMA models is perhaps low due to the exclusion of the trial at a high RoB component. Furthermore, beyond reviewing post-randomization GDM patients' attrition burden from vitamin D-supplemented trial arm/s and its risk, this is plausibly the first study that attempted to distinguish the efficacy between vitamin D and its co-supplemented forms in GDM patients.
Despite these strengths, this study also had a few limitations. This review could not incorporate non-English language publications (if any) as the review authors are competent in handling publications in the English language only. The anticipated generalizability of the evidence generated in this study was low due to the homogenous nature of the study population. Although the prevalence meta-analysis estimate appeared weak due to its inclusion of a trial with a high RoB component, the sensitivity analysis did not observe any fluctuation upon excluding the trial from the model.
The low prevalence of post-randomization attrition of GDM patients from the vitamin D-supplemented intervention arms in RCTs suggests good adherence to the supplement and might encourage trialists across the globe to conduct identical efficacy trials. Given the substantial burden of vitamin D deficiency and insufficiency in Iranian pregnant females[40] (and most trials included in this review were from Iran), from a public health point of view, this study's findings might help the local health authority in reviewing the scope of routine prenatal supplementation of vitamin D and its co-supplemented forms with calcium, omega-3 fatty acids, and probiotics in GDM patients.
In RCTs testing the efficacy of vitamin D supplementation, the post-randomization attrition burden in vitamin D-supplemented GDM patients was low. Prenatal vitamin D and its co-supplemented form with calcium, omega-3 fatty acids, and probiotics each can curb certain perinatal complications' risks in GDM patients and their neonates.
The role of vitamin D in gestational diabetes mellitus (GDM) is not established. Several randomized controlled trials (RCT) have tested it.
The burden and risk of participant attrition from vitamin D receiving treatment arm/s of these trials are unclear. Also, the effect of vitamin D and its co-supplemented forms and other supplements on the mother’s glycemic control and perinatal outcomes remains unclear.
This study aimed to address these issues.
Eligible clinical trials were retrieved by searching the PubMed, Embase, and Scopus databases. The burden and risk of participant attrition got determined by random-effect prevalence and pairwise meta-analysis, respectively. The effect of different nutritional supplements on the perinatal outcomes got estimated by fixed-effect network meta-analysis. All analysis ensued in Stata statistical software (v16).
The database search produced 13 RCTs conducted in Iran and China. The participant attrition from vitamin D treated arms was 6% (95% confidence interval [CI]: 0.03, 0.10), and this risk did not vary from its non-recipient arms. The cesarean section risk decreased with the combined supplementation of vitamin D and calcium [risk ratio (RR): 0.37; 95%CI: 0.18, 0.74]. The vitamin D alone and its co-supplemented forms with calcium and omega-3 fatty acids decreased the risk of newborn- hyperbilirubinemia or hospitalization. The probiotics co-supplemented form of vitamin D decreased newborn hyperbilirubinemia risk (RR: 0.28; 95%CI: 0.09, 0.91). The fasting plasma glucose levels didn’t vary across the compared interventions.
This study suggests that vitamin D supplementation is a relatively well-tolerated intervention in GDM patients resulting in relatively low participant attrition from RCTs testing it. Also, this study suggests that some nutritional supplements can be beneficial in reducing perinatal outcomes.
Given the low burden of participant attrition from the vitamin-supplemented arms of RCTs, future trialists may find the conduct of RCTs with a larger sample size reasonable to produce rigorous results.
| 1. | Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348:g1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 2. | Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018; 131: e49–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29370047. |
| 3. | Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 4. | Lu M, Xu Y, Lv L, Zhang M. Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstet. 2016;293:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 5. | Saha S, Saha S. Changes in anthropometric and blood 25-hydroxyvitamin D measurements in antenatal vitamin supplemented gestational diabetes mellitus patients: a systematic review and meta-analysis of randomized controlled trials. J Turk Ger Gynecol Assoc. 2021;22:217-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 6. | Mavridis D, White IR. Dealing with missing outcome data in meta-analysis. Res Synth Methods. 2020;11:2-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 7. | Akbari M, Moosazaheh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M, Jamilian M, Kolahdooz F, Asemi Z. The Effects of Vitamin D Supplementation on Glucose Metabolism and Lipid Profiles in Patients with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm Metab Res. 2017;49:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Saha S, Saha S. A comparison of the risk of cesarean section in gestational diabetes mellitus patients supplemented antenatally with vitamin D containing supplements versus placebo: A systematic review and meta-analysis of double-blinded randomized controlled trials. J Turk Ger Gynecol Assoc. 2020;21:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 9. | DRI – Dietary Reference Intakes – Calcium and Vitamin D20122 DRI – Dietary Reference Intakes – Calcium and Vitamin D. Institute of Medicine of the National Academies, ISBN: 13‐978‐0‐309‐16394‐1. Nutr Food Sci [Internet]. 2012; 42: 131–131 . [DOI] [Full Text] |
