Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.100896
Revised: February 19, 2025
Accepted: March 17, 2025
Published online: June 25, 2025
Processing time: 223 Days and 3.1 Hours
Kidney is the vital organ that plays a great role in maintaining an optimal internal environment. The normal kidney function can be altered by physical injury or disease. Currently, chronic kidney disease (CKD) is an increasing major health problem worldwide. In 2017, it was ranked as the 12th leading cause of death and is expected to rise to the 5th ranked cause of death by 2040. Therefore, early detection, increasing patients' awareness and treatment of CKD are required to hold the problem. However, despite its higher prevalence of hospitalized mor
To determine the magnitude, and associated factors of CKD in WKUSTH, Ethiopia.
Institutional based cross-sectional study with secondary data was conducted from November 15, 2021 to February 28, 2022 at WKUSTH. Three hundred forty five (345) participants were selected by a convenient sampling technique. Creatinine and urea were measured using cobas311 fully automated chemistry analyzer and estimated glomerular filtration rate (eGFR) was calculated using CKD epidemiology collaboration formula. Socio-demographic and clinical data were collected by using a pretested questionnaire. Data were coded and entered into EpiData 3.1 version and exported to STATA version 14 for analysis. Bivariate analysis was used to screen candidate variables for multivariate analysis. In the multivariate analysis a P value < 0.05 were considered statistically significant.
The magnitude of CKD by impaired eGFR were 54 (15.7%) (95%CI: 0.116-0.194). In multivariable analysis, older age [adjusted odds ratio (AOR) = 5.91, 95%CI: 2.41-14.47)], hypertension (AOR =10.41, 95%CI: 4.55-23.81), diabetes mellitus (AOR = 5.90, 95%CI: 2.14-16.23), high body mass index (AOR = 3.0, 95%CI: 1.30-7.27), and anemia (AOR = 2.94, 95%CI: 1.26-6.88) were independently associated with CKD.
The magnitude of CKD among adult patients admitted to WKUSTH was high. Hence, researchers need to do a population-based study and longitudinal study on the magnitude of CKD, associated factors. Estimation of GFR for all hospitalized patients might help to early detection of CKD and prevent complications.
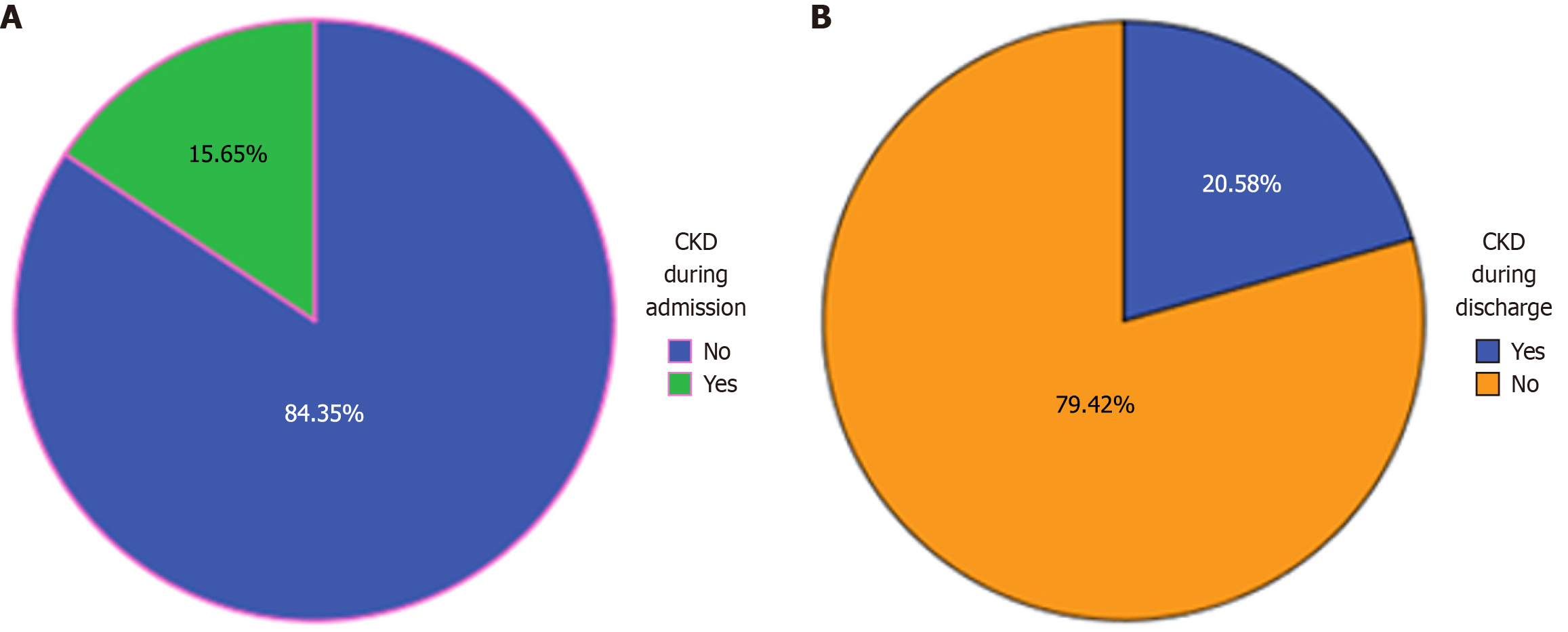
Core Tip: The core tip of this manuscript is to assess chronic kidney disease (CKD) and associated factors at Wolkite University Specialized Hospital. Accordingly, during admission 54 (15.7%) medical ward admitted patients with 95%CI: 0.116-0.194) had CKD by impaired estimated glomerular filtration rate (eGFR). While during discharge 71 (20.58%) medical ward admitted patients with 95%CI: 0.165-0.249) of the patients had CKD by impaired eGFR. This implies about 4.95% of the admitted patients develop kidney disease in hospital during their stay. Therefore, especially, for this resource limited country, screening of kidney disease for admitted patients is essential.
- Citation: Habtu BF, Obsa F, Cheneke W, Asaye S, Nuru A, Hajikelil Z. Assessment of chronic kidney disease and associated factors at Wolkite University Specialized Hospital: A cross-sectional study. World J Nephrol 2025; 14(2): 100896
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/100896.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.100896
The kidneys are highly active metabolic organs that are essential for preserving a stable internal environment. One of their primary functions is regulating the body's fluid and electrolyte balance through processes such as filtration, reabsorption, and the elimination of waste products[1]. The kidneys generate urine through glomerular filtration, followed by the tubular reabsorption of solutes and water. However, physical injury or disease can disrupt this normal function, potentially resulting in acute or chronic kidney disease (CKD)[2]. A slow decline of kidney function or persistent kidney dysfunction can lead to CKD[3].
CKD is a non-communicable disease characterized by kidney damage or a glomerular filtration rate (GFR) below 60 mL/minute/1.73 m² for a minimum of three months[4]. When the kidneys become damaged, they lose their ability to effectively filter blood and eliminate waste, resulting in the buildup of toxic substances and excess fluid in the body[5]. A GFR of less than 60 mL/minute/1.73 m² serves as the primary biomarker for impaired kidney function, while a urine albumin-to-creatinine ratio greater than 30 mg/g is the key indicator of kidney damage[6].
A GFR below 60 mL/minute/1.73 m² indicates a loss of at least half of kidney function and is linked to a higher risk of systemic complications[7,8]. Therefore, assessing GFR is valuable for the early detection of renal impairment, monitoring kidney function, and determining appropriate drug dosages[9]. Creatinine clearance is the typical method for determining GFR, which is calculated from serum creatinine concentration, a 24-hour collected urine creatinine concentration, and a 24-hour measured urine volume. However, the feasibility of precise urine collection is a major limitation of creatinine clearance as a measure of GFR; hence, GFR is mathematically estimated. For this study, CKD epidemiology collaboration (CKD-EPI) equation is used to estimate GFR[9].
Globally, the burden of CKD is on the rise, leading to increased hospitalization, morbidity, and mortality[10]. In 2017, 1.2 million people were died from CKD with estimated increment of this number up to 4.0 million in a worst-case scenario by 2040 and it was ranked as the 12th leading cause of death in 2017 and is anticipated to become the 5th leading cause by 2040[11]. CVD, hypertension, anemia, malnutrition, and mineral and bone disorders are some of the complications of CKD[12].
CKD is a significant public health issue and poses a substantial economic burden. It leads to higher hospitalization rates, increased morbidity, premature mortality, and reduced productivity for both patients and their caregivers[13,14]. Patients with CKD also have a high risk of progression to end-stage renal disease (ESRD). The management of ESRD is extremely costly, as it necessitates either dialysis or a kidney transplant[6]. Undergoing dialysis imposes a significant burden, both in terms of reduced quality of life and financial costs[15]. In developed nations, the treatment of ESRD accounts for over 2%-3% of their yearly healthcare budget[16]. Their annual medical expenses for managing ESRD range from $20110 to $100593 per patient[13].
Even though both the incidence and prevalence of CKD appear to be increasing globally, the rate of increase is much higher in African countries; this is probably a result of poverty, a high incidence of non-communicable and communi
Thus, early detection of CKD is crucial, especially in this resource-limited country, where access to renal replacement therapy is severely restricted, to help prevent or slow its progression to ESRD. However, despite its high prevalence and its subsequent increased hospitalized morbidity and mortality, there is a scarcity of data in Ethiopia especially in study area hospitalized patients. Hence, the finding of this research will serve as a foundational step for future research, such as longitudinal or community-based studies.
A facility based cross-sectional study with secondary data was conducted from November 15, 2021, to February 28, 2022, in Wolkite University Specialized Hospital (WKUSTH). WKUSTH is located in the Central Ethiopia, Gurage Zone, Wolkite located 158 km South West of the capital city of Ethiopia, Addis Ababa, on the way to Jimma. The hospital is situated in Gubreye sub-city, 14 km east of Wolkite town. Currently, WKUSTH is offering outpatient, inpatient, surgical, gynecological, and pediatric services.
Aged 18 years or older patients admitted to the medical wards of WKUSTH during the study period and signed on the consent sheet were included. However, patients on the intensive care unit who were unconscious, younger than 18 years old, amputated patient, pregnant women, admitted due to malnutrition, or morbidly obese patient were excluded. The sample size was determined by using a single population proportion formula [N = (Zα/2)2 × P × (1-P)/d2] with an assumption of a 95% confidence level, a 5% margin of error (d), (P = 33.9%) prevalence CKD at Desse referral hospital, Ethiopia[19], (N) = (1.96)2 × 0.339 × (0.661)/(0.05)2, (N) = 345. During the study period, 997 patients were admitted to the adult medical ward. From these admitted patients, a total of 345 patients were selected using consecutive sampling technique.
Experienced nurses and laboratory professionals were trained on the study protocol and data collection format. After detailed information about the study was given, written consent was obtained from all subjects who were included in the study. Data were collected from patients and their medical charts using a pretested, semi-structured face-to-face interview questionnaire that was developed from the World Health Organisation STEPS surveillance manual[20]. The data collection tool contains socio-demographic information, clinical information, lifestyle behaviors, anthropometric measurements, blood pressure measurements, and laboratory findings. Patients were interviewed to collect data on socio-demographic characteristics, clinical information, and lifestyle behaviors by a trained nurse. The clinical information of the patient or comorbidities, like hypertension, diabetes mellitus (DM), cardiovascular disease, and human immunodeficiency virus/acquired immunodeficiency syndrome information, was confirmed by reviewing their medical chart.
Trained clinical nurses took blood pressure and performed anthropometric measurements. After measuring each subject's height and weight, the body mass index (BMI) for each was determined by dividing the weight in kg by the height in m2 and categorized as normal weight (18.5-24.9 kg/m²), overweight (25.0-29.9 kg/m²), and obese (≥ 30 kg/m²). Following at least ten minutes of rest, the patient's blood pressure was measured, and hypertension was defined as being on antihypertensive medication (history of hypertension) or having a systolic and/or diastolic blood pressure ≥ 140/90 mm/Hg. All comorbidities were defined as present if they were documented in the medical records.
Seven mL of venous blood was collected during admission and discharge by trained laboratory professional following standard operation procedure. Five mL of the blood was collected in each study participant during admission and discharge in sterile serum-separating tubes. The collected sample was left to form a clot at room temperature for 30 minutes, and then centrifuged. Biochemical tests such as creatinine and urea levels were analyzed by using cobas c 311 analyzer (cobas-roche Company, Germany) automated clinical chemistry analyzer according to the manufacturer’s instructions and procedures in the laboratory. CKD-EPI Creatinine Equation (2021) was used to calculate GFR. Those having estimated GFR (eGFR) < 60 ml/min/1.73 m2 both during admission and during discharge are considered as having CKD. Two ml of the blood was collected by EDTA tube for hemoglobin determination and determined using CELL DYNE 1800 hematology analyzer and Anemia is defined as hemoglobin < 13 g/dL in men and < 12 g/dL in women. All laboratory measurements were done following the standard procedures recommended by the manufacturer.
Data were coded and entered into EpiData version 3.1 for further data cleaning and to allow consistency and eliminate discrepancies. Then after, it was exported to STATA version 14 software for analysis. Both bivariate and multivariate logistic regression was done. All Variables with a P value of less than 0.25 in the bivariate analysis were included in multivariate logistic regression. A P value < 0.05 was considered statistically significant. Finally, the result was presented using figures, charts, and table.
Questionnaire data quality control was assured by reviewing and checking for errors, completeness, accuracy, and consistency during data collection and before entry into EpiData, and corrective measures were taken. A pretest was done on 5% of the sample size in Butajira hospital. The expiration date of the reagents and lipemic and hemolysis of every sample were checked. Both normal and pathological control was done every day before sample analysis.
A total of 345 individuals participated with 100% response rate. Of the participants, 176 (51.0%) were male, 154 (44.64%), were in the age group above 59 years. The age (mean + SD) of the study participant was 51.64 years ± 17.73 years. Two-third of the participants 216 (62.6%) live in the rural area. About 249 (72.2%) of the study participants were married. Regarding educational status, 160 (46.4%) of the study participants had no formal education. Gender illustrates CKD higher in the male participants 29 (53.7%) than female 25 (46.3%). CKD was also more common on aged admitted patients compared to the counterparts [42 (77.8%) vs 12 (22.2%)] (Table 1).
| Variable | Category | Chronic kidney disease | P value | ||
| Yes (n = 54) | No (n = 291) | Total | |||
| Age | 18-59 | 12 (22.2) | 179 (61.5) | 191 (55.4) | 0.000 |
| ≥ 60 | 42 (77.8) | 112 (38.5) | 154 (44.6) | ||
| Sex | Male | 29 (53.7) | 147 (50.5) | 176 (51.0) | 0.389 |
| Female | 25 (46.3) | 144 (49.5) | 169 (49.0) | ||
| Place of residence | Urban | 18 (33.3) | 111 (38.1) | 129 (37.4) | 0.305 |
| Rural | 36 (66.7) | 180 (61.9) | 216 (62.6) | ||
| Marital status | Married | 38 (70.4) | 211 (72.5) | 249 (72.2) | 0.431 |
| Single | 16 (29.6) | 80 (27.5) | 96 (27.8) | ||
| Place of residence | Urban | 52 (39.4) | 31 (36.9) | 83 (38.4) | |
| Rural | 80 (60.6) | 53 (63.1) | 133 (61.6) | ||
| Educational status | Illiterate | 33 (61.1) | 127 (43.6) | 160 (46.4) | 0.093 |
| Primary | 8 (14.8) | 76 (26.1) | 84 (24.3) | ||
| Secondary | 10 (18.5) | 59 (20.3) | 69 (20) | ||
| Diploma and above | 3 (5.6) | 29 (10) | 32 (9.3) | ||
| Religion | Christian | 29 (53.7) | 105 (36.1) | 134 (38.8) | 0.012 |
| Muslim | 25 (46.3) | 186 (63.9) | 211 (61.2) | ||
| Occupation | Unemployed | 6 (11.1) | 31 (10.7) | 37 (10.7) | 0.539 |
| Government | 5 (9.3) | 29 (10) | 34 (9.9) | ||
| Private | 11 (20.4) | 81 (27.8) | 92 (26.7) | ||
| Farmer | 29 (53.7) | 120 (41.2) | 149 (43.2) | ||
| Daily labor | 2 (3.7) | 13 (4.5) | 15 (4.3) | ||
| House wife | 1 (1.9) | 17 (5.8) | 18 (5.2) | ||
In this study, the primary clinical diagnosis for admission was hypertension, accounting for 86 cases (24.97%), followed by anemia with 82 cases (23.8%), diabetes with 53 cases (15.4%), and cardiac issues with 38 cases (11.0%). CKD was more prevalent among participants with hypertension (39 cases, 72.2%), DM (21 cases, 38.9%), and those who were overweight (28 cases, 51.9%) (Table 2).
| Variable | Category | CKD | P value | ||
| Yes (n = 54) | No (n = 191) | Total | |||
| Family history of CKD | Yes | 7 (13.0) | 2 (0.7) | 9 (2.6) | 0.000 |
| No | 47 (87.0) | 289 (99.3) | 336 (97.4) | ||
| Hypertension | Yes | 39 (72.2) | 47 (16.2) | 86 (24.9) | 0.000 |
| No | 15 (27.8) | 244 (83.8) | 259 (75.1) | ||
| diabetes mellitus | Yes | 21 (38.9) | 32 (11.0) | 53 (15.4) | 0.000 |
| No | 33 (61.1) | 259 (89.0) | 292 (84.6) | ||
| Cardiac problem | Yes | 11 (20.4) | 27 (9.3) | 38 (11.0) | 0.020 |
| No | 43 (79.6) | 264 (90.7) | 307 (89.0) | ||
| Human immunodeficiency virus status | Yes | 1 (1.9) | 9 (3.1) | 10 (2.9) | 0.518 |
| No | 53 (98.1) | 282 (96.9) | 335 (97.1) | ||
| Alcohol consumption | Yes | 10 (18.5) | 21 (7.2) | 31 (9.0) | 0.012 |
| No | 44 (81.5) | 270 (92.8) | 314 (91.0) | ||
| Cigarette smoking | Yes | 7 (13.0) | 12 (4.1) | 19 (5.5) | 0.017 |
| No | 47 (87.0) | 279 (95.9) | 326 (94.5) | ||
| Body mass index | Normal | 19 (35.2) | 231 (79.4) | 250 (72.5) | 0.000 |
| Overweight | 28 (51.9) | 51 (17.5) | 79 (22.9) | ||
| Obese | 7 (13.0) | 9 (3.1) | 16 (4.6) | ||
| Anemia | Yes | 27 (50.3) | 55 (18.9) | 82 (23.8) | 0.000 |
| No | 27 (50.3) | 236 (81.1) | 263 (76.2) | ||
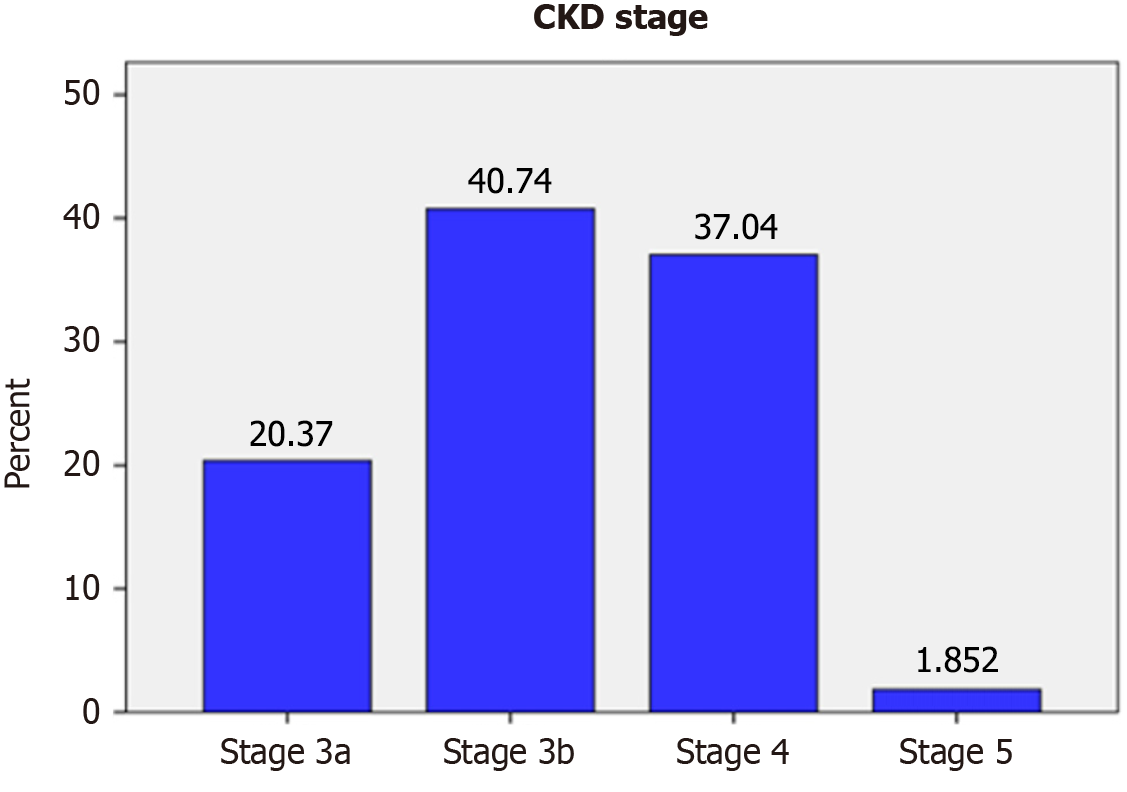
Creatinine levels were measured upon both admission and discharge. At admission, 54 out of the total participants (15.7%) (95%CI: 0.116-0.194) were diagnosed with CKD based on impaired eGFR (Figure 1A). However, by discharge, the number of CKD patients identified by impaired eGFR increased to 71 (20.58%) (95%CI: 0.165-0.249) (Figure 1B). This suggests that approximately 4.9% of admitted patients may have developed acute kidney injury (AKI) during hospitalization. Thus, the prevalence of CKD in this study is 15.7% (95%CI: 0.116-0.194). When categorized by disease stage, the distribution of CKD cases was as follows: (1) Stage 3a: 11 (20.37%); (2) Stage 3b: 22 (40.74%); (3) Stage 4: 20 (37.04%); and (4) Stage 5: 1 (1.85%) (Figure 2).
Before performing logstic regration, the assumptions of the model were verified. The first assumption is that the dependent variable must be categorical. Another assumption is absence of multicollinearity which refers to the relationship among the independent variables. In order to check the existence of multicollinearity, variance inflation factor (VIF) and tolerance (TOL), are calculated and presented in Table 3 below. To confirm the absence of multicollinearity, the value of VIF and TOL should be less than ten and one respectively. As it is shown in the Table 3 below, the value of VIF and TOL are less than ten and one respectively, which implies multicollonearity is not a problem and all the independent variables can be inserted in to the regression model together.
| Model | Collinearity statistics | ||
| Tolerance | Variance inflation factor | ||
| 1 | Sex | 0.864 | 1.157 |
| Age | 0.727 | 1.375 | |
| Family member having chronic kidney disease | 0.905 | 1.105 | |
| History of known hypertension | 0.864 | 1.157 | |
| History of known diabetes mellitus | 0.933 | 1.072 | |
| History of heart problem | 0.883 | 1.132 | |
| Alcohol consumsion | 0.858 | 1.165 | |
| Cigarate smoking | 0.910 | 1.099 | |
| Anemia | 0.915 | 1.093 | |
| Occupation | 0.612 | 1.635 | |
| Education | 0.570 | 1.755 | |
| Body mass index | 0.881 | 1.135 | |
| Place of residence | 0.561 | 1.782 | |
The Omnibus Tests of Model and Hosmer and Lemeshow test are the reliable indication of model fitness in logistic regression. They are interpreted differently. In order to be the model is fit, Omnibus Tests of Model should be significant while Hosmer and Lemeshow test should be insignificant. Accordingly as shown in Tables 4 and 5 below, the value for Omnibus Tests of Model is 0.000 and the Hosmer-Lemeshow Test is 0.542 respectively. So we can conclude that the model is fit.
| χ² | Df | P value | ||
| Step 1 | Step | 154.337 | 13 | 0.000 |
| Block | 154.337 | 13 | 0.000 | |
| Model | 154.337 | 13 | 0.000 | |
After testing the assumptions, bivariate and multivariate logistic regression analyses were conducted to assess the relationship between dependent and independent variables. In the bivariate analysis, several factors showed a significant association with CKD at P <0.25 and were included in the multivariable analysis. These factors included age ≥ 60 years [crude odds ratio (COR) = 5.59, 95%CI: 2.82-11.08], a family history of CKD (COR = 7.32, 95%CI: 1.89-28.22), history of hypertension (COR = 13.49, 95%CI: 6.89-26.44), DM (COR = 5.15, 95%CI: 2.66-9.95), cardiac problems (COR = 3.37, 95%CI: 1.59-7.11), alcohol consumption (COR = 2.92, 95%CI: 1.28-6.61), cigarette smoking (COR = 3.46, 95%CI: 1.29-9.24), BMI ≥ 25 (COR = 3.78, 95%CI: 2.05-6.97), and anemia (COR = 4.29, 95%CI: 2.33-7.89). In the multivariable analysis, older age (adjusted odds ratio (AOR) = 5.91, 95%CI: 2.41-14.47), history of hypertension (AOR = 10.41, 95%CI: 4.55-23.81), DM (AOR = 5.90, 95%CI: 2.14-16.23), high BMI (AOR = 3.0, 95%CI: 1.30-7.27), and anemia (AOR = 2.94, 95%CI: 1.26-6.88) remained independently associated with CKD (Table 6).
| Variable | Category | CKD (n) | Crude OR (95%CI) | Adjusted OR (95%CI) | P value | |
| Yes | No | |||||
| Sex | Male | 29 | 147 | 1.14 (0.63-2.03) | ||
| Female | 25 | 144 | 1 | |||
| Age group | < 60 | 12 | 179 | 1 | 1 | |
| ≥ 60 | 42 | 112 | 5.6 (2.82-11.08) | 5.9 (2.41-14.5) | 0.000 | |
| Residence | Urban | 18 | 111 | 1 | ||
| Rural | 36 | 180 | 1.23 (0.66-2.27) | |||
| Occupational status | Farmer | 29 | 120 | 0.605 (0.34-1.08) | ||
| Other | 25 | 171 | 1 | |||
| Educational status | Illiterate | 33 | 127 | 0.943 (0.27-0.89) | ||
| Literate | 21 | 164 | 1 | |||
| Family member having CKD | Yes | 5 | 4 | 7.3 (1.89-28.22) | 4.0 (0.70-23.19) | 0.118 |
| No | 49 | 287 | 1 | |||
| History of hypertension | Yes | 39 | 47 | 13.5 (6.89-26.44) | 10.4 (4.6-23.81) | 0.000 |
| No | 15 | 244 | 1 | |||
| History of diabetes mellitus | Yes | 21 | 32 | 5.2 (2.66-9.95) | 5.9 (2.14-16.23) | 0.001 |
| No | 33 | 259 | 1 | |||
| History of heart problem | Yes | 13 | 25 | 3.4 (1.59-7.11) | 1.7 (0.58-4.99) | 0.33 |
| No | 41 | 266 | 1 | |||
| Alcohol consumption | Yes | 10 | 21 | 2.9 (1.28-6.61) | 1.4 (0.46-4.31) | 0.533 |
| No | 44 | 270 | 1 | |||
| Cigarette smoking | Yes | 7 | 12 | 3.5 (1.29-9.24) | 1.9 (0.43-8.28) | 0.399 |
| No | 47 | 279 | 1 | |||
| Body mass index | < 25 | 19 | 231 | 1 | 1 | |
| ≥ 25 | 35 | 60 | 3.8 (2.053-6.97) | 3.1 (1.30-7.27) | 0.010 | |
| Anemia | Yes | 27 | 55 | 4.29 (2.33-7.88) | 2.9 (1.26-6.88) | 0.012 |
| No | 27 | 236 | 1 | |||
Currently, various factors such as lifestyle changes and the rising prevalence of non-communicable chronic diseases like hypertension and DM have contributed to CKD for becoming a significant global public health concern. Hospitalized patients, in particular, face a heightened risk of developing CKD due to various factors. In our study, the prevalence of CKD based on impaired eGFR was 15.65% at the time of admission, increasing to 20.58% at discharge. This indicates that approximately 4.9% of hospitalized patients develop kidney disease during their hospital stay. Therefore, screening for kidney disease among admitted patients is crucial. Consequently, this study aimed to assess the prevalence and associated factors of CKD in adult patients admitted to the medical ward at WKUSTH.
Our study indicated that during admission, 54 (15.7%) of patients admitted to the medical ward had CKD based on impaired eGFR, with a 95%CI of 0.116-0.194. This finding was consistent with a study conducted in Botswana 16.3%[21], Uganda 15.3%[22], and London (17.7%)[23]. However, our results were higher than those reported in Canada 8.5%[24] and Brazil 12.7%[25]. This variation could be attributed to differences in population characteristics or a higher proportion of medically complex patients in our study. Conversely, studies from Spain (28.3%)[26], Germany (27.5%)[27], Kenya (38.6%)[28], Guinea (33%)[29], and Jimma University Medical Center (19.2%)[30] reported a higher prevalence of CKD compared to our findings. These discrepancies may be due to, studies conducted in Jimma University Medical Center, Kenya, Spain, and Guinea determined CKD using a single serum creatinine measurement, whereas our study utilized two serum creatinine measurements. This approach helped reduce the overestimation of CKD from 70 cases (20.19%) to 54 cases (15.7%).
In this study, factors associated to CKD included older age, a history of hypertension, DM, BMI, and anemia. Consequently, our findings indicate that hypertensive patients had an approximately tenfold higher risk of developing CKD compared to those without a history of hypertension. This result aligns with a study conducted in Uganda[22], Botswana[21], Kenya[28], Dessie[19], and Jimma[30]. This indicates that patients with previously known hypertensions have higher rates of renal complications.
Our study also identified DM (AOR = 5.90, 95%CI: 2.14-16.23) as a risk factor for CKD. Patients with diabetes had nearly six times the risk of developing CKD compared to those without diabetes. This finding was consistent with a study conducted in Brazil[25], Uganda[22], North-Central Nigeria[31], and Dessie referral hospital[19]. In this study, an age of over 60 years was independently linked to CKD. This finding aligns with previous studies conducted in Brazil[25], Kenya[28], Dessie referral hospital[19], northwest Ethiopia[32], and Jimma University Medical Center[30]. Age-related structural and functional changes in the kidneys or a higher prevalence of renal risk factors, like diabetes, hypertension, and heart disease attributed to the increment of CKD prevalence in aged patients.
Obesity was also identified as a risk factor for CKD, those patients having a BMI greater than 25 were being approximately three times more likely to develop the condition compared to those with a lower BMI. This finding was consistent with a study conducted in Cameroon [33], North-Central Nigeria[31], and Tigray teaching hospitals[34]. Anemia was also independently linked to CKD, aligning with findings from a study conducted in Kenya[28], other factors, such as a family history of kidney disease, a history of heart problems, alcohol consumption, and cigarette smoking, were significant only in the crude analysis.
Although this study has several strengths, it also has some limitations. Since it was conducted in a single hospital using a convenient sampling technique, the findings may not be fully generalizable to the entire population of admitted patients. Additionally, the cross-sectional study design limits our ability to establish a causal relationship between the assessed risk factors and CKD. Furthermore, potential confounding factors such as diet and medication use were not adequately controlled. There is also a possibility of misclassifying AKI as CKD. However, given that hospitalized patients are at a higher risk of developing CKD, our study attempted to diagnose the condition both at admission and discharge. This approach may have helped reduce overestimation and misclassification of CKD.
This study found that the prevalence of CKD among adult patients admitted to the medical ward at WKUSTH was considerably high. Factors such as age over 60 years, high BMI, anemia, and comorbid conditions like hypertension and DM were significantly associated with CKD. Notably, all patients were unaware of their condition at the time of admission, suggesting a potentially high prevalence of kidney disease within the community. To gain a broader understanding, researchers should conduct community-based and longitudinal studies on outpatient populations, incorporating additional confounding variables such as diet and medication history. Estimating GFR for all hospitalized patients could facilitate early CKD detection and help prevent complications. Therefore, healthcare professionals and other stakeholders should provide health education on CKD risk factors, emphasize the benefits of early detection, and regularly monitor GFR in high-risk populations, including hypertensive, elderly, diabetic, anemic, and overweight or obese individuals.
The authors are thankful to Jimma University, Wolkite University Specialized Hospital, study participants, data collectors, and supervisors for the support of the overall process of this study.
| 1. | Bishop ML, Fody EP, Schoeff LE. Clinical Chemistry: Techniques, Principles, and Correlations, Eighth Edition. China: Wolters Kluwer, 2018. |
| 2. | Tanner GA. Kidney function. Medical Physiology-Principles for Clinical Medicine 3rd Edition. In: Rhoades RA, Bell DR, editor. Philadelphia: Lippincott Willams and Wilkins, 2009: 391-418. |
| 3. | Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 1086] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 4. | Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: Part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70:869-876. [PubMed] |
| 5. | Centers for Disease Control and Prevention. Control CfD, Prevention: Chronic kidney disease in the United States. 2021. |
| 6. | Gbaguidi GN, Houehanou CY, Amidou SA, Vigan J, Houinato DS, Lacroix P. Prevalence of abnormal kidney function in a rural population of Benin and associated risk factors. BMC Nephrol. 2021;22:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Girum T, Mesfin D, Bedewi J, Shewangizaw M. The Burden of Noncommunicable Diseases in Ethiopia, 2000-2016: Analysis of Evidence from Global Burden of Disease Study 2016 and Global Health Estimates 2016. Int J Chronic Dis. 2020;2020:3679528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Tegegne B, Demeke T, Amme S, Edmealem A, Ademe S. Knowledge towards Prevention and Early Detection of Chronic Kidney Disease and Associated Factors among Hypertensive Patients at a Chronic Illness Clinic of Jimma Town Public Hospitals. Biomed Res Int. 2020;2020:5969326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | McPherson RA, Msc M, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. Netherlands: Elsevier, 2021. |
| 10. | Haileamlak A. How Can Ethiopia Mitigate the Health Workforce Gap to Meet Universal Health Coverage? Ethiop J Health Sci. 2018;28:249-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4424] [Cited by in RCA: 4368] [Article Influence: 728.0] [Reference Citation Analysis (0)] |
| 12. | Chala G, Sisay T, Teshome Y. Chronic Kidney Disease And Associated Risk Factors Among Cardiovascular Patients. Int J Nephrol Renovasc Dis. 2019;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Elshahat S, Cockwell P, Maxwell AP, Griffin M, O'Brien T, O'Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS One. 2020;15:e0230512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Schrauben SJ, Chen HY, Lin E, Jepson C, Yang W, Scialla JJ, Fischer MJ, Lash JP, Fink JC, Hamm LL, Kanthety R, Rahman M, Feldman HI, Anderson AH; CRIC Study Investigators. Hospitalizations among adults with chronic kidney disease in the United States: A cohort study. PLoS Med. 2020;17:e1003470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Wagnew F, Eshetie S, Kibret GD, Zegeye A, Dessie G, Mulugeta H, Alemu A. Diabetic nephropathy and hypertension in diabetes patients of sub-Saharan countries: a systematic review and meta-analysis. BMC Res Notes. 2018;11:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Kumela Goro K, Desalegn Wolide A, Kerga Dibaba F, Gashe Fufa F, Wakjira Garedow A, Edilu Tufa B, Mulisa Bobasa E. Patient Awareness, Prevalence, and Risk Factors of Chronic Kidney Disease among Diabetes Mellitus and Hypertensive Patients at Jimma University Medical Center, Ethiopia. Biomed Res Int. 2019;2019:2383508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Arogundade FA, Omotoso BA, Adelakun A, Bamikefa T, Ezeugonwa R, Omosule B, Sanusi AA, Balogun RA. Burden of end-stage renal disease in sub-Saharan Africa. Clin Nephrol. 2020;93:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Ashuntantang G, Osafo C, Olowu WA, Arogundade F, Niang A, Porter J, Naicker S, Luyckx VA. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5:e408-e417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Fiseha T, Ahmed E, Chalie S, Gebreweld A. Prevalence and associated factors of impaired renal function and albuminuria among adult patients admitted to a hospital in Northeast Ethiopia. PLoS One. 2021;16:e0246509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | World Health Organization. WHO STEPS surveillance manual: The WHO STEPwise approach to chronic disease risk factor surveillance. 2005. |
| 21. | Rwegerera GM, Bayani M, Taolo EK, Habte D. The prevalence of chronic kidney disease and associated factors among patients admitted at princess marina hospital, Gaborone, Botswana. Niger J Clin Pract. 2017;20:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Kalima N, Gabriel B, Muhindo R, Muyingo A. Chronic kidney disease in patients admitted to the medical ward of Mbarara Regional Referral Hospital in southwestern Uganda: Prevalence and associated factors. IJMBR. 2015;4:107-116. |
| 23. | Annear NM, Banerjee D, Joseph J, Harries TH, Rahman S, Eastwood JB. Prevalence of chronic kidney disease stages 3-5 among acute medical admissions: another opportunity for screening. QJM. 2008;101:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Bohlouli B, Tonelli M, Jackson T, Hemmelgam B, Klarenbach S. Risk of Hospital-Acquired Complications in Patients with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2016;11:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Pinho NA, Silva GV, Pierin AM. Prevalence and factors associated with chronic kidney disease among hospitalized patients in a university hospital in the city of São Paulo, SP, Brazil. J Bras Nefrol. 2015;37:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | de Francisco AL, Fernandez E, Cruz JJ, Casas MT, Gómez-Gerique J, León A, Cava F, Bedini JL, Enguix A, Ripoll E, Borque LA, Fernandez A, Arias M. Under-recognized renal insufficiency in hospitalized patients: implications for care. Eur J Intern Med. 2010;21:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Lüders F, Fürstenberg T, Engelbertz C, Gebauer K, Meyborg M, Malyar NM, Reinecke H. The Impact of Chronic Kidney Disease on Hospitalized Patients With Peripheral Arterial Disease and Critical Limb Ischemia. Angiology. 2017;68:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Mwenda V, Githuku J, Gathecha G, Wambugu BM, Roka ZG, Ong'or WO. Prevalence and factors associated with chronic kidney disease among medical inpatients at the Kenyatta National Hospital, Kenya, 2018: a cross-sectional study. Pan Afr Med J. 2019;33:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Kaba ML, Camara M, Béavogui M, Bah AO, Fousény D, Kourouma ML, Camara A, Diallo AA, Touré YI. Risk factors for chronic kidney disease among patients admitted to the medical wards in Conakry. Saudi J Kidney Dis Transpl. 2016;27:1073-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Adugna T, Merga H, Gudina EK. Impaired glomerular filtration rate, high grade albuminuria and associated factors among adult patients admitted to tertiary Hospital in Ethiopia. BMC Nephrol. 2018;19:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Olanrewaju TO, Aderibigbe A, Popoola AA, Braimoh KT, Buhari MO, Adedoyin OT, Kuranga SA, Biliaminu SA, Chijioke A, Ajape AA, Grobbee DE, Blankestijn PJ, Klipstein-Grobusch K; Ilorin Renal Study Group. Prevalence of chronic kidney disease and risk factors in North-Central Nigeria: a population-based survey. BMC Nephrol. 2020;21:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Damtie S, Biadgo B, Baynes HW, Ambachew S, Melak T, Asmelash D, Abebe M. Chronic Kidney Disease and Associated Risk Factors Assessment among Diabetes Mellitus Patients at A Tertiary Hospital, Northwest Ethiopia. Ethiop J Health Sci. 2018;28:691-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Hamadou B, Boombhi J, Kamdem F, Fitame A, Amougou SN, Mfeukeu LK, Nganou CN, Menanga A, Ashuntantang G. Prevalence and correlates of chronic kidney disease in a group of patients with hypertension in the Savanah zone of Cameroon: a cross-sectional study in Sub-Saharan Africa. Cardiovasc Diagn Ther. 2017;7:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Bahrey D, Gebremedhn G, Mariye T, Girmay A, Aberhe W, Hika A, Teklay G, Tasew H, Zeru T, Gerensea H, Demoz GT. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes. 2019;12:562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/