Published online Dec 25, 2025. doi: 10.5527/wjn.v14.i4.103649
Revised: March 23, 2025
Accepted: April 11, 2025
Published online: December 25, 2025
Processing time: 392 Days and 14.4 Hours
This article comments on Varatharajan et al recent article, highlighting the role of tubulointerstitial damage mechanisms in diabetic nephropathy progression. Evidence suggests a bidirectional interaction between the interstitium, tubular cells, and glomeruli. Renal tubules are highly susceptible to proteinuria, metabolic disorders, and toxins. Since diabetic nephropathy persistently activates inflammatory and fibrotic pathways, epithelial-to-mesenchymal transition mechanisms present promising targets for risk assessment. Periostin, a cellular matrix protein, plays a key role in modulating extracellular interactions. Increased periostin ex
Core Tip: Diabetic kidney disease is characterized by albuminuria and progressive deterioration of renal function; however, traditional markers may fail to categorize a substantial proportion of patients who do not exhibit albuminuria. Tubulointerstitial biomarkers have been proven to be particularly valuable in predicting the onset of albuminuria and the progression of kidney injury in patients with type 2 diabetes. The standardization of biomarker measurements, including neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and periostin, can potentially enhance prognostic indicators for diabetic kidney disease.
- Citation: Alamilla-Sanchez M, Yama Estrella MB, Morales López EF, Delgado Pineda D. Predictive value of biomarkers for tubulointerstitial and glomerular interactions in diabetic nephropathy. World J Nephrol 2025; 14(4): 103649
- URL: https://www.wjgnet.com/2220-6124/full/v14/i4/103649.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i4.103649
Diabetic kidney disease is a leading cause of chronic kidney disease (CKD). Traditional markers such as albuminuria and estimated glomerular filtration rate (eGFR) primarily assess glomerular damage but may fail to detect early tubulointerstitial injury. Studies indicate that up to 47% of patients with type 2 diabetes (T2D) reach CKD stage 3 without al
Biomarkers such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), periostin, and monocyte chemoattractant protein-1 offer a more precise evaluation of renal injury. The predictive power of these biomarkers is adequate, even after adjusting for conventional risk factors such as systolic blood pressure, anemia, serum creatinine, albuminuria, and body mass index[5]. Tubulointerstitial damage often correlates more strongly with eGFR decline than glomerular injury[6,7]. The activation of pathways like Wnt/β-catenin, nuclear factor (erythroid-derived 2)-like factor 2/antioxidant responsive element, and signal transducer and activator of transcription 3/nuclear factor kappa B accelerates renal fibrosis. Experimental models confirm that interventions targeting inflammatory pathways can mitigate fibrosis and CKD progression[8].
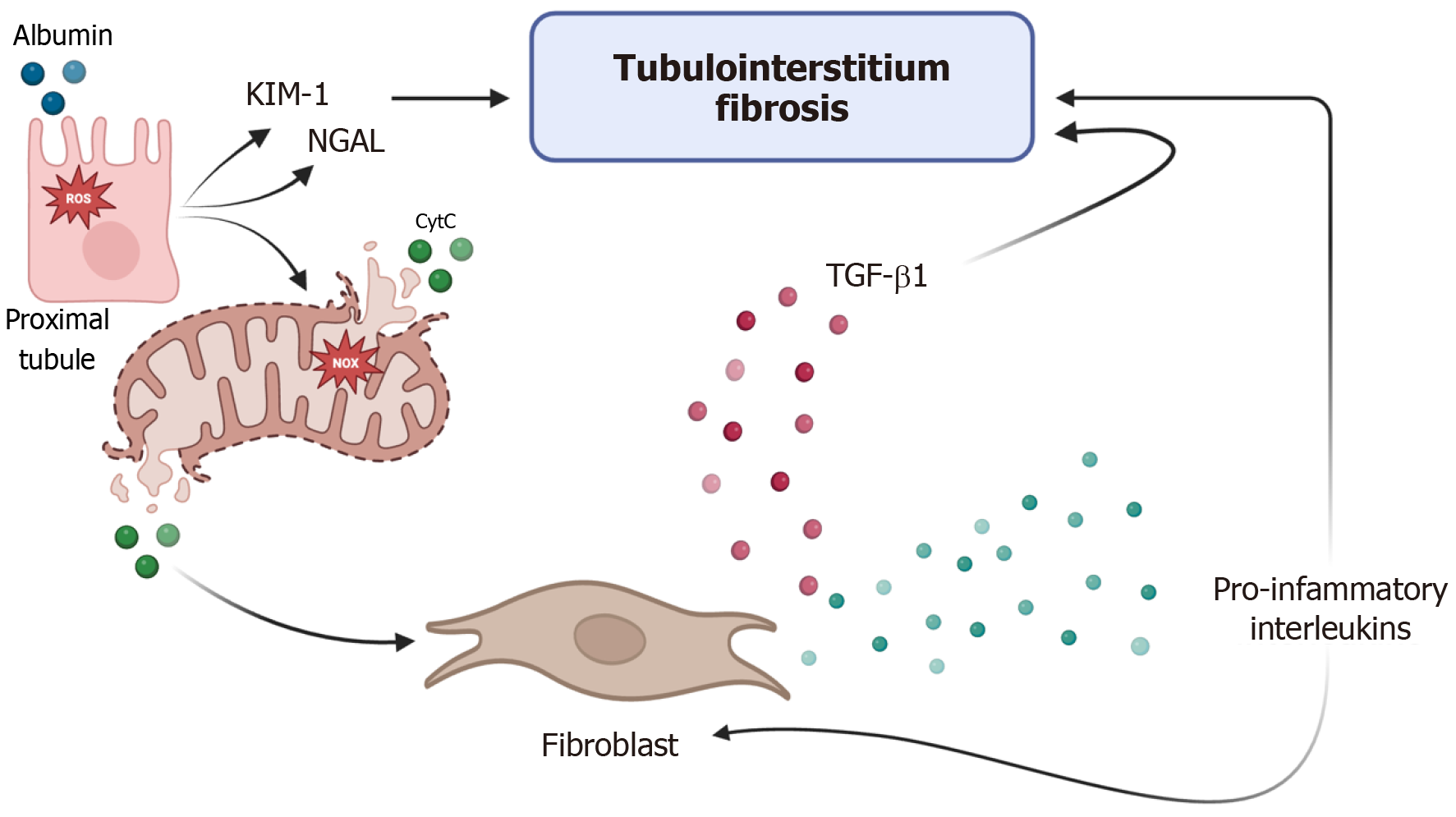
Proteinuria exacerbates tubulointerstitial injury by impairing proximal tubule receptor-mediated endocytosis, leading to oxidative stress and mitochondrial dysfunction[9,10]. This cascade activates fibroblasts and pro-inflammatory cytokines, driving progressive renal fibrosis. KIM-1, a biomarker of proximal tubular injury, further perpetuates this cycle (Figure 1). Inflammation may favor the initiation of harmful mechanisms in tubular cells and the interstitium. Considerable albumin reabsorption by the proximal tubule activates the RAS-related C3 botulinum toxin substrate 1-induced nicotinamide adenine dinucleotide phosphate oxidase pathway. Nicotinamide adenine dinucleotide phosphate oxidase causes mitochondrial dysfunction, increases the production and release of cytochrome C, and triggers cell apoptosis pathways[11-13].
NGAL, a widely studied marker of acute kidney injury, also correlates with CKD progression in diabetes[14]. Elevated urinary NGAL levels predict eGFR decline and albuminuria onset. In a study that included 952 patients with T2D without albuminuria or hypertension, the area under the receiver operating characteristic curve value for the NGAL/Creatinine ratio was 0.51 for the early detection of CKD, thus indicating a direct correlation with the log eGFR (r = 0.28, P < 0.001)[15]. A meta-analysis demonstrated NGAL’s sensitivity (0.79) and specificity (0.87) in detecting diabetic kidney disease[16]. KIM-1 is undetectable in healthy kidneys but is highly expressed in injured proximal tubules[17]. Nephroprotective medications, such as renin-angiotensin-aldosterone system inhibitors, can reduce urine KIM-1 excretion[18]. Its levels correlate with worsening renal function and albuminuria severity[19]. Nephroprotective therapies such as renin-angiotensin-aldosterone system inhibitors have been shown to reduce KIM-1 excretion. Periostin (POSTN) is a 90 kDa cellular matrix protein that was initially described in osteoblasts. It is also known as osteoblast-stimulating factor 2[20]. POSTN modulates the interaction between cells and the extracellular matrix; thus, it plays an essential role in regeneration, remodeling, and fibrosis[21]. Studies reveal increased urinary POSTN levels in T2D patients, independent of albuminuria. Its diagnostic accuracy is high, with an area under the receiver operating characteristic curve value of 0.78-0.99 for distinguishing different diabetic nephropathy stages; the cutoff levels for urine POSTN/Creatinine that showed the best performance were 1.39 ng/mg to distinguish normoalbuminuric T2D patients from healthy controls[22].
Current risk stratification for diabetic nephropathy relies on albuminuria and eGFR. However, tubulointerstitial biomarkers could enable earlier intervention, particularly in normoalbuminuric patients. Standardizing biomarker measurements may enhance prognostic capabilities and guide individualized treatment strategies.
NGAL, KIM-1, and POSTN are promising biomarkers for diabetic nephropathy risk assessment. While currently used for research purposes, they hold potential for early disease stratification and therapeutic guidance.
| 1. | Varatharajan S, Jain V, Pyati AK, Neeradi C, Reddy KS, Pallavali JR, Pandiyaraj IP, Gaur A. Neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and periostin: Novel urinary biomarkers in diabetic nephropathy. World J Nephrol. 2024;13:98880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Persson F, Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl (2011). 2018;8:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 3. | Młynarska E, Buławska D, Czarnik W, Hajdys J, Majchrowicz G, Prusinowski F, Stabrawa M, Rysz J, Franczyk B. Novel Insights into Diabetic Kidney Disease. Int J Mol Sci. 2024;25:10222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 4. | Xing J, Huang L, Ren W, Mei X. Risk factors for rapid kidney function decline in diabetes patients. Ren Fail. 2024;46:2398188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel Tubular Biomarkers Predict Renal Progression in Type 2 Diabetes Mellitus: A Prospective Cohort Study. J Diabetes Res. 2016;2016:3102962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005;S82-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 892] [Article Influence: 42.5] [Reference Citation Analysis (1)] |
| 8. | Zhang JQ, Li YY, Zhang XY, Tian ZH, Liu C, Wang ST, Zhang FR. Cellular senescence of renal tubular epithelial cells in renal fibrosis. Front Endocrinol (Lausanne). 2023;14:1085605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Fatah H, Benfaed N, Chana RS, Chunara MH, Barratt J, Baines RJ, Brunskill NJ. Reduced proximal tubular expression of protein endocytic receptors in proteinuria is associated with urinary receptor shedding. Nephrol Dial Transplant. 2018;33:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Bedin M, Boyer O, Servais A, Li Y, Villoing-Gaudé L, Tête MJ, Cambier A, Hogan J, Baudouin V, Krid S, Bensman A, Lammens F, Louillet F, Ranchin B, Vigneau C, Bouteau I, Isnard-Bagnis C, Mache CJ, Schäfer T, Pape L, Gödel M, Huber TB, Benz M, Klaus G, Hansen M, Latta K, Gribouval O, Morinière V, Tournant C, Grohmann M, Kuhn E, Wagner T, Bole-Feysot C, Jabot-Hanin F, Nitschké P, Ahluwalia TS, Köttgen A, Andersen CBF, Bergmann C, Antignac C, Simons M. Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J Clin Invest. 2020;130:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Erkan E, Devarajan P, Schwartz GJ. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol. 2007;18:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014;25:1237-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 13. | Wolf G, Schroeder R, Ziyadeh FN, Stahl RA. Albumin up-regulates the type II transforming growth factor-beta receptor in cultured proximal tubular cells. Kidney Int. 2004;66:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Mahapatra HS, Kulshreshtha B, Goyal P, Chitkara A, Kumari A, Arora A, Sekhar V, Gupta YP. Comparative diagnostic utility of different urinary biomarkers during pre-albuminuric stages of non-hypertensive type 2 diabetic nephropathy. Indian J Med Res. 2022;156:46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | He P, Bai M, Hu JP, Dong C, Sun S, Huang C. Significance of Neutrophil Gelatinase-Associated Lipocalin as a Biomarker for the Diagnosis of Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press Res. 2020;45:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1381] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 18. | Nielsen SE, Rossing K, Hess G, Zdunek D, Jensen BR, Parving HH, Rossing P. The effect of RAAS blockade on markers of renal tubular damage in diabetic nephropathy: u-NGAL, u-KIM1 and u-LFABP. Scand J Clin Lab Invest. 2012;72:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Żyłka A, Dumnicka P, Kuśnierz-Cabala B, Gala-Błądzińska A, Ceranowicz P, Kucharz J, Ząbek-Adamska A, Maziarz B, Drożdż R, Kuźniewski M. Markers of Glomerular and Tubular Damage in the Early Stage of Kidney Disease in Type 2 Diabetic Patients. Mediators Inflamm. 2018;2018:7659243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014;37:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant. 2012;27:2702-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One. 2015;10:e0124055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/