Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.88946
Peer-review started: October 16, 2023
First decision: November 2, 2023
Revised: November 10, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: March 25, 2024
Processing time: 147 Days and 4.9 Hours
Cholangiocarcinoma is the second most common primary liver malignancy. Its incidence and mortality rates have been increasing in recent years. Hepatitis C virus (HCV) infection is a risk factor for development of cirrhosis and cholangiocarcinoma. Currently, surgical resection remains the only curative treatment option for cholangiocarcinoma. We aim to study the impact of HCV infection on outcomes of liver resection (LR) in intrahepatic cholangiocarcinoma (ICC).
To study the outcomes of curative resection of ICC in patients with HCV (i.e., HCV+) compared to patients without HCV (i.e., HCV-).
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and observational studies to assess the outcomes of LR in ICC in HCV+ patients compared to HCV- patients in tertiary care hospitals. PubMed, EMBASE, The Cochrane Library and Scopus were systematically searched from inception till August 2023. Included studies were RCTs and non-RCTs on patients ≥ 18 years old with a diagnosis of ICC who underwent LR, and compared outcomes between patients with HCV+ vs HCV-. The primary outcomes were overall survival (OS) and recurrence-free survival. Secondary outcomes include perioperative mortality, operation duration, blood loss, intrahepatic and extrahepatic recurrence.
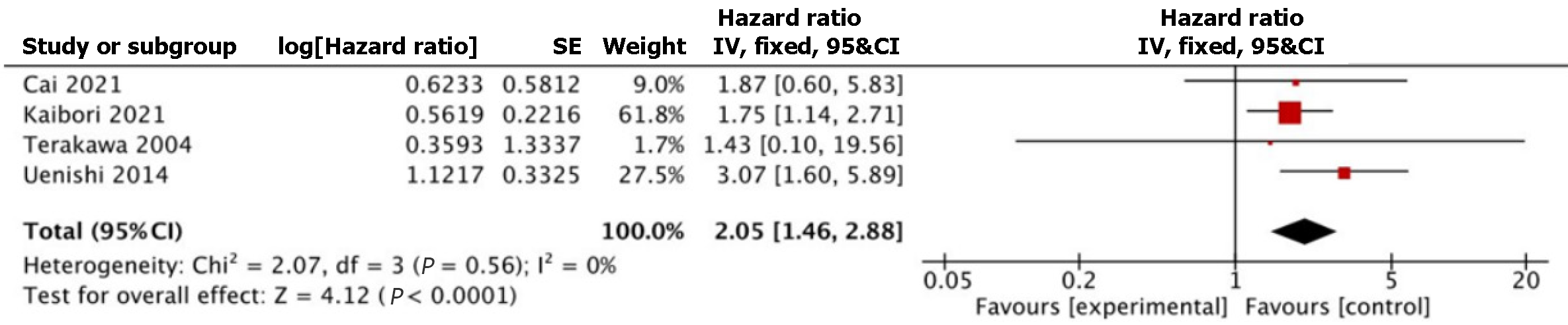
Seven articles, published between 2004 and 2021, fulfilled the selection criteria. All of the studies were retrospective studies. Age, incidence of male patients, albumin, bilirubin, platelets, tumor size, incidence of multiple tumors, vascular invasion, bile duct invasion, lymph node metastases, and stage 4 disease were comparable between HCV+ and HCV- group. Alanine transaminase [MD 22.20, 95%confidence interval (CI): 13.75, 30.65, P < 0.00001] and aspartate transaminase levels (MD 27.27, 95%CI: 20.20, 34.34, P < 0.00001) were significantly higher in HCV+ group compared to HCV- group. Incidence of cirrhosis was significantly higher in HCV+ group [odds ratio (OR) 5.78, 95%CI: 1.38, 24.14, P = 0.02] compared to HCV- group. Incidence of poorly differentiated disease was significantly higher in HCV+ group (OR 2.55, 95%CI: 1.34, 4.82, P = 0.004) compared to HCV- group. Incidence of simultaneous hepatocellular carcinoma lesions was significantly higher in HCV+ group (OR 8.31, 95%CI: 2.36, 29.26, P = 0.001) compared to HCV- group. OS was significantly worse in the HCV+ group (hazard ratio 2.05, 95%CI: 1.46, 2.88, P < 0.0001) compared to HCV- group.
