Published online Sep 20, 2018. doi: 10.5528/wjtm.v7.i1.1
Peer-review started: June 21, 2018
First decision: July 9, 2018
Revised: July 31, 2018
Accepted: August 30, 2018
Article in press: August 30, 2018
Published online: September 20, 2018
Processing time: 92 Days and 3.8 Hours
Although boron has been a chemical element of interest since the ancient times, only a few boron-containing compounds (BCCs) had been used for medicinal purposes before the 21st century. Among these, only boric acid has been explored in multiple therapeutic applications. Hence, it is common to extrapolate from boric acid to all BCCs, supposing a similar biological effect. However, boric acid is just one of dozens of BCCs in nature and thousands available from chemical synthesis. Nowadays, there is a boom in research on new BCCs as potential tools in the prevention, diagnosis and therapy of human disease. We herein discuss the new role of BCCs in drug development, with emphasis on the compounds for which a mechanism of action has been proposed or demonstrated. Because of data gathered in recent years, BCCs have expanded beyond the well-known fields of antimicrobial and antineoplastic agents, now being explored for their possible use as enzyme inhibitors, regulators of protein expression and modulators of the immune response, as well as in biomaterials. We suggest that translational medicine can accelerate the medicinal applications of BCCs, which is especially important for the human diseases that are generating a high global burden.
Core tip: Boron-containing compounds (BCCs) have growing relevance in the biomedical field. The former almost exclusive focus on boric acid has expanded to include a wide range of BCCs in chemical and biomedical studies. The reported findings suggest that research in this field will continue increasing exponentially in the near future. Through translational medicine, the boron atom is being introduced into new compounds to explore its use in the prevention, diagnosis and therapy of multiple pathologies.
- Citation: Farfán-García ED, Castillo-García EL, Soriano-Ursúa MA. More than boric acid: Increasing relevance of boron in medicine. World J Transl Med 2018; 7(1): 1-4
- URL: https://www.wjgnet.com/2220-6132/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v7.i1.1
Boron could have been involved in the origin of life as it is known today[1]. Although some evidence points to the ability of borates to react with and stabilize primordial substances related to nucleic acids, the participation of boron-containing compounds (BCCs) in this sense is not completely clear[2]. Several other effects of boron have been described[3,4], showing that it is of notable importance in biology[5]. However, most information about its relevance to life is linked to the effects of boric acid or borates on organisms[6].
As a result of artificial synthesis, the number of reported BCCs is currently undergoing a rapid increase, especially organoboron compounds (those with boron, carbon, hydrogen and nitrogen in their structure)[4]. There is no doubt about the expanding interest in generating new BCCs for testing in biological systems[3,6].
By far the greatest abundance of data on the biological activity of BCCs comes from the investigation of boric acid[5,6], which has been examined for centuries. Nevertheless, the advances in the last four decades have provided extensive knowledge about the advantages of other BCCs over their boron-free counterparts. Consequently, growing evidence exists about the action of BCCs on membrane, cytoplasmic and nuclear receptors, as well as on enzymes in many biological compartments[4].
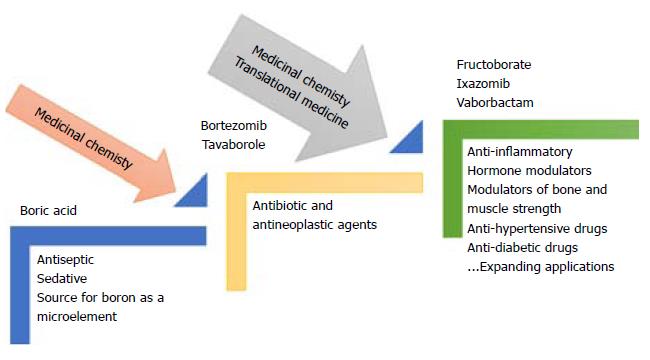
In the last century, research on BCCs has gone far beyond the former limited use of boric acid (the most abundant BCC in nature) as an antiseptic agent. Today BCCs are employed in various therapies, including boron-neutron capture therapy, an antineoplastic drug (bortezomib), and anti-microbial agents (boromycin and tavaborole)[4].
In biology, some key genetic events can be modulated by BCCs (and potential tools are being generated with this aim)[7,8]. Indeed, the absence of boron in the environment or diet is able to disrupt some physiological processes in unicellular organisms, plants and animals[5]. In humans, BCCs participate in many physiological processes, such as the growth and maintenance of bones, wound healing, micronutrient absorption, electrical activity, cell proliferation, protection against some toxic agents (including traditional chemotherapy agents), and the modulation of hormones, antioxidant enzymes and inflammatory biomarkers[9,10]. The greater number of toxicological studies of BCCs has allowed for the identification of potential toxins as well as drugs with diminished risk[6].
Over the last several decades, some BCCs (found in nature or developed with chemical synthesis) have been attractive as enzyme inhibitors, antineoplastic drugs and anti-infectious agents (Table 1)[4]. In the last few years, BCCs have been designed to act on G-protein coupled receptors[11], ionic channels[12], membrane receptors with enzymatic domains[13] and all types of enzymes[14]. BCCs also are being examined as modulators of the flow of genetic information within biological systems[15], the cell-cycle[16], metabolism[17] and inflammatory processes[18]. This modulation frequently implies more than one molecular target.
| Compound | Used since | Reported uses | Reference |
| Boric acid | Antibiotic, antiseptic, sedative | [6] | |
| Bortezomib | 2003 | Treatment of Multiple myeloma (Intravenous) | [16] |
| Calcium Fructoborate | 2011 | Treatment of inflammatory processes | [24] |
| Tavaborole | 2014 | Antifungal (topic to nail) | [27] |
| Ixazomib | 2015 | Treatment of Multiple myeloma (Intravenous and oral) | [28] |
| Crisaborole | 2017 | Inflammation (topical to skin) | [29] |
| Vaborbactam | 2017 | Antibacterial (intravenous) | [30] |
Additionally, pharmacokinetic parameters are used to describe the properties of BCCs. The stability of these compounds in physiological conditions is documented for boron-containing acids, borinates, boronates, boranes and boron-clusters, among other compounds[19]. Some families of membrane transporters have been involved in the ability of BCCs to cross biological membranes, as well as in absorption and excretion[19,20]. The characterization of the partition/distribution coefficient for some BCCs has allowed for the selection of compounds aimed at a specific organ or tissue[19].
The determination of some parameters of the boron atom had led to its inclusion in theoretical assays related to biological effects and drug discovery[21]. Most recently, new breakthroughs in the biomedical field are concerned with boron clusters, boron-containing nanotubes and sheets for drug delivery systems, and biomaterials for covering or substituting tissues in multicellular organisms[22].
In conclusion, the role of boron in biology and medicine has developed far beyond the administration of boric acid to be a source of boron as a microelement in living organisms. BCCs are not only promising compounds for the development of drugs, but also of biomaterials and agents that can act in a preventive and diagnostic sense.
Shortly, a growing number of BCCs will be introduced as drugs due to the attractiveness of their increased potency and efficacy on all the known receptor types targeted for diseases with a high global burden (infectious diseases, cancer, immunological diseases, metabolic disturbances, neurodegeneration, etc.). The leaders in the pharmaceutical industry recognize this trend and are actively involved in the discovery and development of potential drugs; medicinal chemistry and translational medicine have accelerated the development of new drugs (Figure 1).
The role of boron atom in new chemical structures is beyond the development of new drugs as it occurred in the past century with chlorine or fluorine addition[23,24]. Moreover, the recent advances in boron chemistry may result in applications in biology and medicine that were very recently considered science fiction. For instance, BCCs could soon be generated to stabilize DNA or RNA structures, heal individuals with compromising mutations by substitution of boron-containing alpha amino acids[9,15,16,25], participate in smart drug delivery systems, and construct biomaterials with better features than fullerenes and structurally-related materials[22]. It is even feasible that BCCs will eventually be designed to change the appearance of a species[26].
The application of translational medicine is now accelerating the process of developing and testing such compounds for humans. There is no doubt about the relevance of boron in the biomedical field. Whereas some BCCs have the same or a similar mechanism of action as their boron-free counterparts, others show a different interaction with target receptors. The mechanism of action of BCCs is often revealed by pharmacokinetic parameters. In some cases, BCCs have exhibited greater stability due to the strength of the bonds between the boron atom(s) and the carbon, nitrogen or oxygen atoms in their structure. The current expanding diversity of BCCs requires efforts for their classification and the clear definition of their structure-activity relationships in biological systems.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Menendez-Arias L, Santulli G S- Editor: Cui LJ L- Editor: A E- Editor: Song H
| 1. | Kim HJ, Furukawa Y, Kakegawa T, Bita A, Scorei R, Benner SA. Evaporite Borate-Containing Mineral Ensembles Make Phosphate Available and Regiospecifically Phosphorylate Ribonucleosides: Borate as a Multifaceted Problem Solver in Prebiotic Chemistry. Angew Chem Int Ed Engl. 2016;55:15816-15820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 2. | da Silva JA, Holm NG. Borophosphates and silicophosphates as plausible contributors to the emergence of life. J Colloid Interface Sci. 2014;431:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Leśnikowski ZJ. Recent developments with boron as a platform for novel drug design. Expert Opin Drug Discov. 