| 11. | Curtis EM, Moon RJ, Harvey NC, Cooper C. Maternal vitamin D supplementation during pregnancy. Br Med Bull. 2018;126:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Knabl J, Vattai A, Ye Y, Jueckstock J, Hutter S, Kainer F, Mahner S, Jeschke U. Role of Placental VDR Expression and Function in Common Late Pregnancy Disorders. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 13. | Li Q, Xing B. Vitamin D3-Supplemented Yogurt Drink Improves Insulin Resistance and Lipid Profiles in Women with Gestational Diabetes Mellitus: A Randomized Double Blinded Clinical Trial. Ann Nutr Metab. 2016;68:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Karamali M, Bahramimoghadam S, Sharifzadeh F, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2018;43:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 15. | Yazdchi R, Gargari BP, Asghari-Jafarabadi M, Sahhaf F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: a randomized, double-blinded, placebo-controlled clinical trial. Nutr Res Pract. 2016;10:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. 2014;57:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 17. | Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, Sanami M, Asemi Z. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol. 2017;11:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 18. | Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 19. | Saha S, Saha S. The variation in participant attrition between prenatal vitamin D supplemented and not supplemented gestational diabetes mellitus patients: a systematic review and meta-analysis of randomized controlled trials. [Internet]. PROSPERO2020; Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020180634. |
| 20. | Saha S. The prevalence and risk of missing outcome data in prenatal vitamin D supplemented gestational diabetes mellitus patients: a systematic review and meta-analysis protocol. J Ideas Heal. 2020;3:217-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Saha S, Saha S. eP221 Participant attrition and perinatal complications risk in efficacy trials testing vitamin D supplementation in gestational diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials.. [DOI] [Full Text] |
| 22. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51937] [Article Influence: 10387.4] [Reference Citation Analysis (2)] |
| 23. | Higgins JPT GS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. [Internet]. Cochrane Collab. 2011 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 24. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48645] [Article Influence: 2115.0] [Reference Citation Analysis (4)] |
| 25. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5897] [Article Influence: 536.1] [Reference Citation Analysis (1)] |
| 26. | Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5096] [Cited by in RCA: 6203] [Article Influence: 282.0] [Reference Citation Analysis (1)] |
| 27. | Bhavya Swetha RV, Samal R, George CE. The Effect of Vitamin D Supplementation on Improving Glycaemic Control in Diabetic Vitamin D-Deficient Pregnant Women: A Single-Blinded Randomized Control Trial. J Obstet Gynaecol India. 2020;70:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 28. | Hosseinzadeh-Shamsi-Anar M, Mozaffari-Khosravi H, Salami MA, Hadinedoushan H, Mozayan MR. The efficacy and safety of a high dose of vitamin d in mothers with gestational diabetes mellitus: a randomized controlled clinical trial. Iran J Med Sci. 2012;37:159-165. [PubMed] |
| 29. | Huang S, Fu J, Zhao R, Wang B, Zhang M, Li L, Shi C. The effect of combined supplementation with vitamin D and omega-3 fatty acids on blood glucose and blood lipid levels in patients with gestational diabetes. Ann Palliat Med. 2021;10:5652-5658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 30. | Gunasegaran P, Tahmina S, Daniel M, Nanda SK. Role of vitamin D-calcium supplementation on metabolic profile and oxidative stress in gestational diabetes mellitus: A randomized controlled trial. J Obstet Gynaecol Res. 2021;47:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 31. | Asemi Z, Karamali M, Esmaillzadeh A. Favorable effects of vitamin D supplementation on pregnancy outcomes in gestational diabetes: a double blind randomized controlled clinical trial. Horm Metab Res. 2015;47:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Karamali M, Asemi Z, Ahmadi-Dastjerdi M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial. Public Health Nutr. 2016;19:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:2098-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Valizadeh M, Piri Z, Mohammadian F, Kamali K, Amir Moghadami HR. The Impact of Vitamin D Supplementation on Post-Partum Glucose Tolerance and Insulin Resistance in Gestational Diabetes: A Randomized Controlled Trial. Int J Endocrinol Metab. 2016;14:e34312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, Seyed Hosseini E, Haddad Kashani H, Karamali M, Asemi Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab (Lond). 2017;14:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, Asemi Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Jamilian M, Karamali M, Taghizadeh M, Sharifi N, Jafari Z, Memarzadeh MR, Mahlouji M, Asemi Z. Vitamin D and Evening Primrose Oil Administration Improve Glycemia and Lipid Profiles in Women with Gestational Diabetes. Lipids. 2016;51:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Zhang Q, Cheng Y, He M, Li T, Ma Z, Cheng H. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: A randomized controlled trial. Exp Ther Med. 2016;12:1889-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Saha S, Saha S. The risk of morbidities in newborns of antenatal vitamin D supplemented gestational diabetes mellitus patients. Int J Health Sci (Qassim). 2020;14:3-17. [PubMed] |
| 40. | Badfar G, Shohani M, Mansouri A, Soleymani A, Azami M. Vitamin D status in Iranian pregnant women and newborns: a systematic review and meta-analysis study. Expert Rev Endocrinol Metab. 2017;12:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qiong L, China; Wu QN, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH