INTRODUCTION
With over 5 million cases and 5000 dengue-related deaths in 2023, dengue remains a significant public health challenge worldwide. The transmission of dengue viral infection (DVI) from mosquitoes to humans is particularly prevalent in tropical and sub-tropical climates, with the rise in cases linked to global warming[1]. Nigeria, experiencing hyperendemicity from 2009 to 2020, now faces an endemic status across almost all states, with Sokoto state reporting the latest outbreak in the Northwestern region[2-4]. Global urbanization contributes to the disease's spread, which is influenced by factors such as population density, cultural beliefs, water storage practices, hygiene, and water supply accessibility.
The recently recommended Takeda's dengue vaccine by World Health Organization (WHO) experts offers promise in disease eradication. Government initiatives, coupled with improved social and environmental conditions, could alleviate the burden of the disease, particularly in Nigeria. Public awareness campaigns focusing on personal and environmental hygiene, alongside education about the impact of climate change, are crucial steps toward mitigating dengue's impact[5,6]. This paper conducts a comprehensive analysis of the recurrent DVI outbreaks in northern Nigeria, investigating their root causes and presenting updated mortality and natality rates. Additionally, this study proposes innovative solutions to address the frequent outbreaks, emphasising future prevention strategies. A critical evaluation of the recommended Takeda Vaccine is included, highlighting its significant impact in combating the disease within the Nigerian context.
OUTBREAKS OF DENGUE IN NORTHERN NIGERIA AND DISEASE ESTIMATES
Despite the reported occurrence of dengue outbreaks globally, and Africa being among the top leading regions, in Nigeria the disease outbreak has been sporadic[7,8]. Although the initial cases of dengue have been dated to the 1960s[9], there are still limited available surveillance reports on the dengue cases in Nigeria. Based on the studies of Emeribe et al[6], from 2009 to 2020, 30 (3.9%) cases of dengue were attributed to the south-south, 74 (77.1%) to south-east, 534 (37.6%) to north-west, 402 (34.3%) to south-west, 413 (23.5%) to north-central, and 93 (9.2%) to north-east (the least in the country) parts of the country[10]. The most recent dengue fever outbreak in Nigeria was reported in November 2023 in Sokoto state with a total of 13 confirmed cases and 71 suspected cases. Neither case of severe dengue nor death was documented[11]. Moreover, a clear understanding of the dengue burden has not been achieved in Nigeria, just as in other African countries[8]. This is due to the similarity of symptoms with other tropical diseases (such as malaria), insufficient laboratory detection, confirmation capacity, and shortfalls in surveillance and case reporting[12,13]. Generally, dengue fever has been reported to be endemic in Nigeria[10,14].
In Nigeria, there is inadequate surveillance for dengue because it is not very well understood by the medical community, as evidenced by the misdiagnosis and underdiagnosis of the viral infection in numerous unclassified febrile illnesses. However, Nigeria’s dengue disease burden may be drastically underestimated[4]. A summary of prevalence study characteristics of dengue virus (DENV) infection in Nigeria showed the highest prevalence significantly in the south-eastern (77.1%), north-west (34.3%), and north-central (23%) parts of the country, while the north-east and south-south parts had the least at 9.2% and 3.9%, respectively[2,15]. This shows a higher prevalent rate of disease in the southern compared to the northern states. Also, it might be concluded that dengue fever is hyper-endemic in Nigeria.
The most recent outbreak occurred in the north-western part of the country, Sokoto. No fewer than 13 cases were confirmed out of 71 suspected cases. However, no death was reported[2]. The multisectoral National Emerging Viral Hemorrhagic Disease Technical Working Group (NEVHD-TWG), which is directed by the Nigeria Center for Disease Control and Prevention (NCDC), has worked with partners and pertinent stakeholders to conduct a quick risk assessment to guide in-country preparedness efforts. The NEVHD-TWG is in charge of organizing preparations for new viral hemorrhagic fever infections, such as the Ebolavirus disease. Using a dynamic risk assessment, it has been established that the dengue outbreak’s current risk level is moderate because only one state (Sokoto) has reported confirmed cases and out of the 23 Local government areas of the state, only three were affected with no death. Additionally, the state can draw on the knowledge gained from previous DENV outbreaks (2015-2019) to respond to the outbreak[2,16].
GLOBAL EPIDEMIOLOGY OF DENGUE VIRUS DISEASE
DENV disease, though regarded as a 'neglected tropical disease', has been the most important arboviral disease in the world. The disease is widely distributed around the world, in both tropical and subtropical regions and also in both urban and suburban areas, as more than 50% of the world's population live in regions where this disease can potentially occur. The earliest documented symptoms consistent with dengue appeared in a Chinese medical encyclopedia in 992 AD. However, these records were initially published by the China Dynasty centuries earlier (265–420 AD) before being formally edited. The disease was described as 'water poison' and was linked to flying insects[17]. Inadequate vector control, urbanization, excess international travel, climate change, and unavailability of effective antiviral drugs and vaccines to prevent the disease, are what increased the distribution, endemicity, and epidemicity of the DENV around the world[18].
Although the global estimates of DENV disease differ for the last 50 years, the incidence of the disease has increased 50 times annually, thereby making the number of reported cases increasing from 2.2 million in the year 2010 to 3.2 million in 2015. About 3.9 billion people living in 128 countries of the world are at risk of DENV disease, and reports had it that before 1970, severe DENV disease epidemics had occurred in only nine countries, but as of now, the disease is endemic in more than 100 countries of the world, in almost every continent[16]. It was reported that approximately 400 million cases of DENV disease take place each year around the world, where manifestation of symptoms is seen in 96 million of the cases. The WHO reported that 500000 cases of DENV disease occur every year around the globe, leading to approximately 22000 deaths annually, although only 5%-20% of the mortality rate is reported in some regions. The countries that are most affected are those from the Southeast Asian, Western Pacific, and American regions[5,14].
The first outbreak of DENV disease was identified in the year 1779, which occurred in two prominent capital cities, Cairo in Egypt and Jakarta in Indonesia. Moreover, a year later in 1780, another outbreak was confirmed in North America, which was the Philadelphia outbreak[12]. In North and South America, over 1.6 million cases of DENV disease were reported in 2010, where 49000 of the cases were severe cases. The largest outbreak of DENV disease occurred in the United States in 2016, whereover 2.38 million cases were reported, and during this outbreak, the cases were more prevalent in Brazil, with almost 1.5 million cases[18].
Likewise in Africa, the disease was reported in the Eastern, Western, and Southern parts of the continent, since the beginning of the 19th Century[8]. From 1960 to 2010, reviewed data has shown that 22 countries in Africa have reported random and patternless cases of DENV disease, of which 20 reported confirmed cases in the laboratory and two reported clinical cases only[19]. In Asia, the outbreak of DENV disease started in the southeast part of the continent, after World War II, as a result of urbanization[20]. In 1953 and 1956, two cases of dengue outbreaks respectively occurred in the Philippines, which were the first reported cases in Asia. From 2004 to 2010, a large number of cases were reported in Indonesia, which was the second in number of cases after Brazil. Many researchers have agree that the cases of DENV disease will eventually increase in due time to come, due to the globalization and expansion and increased reports being received from the WHO[16].
BIOLOGY OF DENV AND DISEASE DESCRIPTION AND TRANSMISSION
DENV is an arthropod (mosquito) borne pathogen responsible for the causation of the disease termed dengue. DENV is mainly transmitted by a specific species of mosquito called the Aedes mosquito[15,21]. DENV is a flavivirus belonging to the genus Flavivirus and the family Flaviviridae. The different common existing serotypes of the virus are DENV-1, DENV-2, DENV-3, and DENV-4[1]. Dengue fever typically has a sudden onset of symptoms, including high fever, severe headache, pain behind the eyes, muscle and joint pain, nausea, vomiting, and rash. Infection with one serotype of the DENV confers life-long immunity to that serotype but only temporary immunity to the other serotypes, increasing the risk of severe disease upon subsequent infections with different serotypes[5]. Moreover, antigenically different forms of the virus also exist, having varying structural and non-structural proteins[22]. The DENV is a single-stranded positive (+)-sense RNA virus, with an approximate size of 50 nm diameter and 10700 bases. Other similar arthropod-borne families of the virus include Zika, Japanese encephalitis, tick-borne encephalitis, yellow fever, and West Nile viruses[16,23].
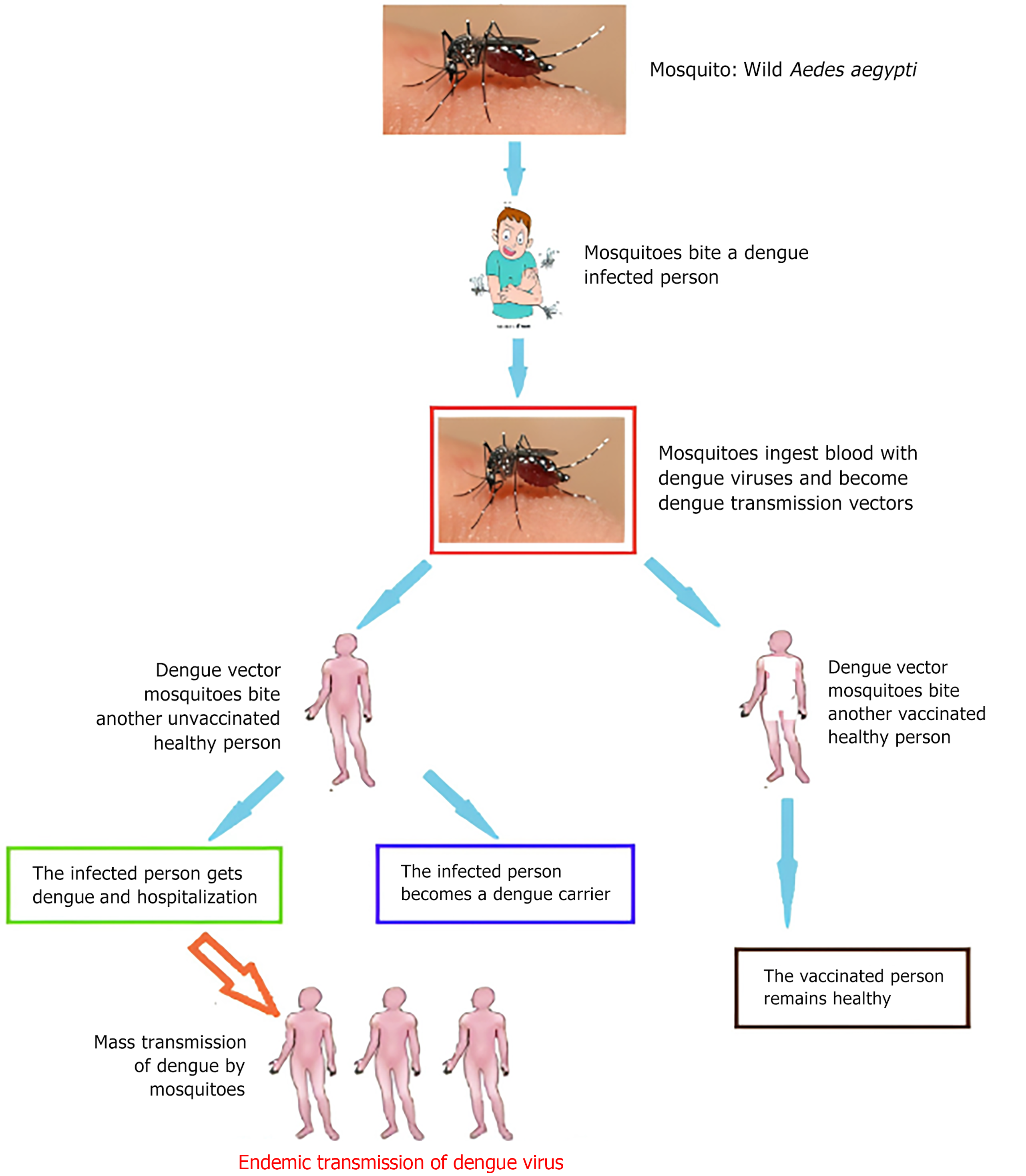
The Aedes species, particularly Aedes aegypti and Aedes albopictus, are common vectors of DENV[15,21]. DENV is primarily transmitted (Figure 1) through the bite of infected Aedes mosquitoes, with humans as the main reservoir and amplifying host. Non-human primates can host the virus but are not the primary mode of human transmission. Transmission of all four DENV serotypes (1, 2, 3, and 4) occurs mainly through human-mosquito-human cycles with mosquitoes being the primary vectors[12].
Figure 1 Dengue transmission cycle.
The transmission cycle of dengue virus typically begins with a mosquito bite and involves various stages such as mosquito bite, blood meal and viral acquisition, transmission to humans, and symptomatic phase until an infected person requires hospitalisation.
Human infection of DENV is following the bite by the Aedes mosquito that has fed on an already infected person[1]. After the feeding, the virus develops within the mosquito gut (having an incubation period of 10-12 d) and then disseminates to other parts of the mosquito. For the rest of the mosquito’s life span, once it has fully become infectious, it can continue to transmit the disease (via horizontal transmission)[23,24]. Even though rare, the vertical (maternal) transmission of the virus from pregnant mothers to their babies has been reported. Other rare modes of transmission are through sharp objects (e.g. needles), and organ and blood product donations[12]. DENV disease ranges from mild and asymptomatic form to severe shock syndrome and severe haemorrhagic fever. The disease can manifest in varying severity, from mild flu-like symptoms to severe forms such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), which can be fatal if not promptly treated. Severe DHF ensues because of a heterologous infection. The severe form of the disease presents a flu-like symptom and a host can be infected multiple times[25]. The most common signs and symptoms are headache, fever, pains (in joints and bones), myalgia, and mucosal bleeding in rare cases[26]. Moreover, life-long immunity to a specific serotype may be conferred[23] coupled with short-term immunity to other related serotypes[5].
UNIQUE ENVIRONMENT AND MOSQUITO VECTOR CHARACTERISTICS IN NORTHERN NIGERIA
Northern Nigeria experiences a tropical climate characterized by high temperatures and seasonal rainfall, creating favourable conditions for the breeding of Aedes mosquitoes and the transmission of the DENV. Urban areas in northern Nigeria, such as Kano, Kaduna, and Sokoto, are particularly susceptible to dengue transmission due to factors such as rapid population growth, unplanned urbanization, and inadequate sanitation infrastructure. The presence of densely populated urban slums with poor housing conditions and limited access to basic amenities can facilitate mosquito breeding and increase human-mosquito contact, amplifying the risk of dengue transmission[18,27].
Aedes mosquitoes, especially Aedes aegypti, are well-adapted to urban environment and are the primary vectors of DENV transmission in northern Nigeria. They have distinctive physical characteristics, including black and white stripes on their legs and body, and prefer to breed in artificial water containers found in and around human dwellings[10]. Common breeding sites for these mosquitoes in northern Nigeria include discarded water storage containers, flowerpots, and other containers that collect stagnant water. They exhibit daytime biting behavior, with peak activity during the early morning and late afternoon, increasing the likelihood of human-mosquito contact and dengue transmission[2].
CAUSES OF DENGUE VIRAL DISEASE IN NORTHERN NIGERIA
Over 2.5 billion population live in dengue-endemic countries worldwide, and roughly 390 million people have been infected with DENV[28,29]. In those regions, approximately 50-100 million new cases are reported each year[9]. DENV is spread by the primary vector, Aedes aegypti, and the less efficient vector, Aedes albopictus. Increases in temperatures are causing the vector to spread all over the world, which thereby facilitates the transmission of dengue to previously unreported countries. This has contributed to the prevalence of dengue fever and other arboviral infections[8]. The disease is most prevalent in tropical and subtropical climates, leaving about one-third of the global population vulnerable[30]. Exposure to DENV causes a variety of clinical conditions that ranges from mild asymptomatic dengue fever to severe DHF and DSS, which can be deadly[25].
The significant spread of DENV in northern Nigeria (Sokoto) is a result of a lack of effective mosquito control and prevention measures. Dengue fever has reemerged as one of the world's most common mosquito-borne diseases. Dengue fever is currently endemic in 128 countries, the majority of which are developing countries. A recent dengue distribution model predicted 390 million dengue infections annually, with 96 million cases emerging[29]. Sokoto has recently reported confirmed cases of dengue from 3 Local government areas[2], thus calling for an urgent need to strengthen sero-surveillance so that authorities can effectively prepare for an outbreak.
Humans contract dengue fever from female Aedes mosquitoes, a vector belonging to the subgenus Stegomyia. Ae. aegypti that has been the primary epidemic carrier in the tropical and subtropical regions while it has been discovered that certain species, including Aspergillus niveus (Ae. Niveus), Aedes albopictus (Ae. Albopictus), Aedes polynesiensis, and members of the Ae. scutellaris complex, exist as secondary vectors[16]. The life cycle of the Aedes mosquito takes up to 8 to 10 days at room temperature, depending on how often it feeds. It has two phases: The terrestrial phase (eggs, adults) and the aquatic phase (larvae, pupae). However, Ae. niveus is only thought of as a sylvatic vector. Ae. albopictus has become an increasingly significant vector due to its ease of adaptation to new habitats, particularly in temperate zones. As a result of its spread to Ae. aegypti-free countries, DENV now have more regions to infect and transmit the disease. But even so, its role in human DENV infections remains negligible[30,16].
RISKF ACTORS FOR DENV
The associated risk factors for DENV infection include travel to endemic areas, poor sanitation, stagnant water, lack of mosquito control measures, urbanization, previous infection, immunocompromised status, and variations in the climates (most especially) of tropical and subtropical regions[3,12]. Other factors contributing to the spread of dengue have been attributed to the influences of evolution of the virus such as globalization, trade, settlement, sanitation, other sociodemographic and economic factors, ecologic factors, and environmental factors[4,23,24]. These factors greatly contribute to the easy development and dissemination of the DENV infection, the viral agent, and infected host and vector, thereby cutting across the three walls of epidemiology (environment, susceptible host, and the disease agent)-the epidemiological triad[26].
CHALLENGES IN CONTROL AND PREVENTION OF DENV DISEASE IN NIGERIA
Control of dengue fever in northern Nigeria faces numerous challenges, including limited resources for vector control programs, inadequate healthcare infrastructure, and low awareness of the disease among healthcare providers and the general population. Integrated vector management approaches, such as larval source reduction, use of insecticides, environmental management, and community engagement, are essential for controlling Aedes mosquito populations and reducing dengue transmission[8]. Public health education campaigns aimed at promoting personal protective measures, such as using insect repellents, wearing long-sleeved clothing, and sleeping under mosquito nets, are crucial for reducing the risk of dengue infection. Strengthening surveillance systems for early detection and reporting of dengue cases, along with improving access to healthcare services and enhancing capacity for clinical management of dengue patients, are critical components of dengue prevention and control efforts in northern Nigeria.
SOLUTIONS TO FREQUENT OUTBREAKS OF DENV DISEASE IN NIGERIA
Government/stakeholders’ intervention in control of DENV disease
The major way of bringing a remedy to the frequent outbreaks of DENV disease is through vector control. To curb this menace, preventative measures have to be taken against Aedes mosquitos which are the vector responsible for the spread of the virus. To achieve this, governmental agencies and other local authorities have roles to play. They must enforce some measures to the public to have an Aedes-free environment, this includes monitoring public parks, construction sites, and swampy areas in both rural and urban areas for any possible breeding of the mosquitoes. Plants growing near roadsides or structures, especially those capable of retaining water, require monitoring. Governments should sponsor regular fogging and fumigation using vector repellents in affected areas.
Safe/healthy community practices
Human behavior is the main cause of the spread of DENV. The best way to curb the spread is through the practice of personal and environmental hygiene. The public should ensure that their homes and surroundings do not support the breeding of Aedes mosquitoes. This can be achieved through draining and clearing of gutters and domestic water bodies to avoid stagnant water which may serve as a breeding site for Aedes mosquitoes; use of insecticides and mosquito repellent; use of insecticide-treated mosquito nets; proper disposal of wastes; and wearing protective clothing to prevent the bite of dengue mosquitoes.
The government must propose innovative solutions to address frequent outbreaks, emphasizing future prevention strategies
In a chemical approach to remedy the spread of DENV, the WHO recommended the use of “Bediocarb” insecticide, which is tested to be effective for the control of Aedes mosquitoes. However, in a biological approach to vector control, the use of Wolbachia bacteria has proven to be effective. The Wolbachia bacteria can be introduced to the male Aedes mosquitoes, and when they mate with their female counterparts, the eggs will not be hatched, thus preventing the reproduction of new breeds. However, the media also have a role to play in curbing the spread of DENV disease. This may involve raising awareness in schools, hospitals, markets, and other crowded places or social gatherings, and enlightening the public about possible preventive measures to avoid the spread of DENV.
EVALUATING THE RECOMMENDED TAKEDA VACCINE AND PROBLEMS OF ITS ACCEPTABILITY IN NIGERIA
Takeda dengue vaccine (TDV/TAK-003) is a live attenuated tetravalent vaccine which is effective on all the four serotypes of DENV (DENV 1-4) and stimulates various parts of the immune system such as antibodies and immune cells to fight against DENV. The vaccine shots are administered in two doses, subcutaneously, with 3 mo apart[31]. The vaccine is engineered with a live attenuated DENV-2 virus, which is the backbone of the genetic compositions of the other strains of the virus.
Takeda Pharmaceutical Company Limited of Japan manufactures TDV/TAK-003, but the idea of its manufacture was primarily designed by the Division of Vector-Borne Diseases of the Centers for Disease Control and Prevention. The vaccine contains a weakened DENV whose virulence is reduced and when administered, the immune system of the body recognizes the dengue proteins in the weakened viruses as 'foreign' and produces antibodies against them, hence preventing the establishment of the virulent DENV in the body which may cause disease. The vaccine is administered in the form of a subcutaneous injection, which is given two times, with 90-d intervals from the first dose. Presently, the second part of the Phase III Tetravalent Immunization of Dengue Efficacy Study is being conducted in Asia and Latin America, to further test the efficacy and safety of the vaccine[32].
However, the vaccine may encounter challenges in its acceptance in Nigeria, particularly in the northern region, compared to other developed countries[33]. These challenges include a lack of knowledge or misperceptions of vaccine administration by the public; influence of religion and ethnicity; political influence; fear of side effects; lack of faith in vaccines; low level of education; and fear and confusion.
TAKEDA VACCINE AND FUTURE PREVENTION STRATEGIES
Takeda vaccine, endorsed by the WHO's Strategic Advisory Group of Experts on Immunization, is recommended for preventing DENV disease[34,35]. This development represents a significant step forward in the fight against dengue fever. However, comprehensive prevention strategies encompassing vector control, public health education, surveillance, healthcare infrastructure[35,36], and ongoing research are crucial to achieving sustainable dengue control and prevention. By integrating these strategies, we can significantly reduce the global burden of dengue and improve public health outcomes both now and in the future, including in Nigeria.
CONCLUSION
Dengue fever remains a significant public health threat in Nigeria, with recent outbreaks in Sokoto state in northern Nigeria, highlighting the need for effective and continual prevention and control measures. The TDV offers a promising solution to prevent dengue fever, but its acceptance and accessibility in Nigeria, particularly in the northern region, pose challenges. To address these challenges, government intervention, community engagement, and innovative vector control strategies are essential. Strengthening surveillance, increasing public awareness, and enhancing healthcare infrastructure are crucial steps in combating dengue fever in Nigeria. With concerted efforts and collaborative initiatives, Nigeria can mitigate the burden of dengue fever and safeguard public health in the future.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society for Microbiology; Nigerian Society for Microbiology; International Society for Infectious Diseases; Nigerian Bioinformatics and Genomics Network.
Specialty type: Virology
Country of origin: Nigeria
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade B
P-Reviewer: Haisheng H S-Editor: Liu H L-Editor: Wang TQ P-Editor: Zhang XD