2016;11:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | Soriano-Ursúa MA, Das BC, Trujillo-Ferrara JG. Boron-containing compounds: chemico-biological properties and expanding medicinal potential in prevention, diagnosis and therapy. Expert Opin Ther Pat. 2014;24:485-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Uluisik I, Karakaya HC, Koc A. The importance of boron in biological systems. J Trace Elem Med Biol. 2018;45:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 6. | Farfán-García ED, Castillo-Mendieta NT, Ciprés-Flores FJ, Padilla-Martínez II, Trujillo-Ferrara JG, Soriano-Ursúa MA. Current data regarding the structure-toxicity relationship of boron-containing compounds. Toxicol Lett. 2016;258:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Scott H, Walmsley RM. Ames positive boronic acids are not all eukaryotic genotoxins. Mutat Res Genet Toxicol Environ Mutagen. 2015;777:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kan SBJ, Huang X, Gumulya Y, Chen K, Arnold FH. Genetically programmed chiral organoborane synthesis. Nature. 2017;552:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 9. | Pizzorno L. Nothing Boring About Boron. Integr Med (Encinitas). 2015;14:35-48. [PubMed] |
| 10. | Abdelnour SA, Abd El-Hack ME, Swelum AA, Perillo A, Losacco C. The vital roles of boron in animal health and production: A comprehensive Review. J Trace Elem Biol Med. 2018;50:296-304. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 11. | Soriano-Ursúa MA, Arias-Montaño JA, Correa-Basurto J, Hernández-Martínez CF, López-Cabrera Y, Castillo-Hernández MC, Padilla-Martínez II, Trujillo-Ferrara JG. Insights on the role of boron containing moieties in the design of new potent and efficient agonists targeting the β2 adrenoceptor. Bioorg Med Chem Lett. 2015;25:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Zhuo RG, Liu XY, Zhang SZ, Wei XL, Zheng JQ, Xu JP, Ma XY. Insights into the stimulatory mechanism of 2-aminoethoxydiphenyl borate on TREK-2 potassium channel. Neuroscience. 2015;300:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Asano T, Nakamura H, Uehara Y, Yamamoto Y. Design, synthesis, and biological evaluation of aminoboronic acids as growth-factor receptor inhibitors of EGFR and VEGFR-1 tyrosine kinases. Chembiochem. 2004;5:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010- 2018). Expert Opin Ther Pat. 2018;28:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Najarro MA, Hackett JL, Macdonald SJ. Loci Contributing to Boric Acid Toxicity in Two Reference Populations of Drosophila melanogaster. G3 (Bethesda). 2017;7:1631-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Gu JJ, Kaufman GP, Mavis C, Czuczman MS, Hernandez-Ilizaliturri FJ. Mitotic catastrophe and cell cycle arrest are alternative cell death pathways executed by bortezomib in rituximab resistant B-cell lymphoma cells. Oncotarget. 2017;8:12741-12753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Zhang T, Hsu FN, Xie XJ, Li X, Liu M, Gao X, Pei X, Liao Y, Du W, Ji JY. Reversal of hyperactive Wnt signaling-dependent adipocyte defects by peptide boronic acids. Proc Natl Acad Sci USA. 2017;114:E7469-E7478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Baldwin AG, Rivers-Auty J, Daniels MJD, White CS, Schwalbe CH, Schilling T, Hammadi H, Jaiyong P, Spencer NG, England H. Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chem Biol. 2017;24:1321-1335.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Ocampo-Néstor AL, Trujillo-Ferrara JG, Abad-García A, Reyes-López C, Geninatti-Crich S, Soriano-Ursúa MA. Boron’s journey: advances in the study and application of pharmacokinetics. Expert Opin Ther Pat. 2017;27:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Thurtle-Schmidt BH, Stroud RM. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc Natl Acad Sci USA. 2016;113:10542-10546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Andrade-Jorge E, Garcia-Avila AK, Ocampo-Nestor AL, Trujillo-Ferrara JG, Soriano-Ursua MA. Advances of Bioinformatics Applied to Development and Evaluation of Boron-Containing Compounds. Curr Org Chem. 2018;22:298-306. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Zhang Z, Penev ES, Yakobson BI. Two-dimensional boron: structures, properties and applications. Chem Soc Rev. 2017;46:6746-6763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Gouverneur V, Seppelt K. Introduction: fluorine chemistry. Chem Rev. 2015;115:563-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Donoiu I, Militaru C, Obleagă O, Hunter JM, Neamţu J, Biţă A, Scorei RI, Rogoveanu OC. Effects of Boron-Containing Compounds on Cardiovascular Disease Risk Factors–A Review. J Trace Elem Med Biol. 2018;50:47-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Schiefner A, Nästle L, Landgraf M, Reichert AJ, Skerra A. Structural Basis for the Specific Cotranslational Incorporation of p-Boronophenylalanine into Biosynthetic Proteins. Biochemistry. 2018;57:2597-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Biswas A, Malferrari S, Kalaskar DM, Das AK. Arylboronate esters mediated self-healable and biocompatible dynamic G-quadruplex hydrogels as promising 3D-bioinks. Chem Commun (Camb). 2018;54:1778-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Markham A. Tavaborole: first global approval. Drugs. 2014;74:1555-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, Hui AM, Gupta N, Di Bacco A, Yu J. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Zane LT, Hughes MH, Shakib S. Tolerability of Crisaborole Ointment for Application on Sensitive Skin Areas: A Randomized, Double-Blind, Vehicle-Controlled Study in Healthy Volunteers. Am J Clin Dermatol. 2016;17:519-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of the β-Lactamase Inhibitor Vaborbactam (RPX7009) in Healthy Adult Subjects. Antimicrob Agents Chemother. 2016;60:6326-6332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |