Published online Dec 25, 2025. doi: 10.5527/wjn.v14.i4.110896
Revised: July 7, 2025
Accepted: October 11, 2025
Published online: December 25, 2025
Processing time: 188 Days and 17.2 Hours
Kidney transplantation remains the best treatment option for patients with end-stage kidney disease, offering superior outcomes and improved quality of life. However, as in the general population, cardiovascular disease remains the lead
Core Tip: Kidney transplantation offers improved quality of life and survival advantages in addition to a reduced cardiovascular disease risk overall compared to remaining on long-term dialysis. However, kidney transplant recipients continue to experience a higher risk of adverse cardiovascular disease outcomes compared to the general population, with many of these risk factors worsened by and specific to their transplant.
- Citation: Belal AA, Santos Jr AH, Kazory A. Non-traditional cardiovascular risk factors after kidney transplantation. World J Nephrol 2025; 14(4): 110896
- URL: https://www.wjgnet.com/2220-6124/full/v14/i4/110896.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i4.110896
Despite advancements in kidney transplantation surgery and follow-up, cardiovascular death continues to be the most common cause of post-transplant mortality with a functioning graft among kidney transplant recipients (KTRs)[1]. A retrospective analysis of 210327 first-time KTRs between 1996 and 2014 found a mortality rate of 3.2% at 1-year post-transplant, with cardiovascular deaths being responsible for 24.7% of these deaths[2]. This is consistent with other patients with chronic kidney disease (CKD) being of an elevated risk for cardiovascular disease[3]. While there is a mortality benefit of kidney transplant over recipients remaining on dialysis, kidney transplant recipients continue to have increased overall mortality when compared to the general population[4]. KTRs continue to experience up to 10 times the rate of cardiac death and 50 times the rate of fatal or non-fatal cardiovascular events to that of the general population as these recipients’ renal function, on average, remains lower than that of the general population[5]. This is at least in part due to the challenges controlling traditional risk factors (e.g., hypertension, diabetes, dyslipidemias) in KTRs[6].
Established traditional cardiovascular risk factors are certainly present in the subset of the population with kidney transplants, with some risk factors being of high prevalence. For example, hypertension is present in up to 90% of KTRs based on a retrospective cohort study of 1666 patients followed for 5 years post-transplant with hypertension found to be closely associated with increased adjusted risk for graft failure and death[7]. The prevalence of dyslipidemias among KTRs is also high compared to the general population, particularly with elevated total cholesterol and low-density lipoprotein (LDL) levels, though triglycerides are also often times increased on fasting lipid panels[8]. Given that diabetes continues to be the leading cause of end-stage kidney disease, it too is highly prevalent in KTRs and is associated with an increased risk of cardiovascular events post-transplant, particularly when uncontrolled[4,9]. Transplant-specific risk factors that exacerbate traditional risk factors such as hypertension for KTRs include factors related to their immunosuppression, donor factors, and if they have experienced episodes of rejection (particularly vascular rejection episodes associated with angiotensinogen 1 receptor antibodies), and transplant renal artery stenosis[10]. These KTRs may also be at risk of poorly controlled hypertension in the perioperative period due to volume overload, particularly in the setting of slow or delayed graft function[9].
Additional non-traditional cardiovascular risk factors in patients with CKD, such as kidney function impairment, CKD-mineral bone disease (CKD-MBD), proteinuria, and anemia are also represented in the KTR population[11]. In a retrospective post hoc analysis of a trial including 3676 renal transplant recipients, it was found that each 5 mL/minute/1.73 m2 higher estimated glomerular filtration rate below a threshold of 45 mL/minute/1.73 m2 was independently associated with a 15% lower risk of both cardiovascular disease and death[12]. With regards to CKD-MBD, hyperparathyroidism is often persistent post-transplant with associated poorer allograft and patient survival[13]. Anemia is observed in 20%-45% of KTRs and is often multifactorial from suboptimal allograft function to the utilization of immunosuppressants and prophylactic medications[14]. Finally, proteinuria is also noted in upwards of 20% of KTRs, and as in the general population, it is a potent independent risk factor for cardiovascular disease[15,16]. In a study of 532 KTRs, the development of proteinuria greater than 0.5 g/day was found to be associated with significantly lower five- and ten-year survival of 85% and 60% compared to 90% and 83% for patients without significant proteinuria with the presence of post-transplant cardiovascular disease and ischemic heart disease significantly higher in the proteinuria group as well (45.9% and 24% compared to 28% and 13%)[17].
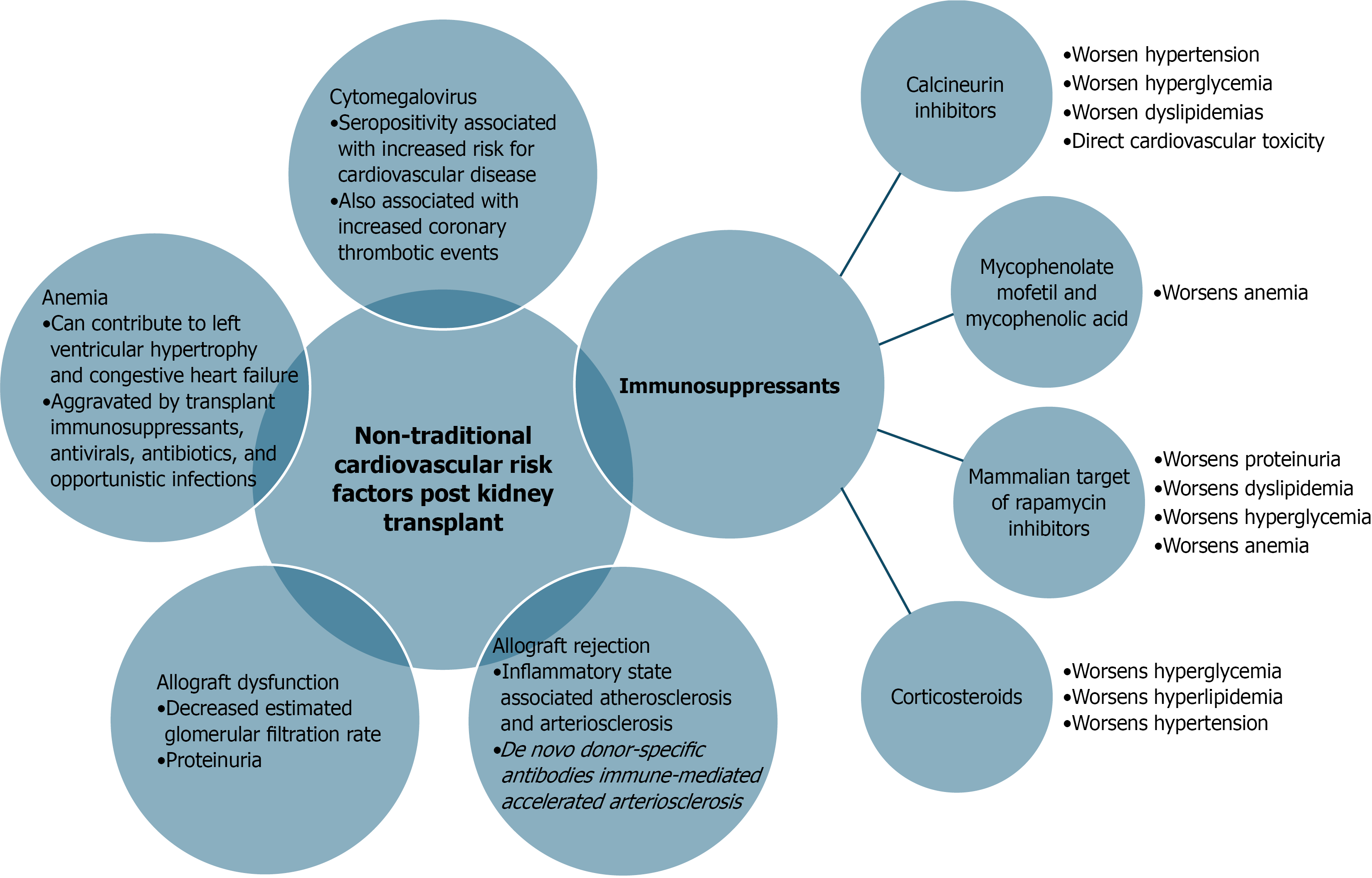
Regarding transplant-specific risk factors for cardiovascular disease in KTRs, we have touched on both induction and maintenance immunosuppression and their impact on patients’ cardiovascular risk but will expound upon it here as well as in Figure 1. Based on 2023 data, 93% of adult KTRs received induction immunosuppression, with 75% of these being given T-cell-depleting agents and maintenance immunosuppression being a triple therapy regimen of tacrolimus, mycophenolate, and steroids was the most common regimen for initial immunosuppression for 68% of recipients[18].
Calcineurin inhibitors (CNIs) such as tacrolimus and cyclosporine have nuanced mechanisms of action as it relates to their hypertensive properties, including activation of the renin-angiotensin system, increased activity of the thiazide-sensitive sodium chloride cotransporters as well as vasoconstrictive effects by way of increased endothelin production in larger preglomerular arteries[19]. There has also been found to be intragroup variability for CNIs in regards to the effect on systemic vascular resistance and sodium-dependent hypertension with clinically relevant outcomes of less hypertension for patients treated with tacrolimus when compared to cyclosporine[20,21]. CNI-associated dyslipidemias have also been observed due to the interference of LDL cholesterol binding to the LDL receptor, thus reducing its clearance[22]. These dyslipidemias too have intraclass variability between tacrolimus and cyclosporine, with tacrolimus-based regimens having less incidence of hyperlipidemias at 6 months post-transplant compared to cyclosporine-based regimens[23]. The use of CNIs also imposes impairment in insulin secretion with pancreatic islet-cell toxicity thus, CNIs are also associated with glucose intolerance and post-transplant diabetes mellitus (PTDM) again with intraclass variability in incidence, though tacrolimus-based regimens portend greater numerical incidence of PTDM compared to cyclosporine-based regimens[22,24]. Beyond these traditional metabolic cardiovascular risk factors exacerbated by the use of CNIs, the use of CNIs has been found to be associated with direct cardiovascular toxicity by such mechanisms and their deleterious effects on endothelial dysfunction and arterial stiffness[25]. Concerning post-transplant proteinuria, CNIs appear to have antiproteinuric effects by way of stabilization of the kidney filtration barrier and inhibition of intracellular signaling of nuclear factor activated T-cells that can result in podocyte damage and increased proteinuria[26,27].
The antimetabolite mycophenolate mofetil or its active metabolite mycophenolic acid has been well studied in its association with the development of dose-dependent anemia in KTRs by way of bone marrow aplasia as well as pure red cell aplasia likely due to their antiproliferative effects[28]. Anemia due to pure red cell aplasia in patients treated with tacrolimus and azathioprine have also rarely been observed[29,30]. Additional triggers for worsening anemia for KTRs include opportunistic viral infections [e.g. Parvovirus B19, Epstein-Barr virus, cytomegalovirus (CMV)], use of mammalian target of rapamycin (mTOR) inhibitors, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and prophylactic medications such as valganciclovir and trimethoprim- sulfamethoxazole[28,31]. Chronic anemia has been associated with the development of congestive heart failure in KTRs, as it may lead to maladaptive left ventricular hypertrophy (LVH) and cavitary enlargement[32]. This LVH is highly associated with increased cardiovascular morbidity and mortality[25].
Corticosteroids have also been strongly associated with metabolic derangements post-kidney transplant, particularly with resultant hyperglycemia and hyperlipidemia[33,34]. Corticosteroid utilization as part of the KTR immunosuppression regimen has also been associated with increased average blood pressure readings that are improved alongside their corresponding lipid profiles and incidence of PTDM with corticosteroid reduction[35].
Turning to the less often but still commonly utilized mTOR inhibitors, this class of immunosuppressants has been well known to have an association with worsening proteinuria, often times with nephrotic syndrome[36]. The hypothesis on the mechanism of worsening proteinuria with mTOR inhibitor initiation in KTR is that it may be due to induction of de novo focal segmental glomerulosclerosis by the mTOR inhibitor causing dose dependent changes on the podocyte cytoskeleton and slit diaphragm due to reduction in CD2 associated protein (CD2ap), nephrin, WT1 protein, and podocin protein expression[37]. The use of mTOR inhibitors has also been implicated in PTDM and dyslipidemia[38,39].
Kidney allograft rejection is associated with inflammation, and an inflammatory state has been found in clinical studies to lead to atherosclerosis and arteriosclerosis[40,41]. The process of transplantation in itself an inflammatory process beginning with the initial ischemia-reperfusion injury with the initial surgical organ acquisition and allograft perfusion that promotes the release of damage-associated molecular patterns that activate the alloimmune recognition and sensitization processes by the host’s innate and adaptive immune system[42]. The circulation of so called de novo donor-specific antibodies that typify antibody-mediated rejection episodes in KTR has also been pointed to as immune-mediated cause of accelerated arteriosclerosis[43]. A recent retrospective cohort study of 553 KTRs conducted in Bangkok, Thailand, found that the risk of a post-transplant cardiovascular event was increased by a factor of 3 for a history of a T-cell mediated rejection and by a factor of 3.38 for a history of antibody mediated rejection[44]. The role on a non-HLA antibody in acute rejection and hypertension has also been previously elucidated. In a seminal study, Dragun et al[45] demonstrated that KTRs with vascular transplant rejection and accelerated hypertension without anti-HLA antibody had pathogenic antibodies against some angiotensin type 1 receptor epitopes.
CMV is a DNA virus from the Herpesviridae, with strong evidence available that it is a significant risk factor for cardiovascular disease[46]. This assertion is built on prior studies that atherosclerosis is the underlying disorder in multiple aspects of cardiovascular disease[47]. Previous infection with CMV has been associated with an increased risk of coronary thrombotic events after stent placement in immunocompetent individuals as well[48,49]. The use of mTOR inhibitors over other immunosuppressants has been associated with reduced rates of CMV infection following kidney transplantation, as mTOR inhibitors are thought to improve T-cell fitness[50].
As can be gleaned through the course of this overview, there are several transplant specific risk factors for cardiovascular disease in KTRs that can be attributed to the ongoing need for immunosuppression. Thus, we are in agreement with the assertion that as much as possible the regimen should be tailored to the specific clinical characteristics of the KTRs[51]. Patients of higher cardiovascular and lower immunological risk may be given consideration for a steroid-free regimen or early steroid discontinuation regimen for maintenance. Given the dangers detailed above of the aforementioned immunosuppressants (e.g., particularly of CNIs), treatment with novel immunosuppressant agent belatacept has been seen as a promising alternative to permit CNI avoidance while minimizing risk for allograft rejection in select patients of lower immunological risk for rejection[52]. Belatacept is a blocker of the second signal in the pathway for T-cell activation by binding to CD80/CD86 and improved graft outcomes with positive effects on the cardiovascular risk profile when compared to CNIs, albeit with increased incidence of early acute rejections[53,54]. On long-term analysis, the use of belatacept was associated with an increased incidence of proteinuria and increased CMV reactivation infections in KTRs when compared to CNI-based regimens[55,56]. In conclusion, further investigation on additional means to stratify cardiovascular risk in KTRs needs to occur to customize therapy plans and optimize the need for immunosuppressants while mitigating potential adverse events.
| 1. | Burton H, Iyamu Perisanidou L, Steenkamp R, Evans R, Mumford L, Evans KM, Caskey FJ, Hilton R. Causes of renal allograft failure in the UK: trends in UK Renal Registry and National Health Service Blood and Transplant data from 2000 to 2013. Nephrol Dial Transplant. 2019;34:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, Navaneethan SD, Ramanathan V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996-2014). Am J Nephrol. 2018;48:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3261] [Cited by in RCA: 3194] [Article Influence: 199.6] [Reference Citation Analysis (0)] |
| 4. | Kramer A, Pippias M, Noordzij M, Stel VS, Afentakis N, Ambühl PM, Andrusev AM, Fuster EA, Arribas Monzón FE, Åsberg A, Barbullushi M, Bonthuis M, Caskey FJ, Castro de la Nuez P, Cernevskis H, des Grottes JM, Garneata L, Golan E, Hemmelder MH, Ioannou K, Jarraya F, Kolesnyk M, Komissarov K, Lassalle M, Macario F, Mahillo-Duran B, Martín de Francisco AL, Palsson R, Pechter Ü, Resic H, Rutkowski B, Santiuste de Pablos C, Seyahi N, Simic Ogrizovic S, Slon Roblero MF, Spustova V, Stojceva-Taneva O, Traynor J, Massy ZA, Jager KJ. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: a summary. Clin Kidney J. 2018;11:108-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Liefeldt L, Budde K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl Int. 2010;23:1191-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Birdwell KA, Park M. Post-Transplant Cardiovascular Disease. Clin J Am Soc Nephrol. 2021;16:1878-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O'Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 292] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Kasiske B, Cosio FG, Beto J, Bolton K, Chavers BM, Grimm R Jr, Levin A, Masri B, Parekh R, Wanner C, Wheeler DC, Wilson PW; National Kidney Foundation. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant. 2004;4 Suppl 7:13-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Aziz F, Jorgenson M, Garg N, Parajuli S, Mohamed M, Raza F, Mandelbrot D, Djamali A, Dhingra R. New Approaches to Cardiovascular Disease and Its Management in Kidney Transplant Recipients. Transplantation. 2022;106:1143-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Aziz F, Clark D, Garg N, Mandelbrot D, Djamali A. Hypertension guidelines: How do they apply to kidney transplant recipients. Transplant Rev (Orlando). 2018;32:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol. 2019;32:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, Kasiske BL, Kim SJ, Kusek JW, Bostom AG. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12:2437-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Pihlstrøm H, Dahle DO, Mjøen G, Pilz S, März W, Abedini S, Holme I, Fellström B, Jardine AG, Holdaas H. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation. 2015;99:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Hiremath S, Fergusson D, Doucette S, Mulay AV, Knoll GA. Renin angiotensin system blockade in kidney transplantation: a systematic review of the evidence. Am J Transplant. 2007;7:2350-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Fernández-Fresnedo G, Plaza JJ, Sánchez-Plumed J, Sanz-Guajardo A, Palomar-Fontanet R, Arias M. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant. 2004;19 Suppl 3:iii47-iii51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Peddi VR, Dean DE, Hariharan S, Cavallo T, Schroeder TJ, First MR. Proteinuria following renal transplantation: correlation with histopathology and outcome. Transplant Proc. 1997;29:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Fernández-Fresnedo G, Escallada R, Rodrigo E, Piñera C, de Francisco AL, Cotorruelo JG, Sanz de Castro S, Arias M. Proteinuria is an independent risk factor of cardiovascular disease in renal transplant patient. Transplant Proc. 2002;34:367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lentine KL, Smith JM, Lyden GR, Miller JM, Booker SE, Dolan TG, Temple KR, Weiss S, Handarova D, Israni AK, Snyder JJ. OPTN/SRTR 2023 Annual Data Report: Kidney. Am J Transplant. 2025;25:S22-S137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 72] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 19. | Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. 2012;25:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Textor SC, Wiesner R, Wilson DJ, Porayko M, Romero JC, Burnett JC Jr, Gores G, Hay E, Dickson ER, Krom RA. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, Moons P, Borm G, Hilbrands LB. Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. Am J Transplant. 2004;4:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Morales JM, Domínguez-Gil B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol. 2006;17:S296-S303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, Metzger R, Shield C 3rd, Rocher L, Scandling J, Sorensen J, Mulloy L, Light J, Corwin C, Danovitch G, Wachs M, van Veldhuisen P, Salm K, Tolzman D, Fitzsimmons WE. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation. 2000;69:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, Hardy M, Metzger R, Shield C 3rd, Rocher L, Scandling J, Sorensen J, Mulloy L, Light J, Corwin C, Danovitch G, Wachs M, VanVeldhuisen P, Leonhardt M, Fitzsimmons WE. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 2003;75:2048-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Paccagnella C, Andreola S, Gambaro A, Gambaro G, Caletti C. Immunosuppressive Therapy-Related Cardiovascular Risk Factors in Renal Transplantation: A Narrative Review. Cardiorenal Med. 2025;15:209-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 786] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 27. | Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179:1719-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Yang Y, Yu B, Chen Y. Blood disorders typically associated with renal transplantation. Front Cell Dev Biol. 2015;3:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Gregoor PS, Weimar W. Tacrolimus and pure red-cell aplasia. Am J Transplant. 2005;5:195-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Zuber J, Beldjord K, Casadevall N, Thervet E, Legendre C, Varet B. Immune-mediated pure red cell aplasia in renal transplant recipients. Haematologica. 2008;93:1750-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Khalil MAM, Khalil MAU, Khan TFT, Tan J. Drug-Induced Hematological Cytopenia in Kidney Transplantation and the Challenges It Poses for Kidney Transplant Physicians. J Transplant. 2018;2018:9429265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol. 2002;13:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int J Mol Sci. 2021;22:623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 34. | Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 357] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Kishikawa H, Nishimura K, Soda T, Yamanaka K, Hirai T, Kyo M, Takeda M, Fujisawa M, Kokado Y, Ichikawa Y. Low-dose steroid maintenance for renal transplant recipients. Transplant Proc. 2010;42:4030-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Morelon E, Kreis H. Sirolimus therapy without calcineurin inhibitors: Necker Hospital 8-year experience. Transplant Proc. 2003;35:52S-57S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Stallone G, Infante B, Pontrelli P, Gigante M, Montemurno E, Loverre A, Rossini M, Schena FP, Grandaliano G, Gesualdo L. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation. 2011;91:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, Strom EH, Jardine A, Midtvedt K, Machein U, Ulbricht B, Karpov A, O'Connell PJ; ASCERTAIN Investigators. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation. 2011;92:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Shivaswamy V, Boerner B, Larsen J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr Rev. 2016;37:37-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Ponticelli C, Campise MR. The inflammatory state is a risk factor for cardiovascular disease and graft fibrosis in kidney transplantation. Kidney Int. 2021;100:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, Duong van Huyen JP, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 838] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 42. | Li Q, Lan P. Activation of immune signals during organ transplantation. Signal Transduct Target Ther. 2023;8:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 43. | Loupy A, Vernerey D, Viglietti D, Aubert O, Duong Van Huyen JP, Empana JP, Bruneval P, Glotz D, Legendre C, Jouven X, Lefaucheur C. Determinants and Outcomes of Accelerated Arteriosclerosis: Major Impact of Circulating Antibodies. Circ Res. 2015;117:470-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Amornkanjanawat P, Kerr SJ, Wuttiputhanun T, Townamchai N, Leelahavanichkul A, Tantiyavarong P, Praditpornsilpa K, Eiam-Ong S, Avihingsanon Y, Udomkarnjananun S. Kidney Allograft Rejection as an Independent Nontraditional Risk Factor for Post-Transplant Cardiovascular Events. Kidney360. 2025;6:1176-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 664] [Article Influence: 31.6] [Reference Citation Analysis (10)] |
| 46. | Wang H, Peng G, Bai J, He B, Huang K, Hu X, Liu D. Cytomegalovirus Infection and Relative Risk of Cardiovascular Disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A Meta-Analysis of Prospective Studies Up to 2016. J Am Heart Assoc. 2017;6:e005025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 47. | Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6994] [Cited by in RCA: 7502] [Article Influence: 1250.3] [Reference Citation Analysis (0)] |
| 48. | Neumann FJ, Kastrati A, Miethke T, Pogatsa-Murray G, Seyfarth M, Schömig A. Previous cytomegalovirus infection and risk of coronary thrombotic events after stent placement. Circulation. 2000;101:11-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Kazory A, Ducloux D, Coaquette A, Manzoni P, Chalopin JM. Cytomegalovirus-associated venous thromboembolism in renal transplant recipients: a report of 7 cases. Transplantation. 2004;77:597-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Kaminski H, Marseres G, Yared N, Nokin MJ, Pitard V, Zouine A, Garrigue I, Loizon S, Capone M, Gauthereau X, Mamani-Matsuda M, Coueron R, Durán RV, Pinson B, Pellegrin I, Thiébaut R, Couzi L, Merville P, Déchanet-Merville J. mTOR Inhibitors Prevent CMV Infection through the Restoration of Functional αβ and γδ T cells in Kidney Transplantation. J Am Soc Nephrol. 2022;33:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Reggiani F, Moroni G, Ponticelli C. Cardiovascular Risk after Kidney Transplantation: Causes and Current Approaches to a Relevant Burden. J Pers Med. 2022;12:1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 52. | Schulte K, Vollmer C, Klasen V, Bräsen JH, Püchel J, Borzikowsky C, Kunzendorf U, Feldkamp T. Late conversion from tacrolimus to a belatacept-based immuno-suppression regime in kidney transplant recipients improves renal function, acid-base derangement and mineral-bone metabolism. J Nephrol. 2017;30:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 738] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 54. | Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial Mdel C, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyó J. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 55. | Divard G, Aubert O, Debiais-Deschamp C, Raynaud M, Goutaudier V, Sablik M, Sayeg C, Legendre C, Obert J, Anglicheau D, Lefaucheur C, Loupy A. Long-Term Outcomes after Conversion to a Belatacept-Based Immunosuppression in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2024;19:628-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 56. | Zuber J, Leon J, Déchanet-Merville J, Kaminski H. Belatacept-related cytomegalovirus infection: Advocacy for tailored immunosuppression based on individual assessment of immune fitness. Am J Transplant. 2025;25:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/