This meta-analysis demonstrated significantly worse OS in HCV+ patients with ICC who underwent curative resection compared to HCV- patients.
Core Tip: Impact of hepatitis C virus (HCV) infection on survival outcomes in patients with intrahepatic cholangiocarcinoma (ICC) undergoing curative resection remains unclear. This is the first systematic review and meta-analysis comparing outcomes of surgical resection of ICC in HCV-positive patients vs HCV-negative patients. Our primary outcomes include overall survival (OS) and recurrence-free survival; secondary outcomes include perioperative mortality, operation duration, blood loss and recurrence. Our review and analysis demonstrated worse OS in HCV-positive patients compared to HCV-negative patients.
- Citation: Cheo FY, Chan KS, Shelat VG. Outcomes of liver resection in hepatitis C virus-related intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. World J Virol 2024; 13(1): 88946
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/88946.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.88946
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy accounting for 15% of all primary liver malignancy, after hepatocellular carcinoma (HCC)[1]. Though rare, its incidence and mortality rates have increased in recent years[2,3]. Incidence amongst males increased from 1.51 per 100000 in 1993-1997 to 4.07 per 100000 in 2013-2017 and incidence amongst females increased from 1.73 per 100000 to 2.95 per 100000 respectively[4]. Mortality rates in cholangiocarcinoma have been reported to be up to 2 deaths per 100,000 in the United States, with mortality rates 3 times higher in Asia, and are still increasing[5]. Surgery remains the only potentially curative treatment modality in resectable ICC. However, the presentation for ICC is non-specific and patients may be diagnosed late; a retrospective study on patients with ICC demonstrated that 54% of ICCs were unresectable at diagnosis[6].
Common causes of ICC include cirrhosis, alcohol, hepatotoxins, chronic viral hepatitis, hepatolithiasis and liver fluke infections[7,8]. Patients with hepatitis C virus (HCV) have a 2-fold increase in risk of developing ICC compared to the general population[9]. To add on, a meta-analysis by Wang et al[10] in 2016 on 2842 patients with ICC showed that HCV was associated with worse survival [hazard ratio (HR) 2.64, 95% confidence interval (CI): 1.77-3.93] compared to controls. However, their study included patients who received various forms of treatment, ranging from curative surgery to palliative treatment. In 50 patients who received liver resection (LR) for ICC, Hai et al[11] however showed that HCV was not a predictor of survival following LR. While HCV is a significant risk factor for ICC, the prognostic significance of HCV remains uncertain for patients with ICC following LR. This study aims to perform a systematic review and meta-analysis to compare the survival between patients with HCV infection (i.e. HCV+) vs those without HCV (i.e. HCV-) in ICC following LR.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) guidelines[12]. The protocol for this systematic review and meta-analysis was registered at PROSPERO (Ref no: CRD42023459605). A systematic search of the following databases (PubMed, EMBASE, The Cochrane Library and Scopus) was conducted for studies published from inception to 19th August 2023. A combination of the following search terms was used: “cholangiocarcinoma” or “bile duct cancer”, and “hepatectomy” or “liver resection”, and “hepatitis C” or “HCV”. The search was restricted to the title, abstract and keywords. The complete search strategy is appended in Supplementary Table 1. Search strategies for other databases were modified from the initial search strategy done on PubMed based on the database requirements.
Included studies were randomised controlled trials (RCTs) and non-RCTs on patients ≥ 18 years old with a diagnosis of ICC who underwent LR, and compared outcomes between patients with HCV+ vs HCV-. Exclusion criteria were studies: (1) On other types of liver malignancies (e.g., HCC) or underwent liver transplantation (LT); (2) single-arm studies without comparison; (3) which did not include our outcome of interest; (4) on the same cohort of patients; and (5) based on article type (non-English studies, conference abstracts, case report or series, editorials, expert opinions, and review articles without original data). There were no studies which reported on the same cohort of patients. LR was defined as any form of surgical resection of the liver, including wedge resection, anatomical resection such as minor LR and major LR, and non-anatomical resection. HCV+ was defined as presence of anti-HCV antibodies detected on serology.
All cross-references were screened for potentially relevant studies not identified by the initial literature search. After removing duplicates, two authors screened abstracts for potential inclusion screening independently (Cheo FY and Chan KS). The included studies' full texts were reviewed and selected based on the inclusion and exclusion criteria. All discrepancies were resolved after review by the senior author (Shelat VG).
Data extraction was independently performed by two authors (Cheo FY and Chan KS). The following variables were extracted from each study: Publication details (name of first author, publication year and country), study characteristics (sample size, sex, age, Child-Pugh score, presence of cirrhosis, baseline tumor markers (alpha-fetoprotein, carbohydrate antigen 19-9, carcinoembryonic antigen, and tumor size). Our primary outcomes were overall survival (OS) and recurrence free survival (RFS). Our secondary outcomes were perioperative outcomes, including mortality, operation duration, blood loss, and tumor recurrence.
Two authors (Cheo FY and Chan KS) independently assessed the included studies' quality. Observational studies were assessed using the modified Newcastle-Ottawa scale (Supplementary Table 2)[13]. No RCTs were included in this study. Only observational studies with sufficient quality (articles with a score >6) were included. Disagreements between authors were resolved by discussion with the senior author (Shelat VG).
Study variables were extracted to Microsoft Excel 365 (Microsoft®, Washington, United States). Categorical variables were described as n (%), and continuous variables were expressed as mean ± SD, or median [interquartile range (IQR)] unless otherwise specified. For continuous variables expressed only in median and range or IQR, mean and SD were estimated from median and range values using methods described by Wan et al[14]. Meta-analysis was performed using RevMan 5.4 (Review Manager 5.4, The Nordic Cochrane Centre, Copenhagen, Denmark). For cumulative OS and RFS, HR and standard error (SE) were estimated indirectly according to the methods described by Parmar et al[15]. Pooled HR was calculated through the inverse-variance method using the natural logarithm of HR [ln(HR)] and SE[16]. For studies that used univariate and multivariate analysis to assess the impact of HCV infection, the effect size from the multivariate analysis was used in our pooled analysis. Dichotomous outcomes were pooled and calculated using the Mantel-Haenszel method and expressed as odds ratio (OR) with 95%CI. Continuous outcomes were pooled and calculated using the inverse variance method and expressed as mean difference (MD) with 95%CI. Heterogeneity was assessed using Cochrane's Q and quantified by I2. If data was heterogenous (defined as I2 > 50%), a random-effects model using the DerSimonian and Laird approach was used. Statistical significance was defined as P < 0.05. Publication bias was investigated using funnel plots. Due to low sample size, quantitative analysis was not performed for short-term intra-operative and post-operative outcomes.
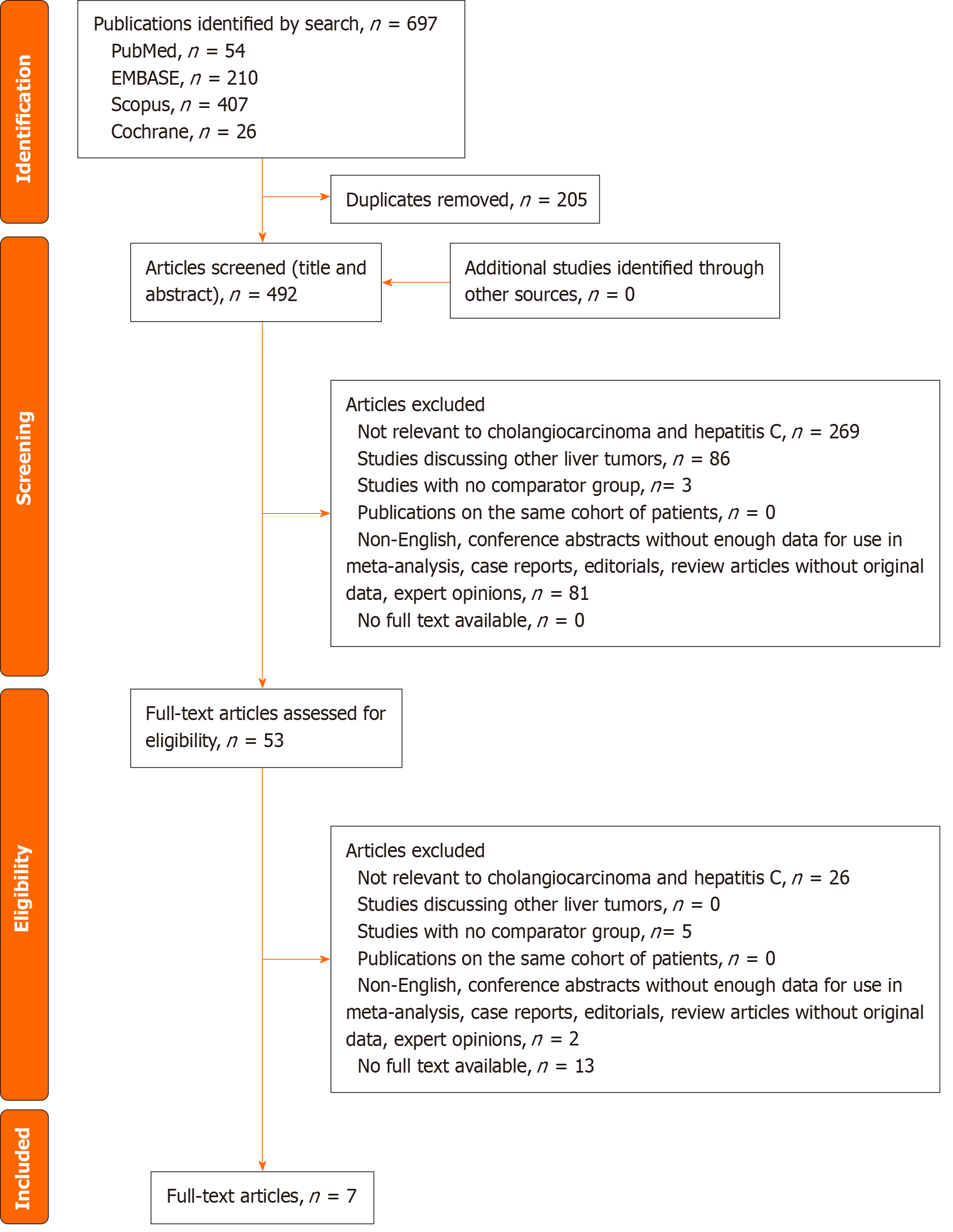
The systematic search identified 697 articles from the four databases. There were 492 articles after removal of the duplicates. Titles and abstracts of all the identified articles were screened. The remaining 53 articles underwent full-text review, of which seven articles were included in the final analysis[11,17-22]. The PRISMA diagram for the study selection process is appended in Figure 1. The funnel plots are appended in Supplementary Figure 1.
There were seven studies with 1181 patients (HCV+ n = 205, HCV- n = 976)[11,17-22]. Kaibori et al[19] performed propensity score matching (PSM) analysis to derive their cohorts; only the PSM cohort was analysed in our study. Uenishi et al[20] performed univariate and multivariate analyses on the impact of HCV infection on outcomes of surgical resection in cholangiocarcinoma, of which outcomes of the multivariate analysis was included in our study. Yang et al[21] reported on OS of HCV+ and HCV- groups in the early relapse subgroup (within 24 mo), which we excluded from our quantitative analysis of OS due to selection bias and misrepresentation of the entire cohort of ICC. While the study by Hai et al[11] performed Kaplan-Meier analysis on OS, HR and SE could not be estimated due to the lack of clarity of the Kaplan-Meier curve; clinical demographics and other outcomes were still included. The study by Terakawa et al[22] was reported in Japanese; however, as the tables and figures were in English, relevant data such as survival outcomes were included in our study to avoid dilution to power and sample size.
The study characteristics and patient demographics of individual studies were summarized in Table 1. The overall mean age was 65.0 years, and 17.2% (n = 46/267) patients had cirrhosis. There were 17.7% (n = 55/311) patients with multiple tumors on diagnosis, and the mean tumor size ranged from 3.6-6.4 cm. 12.5% of patients (n = 16/128) had synchronous HCC lesions. Pooled analysis showed that age, incidence of male patients, albumin, bilirubin, platelets, tumor size, incidence of multiple tumors, vascular invasion, bile duct invasion, lymph node metastases, stage 4 disease were comparable between HCV+ and HCV- (Table 2). However, alanine transaminase (ALT) and aspartate transaminase (AST) levels were significantly higher in HCV+ (ALT: MD +22.2, 95%CI: 13.75, 30.65; AST: MD +27.27, 95%CI: 20.20, 34.34) compared with HCV-. There were also more patients with cirrhosis (OR 5.78, 95%CI: 1.38, 24.14, P = 0.02), poorly differentiated disease (OR 2.55, 95%CI: 1.34, 4.82, P = 0.004), and concomitant HCC (OR 8.31, 95%CI: 2.36, 29.26, P = 0.001) in the HCV+ group compared to HCV- group.
| No. | Ref. | Study design | Study period | Country | Sample size, n (%) | Age, yr | Males, n (%) | Tumor size, cm | Cirrhosis, n | Tumor stage | Tumor grade |
| 1 | Hai et al[11], 2005 | Retrospective | Jan 1997-Dec 2002 | Japan | HCV+: 17; | HCV+: 69.0 ± 4.9; HCV-: 60.6 ± 12.4 | HCV+: 10; HCV-: 13 | HCV+: 3.6 ± 2.3; HCV-: 6.4 ± 4.5 | NR | HCV+:I: 4, II: 4, III: 3, IV: 6; HCV-: I: 0, II: 6, III: 7, IV: 8 | NR |
| 2 | Kaibori et al[19], 20211 | Retrospective | Jan 2000-Dec 2007 | Japan | HCV+: 102; HCV-: 102 | HCV+: ≥ 70: 56/102; HCV-: ≥ 70: 59/102 | HCV+: 64; HCV-: 74 | HCV+: ≥ 3.5cm: 69/102; HCV-: ≥ 3.5cm: 61/102 | HCV+: 24; HCV-: 8 | NR | HCV+:well: 14, moderate: 49, poor: 27; HCV-: well: 21, moderate: 53, poor: 11 |
| 3 | Uenishi et al[20], 2014 | Retrospective | Jan 2000-Dec 2011 | Japan | HCV+: 33 HCV-: 57 | HCV+: 66.9 ± 9.0; HCV-: 64.3 ± 11.2 | HCV+: 23; HCV-: 38 | HCV+: 4.7 ± 1.7; HCV-: 4.8 ± 2.6 | HCV+: 14; HCV-: 3 | HCV+:I: 12, II: 7, III: 5, IV: 9; HCV-: I: 18, II: 9, III: 5, IV: 25 | HCV+: poor: 7; HCV-: poor: 7 |
| 4 | Cai et al[18], 2021 | Retrospective | Dec 2008-Dec 2017 | China | HCV+: 3; HCV-: 527 | NR | NR | NR | NR | NR | NR |
| 5 | Ariizumi et al[17], 2011 | Retrospective | 1989-2008 | Japan | HCV+: 42; HCV-: 92 | NR | NR | NR | NR | NR | NR |
| 6 | Yang et al[21], 2019 | Retrospective | Jan 2005-Dec 2011 | China | HCV+: 1; HCV-: 167 | NR | NR | NR | NR | NR | NR |
| 7 | Terakawa et al[22], 2004 | Retrospective | Jan 1992-Dec 2001 | Japan | HCV+: 7; HCV-: 10 | HCV+:64.0 ± 3.0; HCV-: 66.0 ± 3.0 | HCV+: 4; HCV-: 8 | HCV+: 5.0 ± 1.2; HCV-: 5.1 ± 1.0 | NR | HCV+:II: 1, III: 3, IV: 3HCV-: II: 2, III: 4, IV: 4 | HCV+:well: 1, moderate: 4; HCV-: well: 1, moderate: 5 |
| No. | Study variables and/or outcomes | No. of data sets | Total number of patients, n (HCV+/HCV-) | No. of patients (%) | Effect size, OR (95%CI)/MD (95%CI)/HR (95%CI)1 | P value | I2, % | Model used | |
| HCV+ | HCV- | ||||||||
| Demographics and histopathological findings | |||||||||
| 1 | Age, yr | 4 | 349 (159/190) | NA | 2.55 (-3.09, 8.20) | 0.38 | 82 | RE | |
| 2 | Male | 4 | 349 (159/190) | 101 (63.5) | 133 (70.0) | 0.74 (0.47, 1.17) | 0.20 | 0 | FE |
| 3 | ALT, IU/L | 2 | 294 (135/159) | NA | 22.20 (13.75, 30.65) | < 0.00001a | 0 | FE | |
| 4 | AST, IU/L | 2 | 294 (135/159) | NA | 27.27 (20.20, 34.34) | < 0.00001a | 0 | FE | |
| 5 | Albumin, g/L | 2 | 294 (135/159) | NA | -0.11 (-0.34, 0.12) | 0.34 | 71 | RE | |
| 6 | Bilirubin, umol/L | 2 | 294 (135/159) | NA | -0.07 (-0.33, 0.19) | 0.61 | 84 | RE | |
| 7 | Platelets, 104/mm3 | 2 | 294 (135/159) | NA | -1.96 (-5.88, 1.96) | 0.33 | 71 | RE | |
| 8 | Tumor size, cm | 3 | 145 (57/88) | NA | -0.61 (-1.79, 0.57) | 0.31 | 62 | RE | |
| 9 | Multiple tumors | 3 | 311 (142/169) | 25 (17.6) | 30 (17.8) | 1.12 (0.61, 2.06) | 0.70 | 0 | FE |
| 10 | Cirrhosis | 2 | 294 (135/159) | 35 (25.9) | 11 (6.9) | 5.78 (1.38, 24.14) | 0.02a | 69 | RE |
| 11 | Vascular invasion | 3 | 145 (57/88) | 20 (35.1) | 36 (40.9) | 0.76 (0.37, 1.54) | 0.45 | 0 | FE |
| 12 | Bile duct invasion | 2 | 294 (135/159) | 53 (39.3) | 64 (40.3) | 0.86 (0.53, 1.39) | 0.53 | 0 | FE |
| 13 | Lymph node metastases | 4 | 349 (159/190) | 41 (25.8) | 54 (28.4) | 0.85 (0.53, 1.37) | 0.51 | 15 | FE |
| 14 | Stage 4 | 3 | 145 (57/88) | 18 (31.6) | 37 (42.0) | 0.64 (0.32, 1.28) | 0.21 | 0 | FE |
| 15 | Poorly differentiated | 2 | 273 (127/146) | 34 (26.8) | 18 (12.3) | 2.55 (1.34, 4.82) | 0.004a | 0 | FE |
| 16 | Simultaneous HCC lesions | 2 | 128 (50/78) | 13 (26.0) | 3 (3.8) | 8.31 (2.36, 29.26) | 0.001a | 0 | FE |
| Outcomes | |||||||||
| 17 | Overall survival | 4 | 841 (145/696) | NA | 2.05 (1.46, 2.88) | <0.0001a | 0 | FE | |
Table 3 summarizes the survival outcomes reported in individual studies. Four studies involving 841 patients (HCV+ n = 145, HCV- n = 696) reported on OS[18-20,22]. Pooled HR showed statistically significantly worse OS in the HCV+ group (HR 2.05, 95%CI: 1.46, 2.88, P < 0.0001) (Figure 2). Heterogeneity was not significant among the studies (I2 = 0%, P = 0.56). The study by Cai et al[18] had very few HCV+ patients compared with HCV- patients (HCV+ n = 3, HCV- n = 527). In view of this, sensitivity analysis was performed to exclude their study; OS remained significantly worse in HCV+ group (HR 2.07, 95%CI: 1.45, 2.96, P < 0.0001) compared to HCV- group.
| No. | Ref. | 1-yr OS, % | 1-yr DFS, % | 3-yr OS, % | 3-yr RFS, % | 3-yr DFS, % | 5-yr OS, % | 5-yr RFS, % |
| 1 | Hai et al[11], 2005 | HCV+: 70.9; HCV-: 75.6 | HCV+: 55.7; HCV-: 49.0 | HCV+: 41.4; HCV-: 30.1 | NR | HCV+: 27.9; HCV-: 32.7 | NR | NR |
| 2 | Kaibori et al[19], 20211 | NR | NR | NR | NR | NR | HCV+: 32.2; HCV-: 44.7 | HCV+: 25.0; HCV-: 31.3 |
| 3 | Uenishi et al[20], 2014 | NR | NR | HCV+: 30.6; HCV-: 65.6 | HCV+: 29.9; HCV-: 31.4 | NR | HCV+: 21.9; HCV-: 32.8 | HCV+: 22.4; HCV-: 20.6 |
| 4 | Cai et al[18], 2021 | NR | NR | NR | NR | NR | NR | NR |
| 5 | Ariizumi et al[17], 2011 | NR | NR | NR | NR | NR | HCV+: 53; HCV-: 32 | NR |
| 6 | Yang et al[21], 2019 | NR | NR | NR | NR | NR | NR | NR |
| 7 | Terakawa et al[22], 2004 | NR | NR | NR | NR | NR | NR | NR |
There were 2 studies involving 294 patients (HCV+ n = 135, HCV- n = 159) which reported on RFS[19,20]. Meta-analysis was not performed for RFS due to the small sample size and limitations in interpretation. Kaibori et al[19] reported significantly worse RFS in the HCV+ group (HR 1.61, 95%CI: 1.09, 2.38, P = 0.016) compared to the HCV- group. Uenishi et al[20] reported comparable RFS between the HCV+ group and HCV- group (HR 1.59, 95%CI: 0.74, 3.41, P = 0.24).
There was one study which reported on incidence of post-operative mortality. Uenishi et al[20] reported in-hospital mortality of 13% in HCV+ group (n = 3/33) and 4.8% in HCV- group (n = 1/57). However this did not reach statistical significance (P = 0.609).
There was one study which reported on operative time and intraoperative blood loss. Terakawaet al[22] reported mean operative duration of 359 ± 74 min in the HCV+ group compared to 336 ± 34 min in the HCV- group. They additionally reported mean intraoperative blood loss of 2037 ± 577 mL in HCV+ group compared to 1226 ± 269 mL in HCV- group. However, no comparative statistical analysis was performed to compare between HCV+ and HCV- groups[22].
There were two studies which reported on tumor recurrence post-LR; Yang et al[21] reported comparable tumor recurrence (both intrahepatic and extrahepatic) in HCV+ group and HCV- group (HR 3.28, 95%CI: 0.80, 13.51, P = 0.098) . Kaibori et al[19] showed comparable incidence of intrahepatic recurrence [HCV+: 36% (n = 33/92), HCV-: 30% (n = 28/94); P = 0.467] and extrahepatic recurrence [HCV+: 36% (n = 25/69) vs HCV-: 27% (n = 18/67); P = 0.322] between HCV+ and HCV- groups.
Hepatitis B virus (HBV) and HCV infection are significant risk factors involved in the pathogenesis of cholangiocarcinoma. Interestingly, while HBV infection has been shown to provide favourable prognosis for patients with cholangiocarcinoma, HCV+ is associated with shorter OS compared to HCV- patients[10]. Our study similarly showed that HCV+ is associated with worse OS in ICC following LR. However, there is limited data on peri-operative outcomes.
Risk factors for ICC include biliary tract diseases such as primary sclerosing cholangitis, recurrent pyogenic chol-angitis, primary biliary cirrhosis, congenital malformations of the bile duct (i.e choledochal cysts), cirrhosis and chemical exposure[23]. Incidence of HCV has been reported to be 13.8%-23.1% in ICC[24,25]. Various mechanisms have been proposed on the role of HCV in the pathogenesis of ICC[26]. One postulation is that cholangiocytes and hepatocytes share the same liver progenitor cell; cholangiocytes express receptors which are susceptible to HCV infection[27]. Another postulation is that the initial HCV infection of hepatocytes result in transdifferentiation of hepatocytes into cholangiocytes[28]. Interaction of cholangiocytes with HCV protein induces chronic biliary inflammation with resulting development of ICC.
HCV is a significant risk factor in the development of cholangiocarcinoma[29]. Globally, HCV is strongly associated with cholangiocarcinoma, especially in the Western populations[9]. Therefore, an understanding of its impact on outcomes helps to guide clinical decisions and development of treatment pathways. A previous meta-analysis by Wang et al[10] explored the impact of HCV infection on survival outcomes in patients with ICC, regardless of treatment modality, and showed poorer prognosis in HCV+ patients. However, we wish to understand the implications of HCV on long-term outcomes following curative LR in ICC. Since then, more studies comparing LR outcomes in ICC between HCV+ and HCV- groups have been published. This updated meta-analysis included five new studies with 1053 patients; we showed that HCV+ patients had worse OS compared to HCV- in patients who received curative LR for ICC[17-19,21,22]. We hypothesize potential reasons for these observations which are discussed below.
Several factors prognosticate OS and RFS in ICC following LR, including cirrhosis, positive surgical margins, tumor morphology patterns, tumor size, nodal involvement, and vascular invasion[30,31]. Chronic HCV is recognised as a significant precursor to liver cirrhosis, due to its process of chronic hepatocellular injury leading to chronic inflammation, resulting in scarring and fibrosis[32]. Cirrhosis has been associated with worse short-term and long-term survival; for instance, Zaydfudim et al[33] reported higher postoperative mortality (OR = 2.24; 95%CI: 1.16, 4.34, P = 0.016) in patients with cirrhosis; Sasaki et al[34] reported worse 5-year disease-specific survival (75.4% in patients with normal liver function vs 59.1% in patients with cirrhosis, P = 0.04) in cirrhotic patients as well. Liver cirrhosis is also a risk factor of tumor recurrence in cholangiocarcinoma; Tsilimigras et al[35] reported a significant association between cirrhosis and very early recurrence (within 6 mo after resection) of ICC post-LR (OR 2.06, 95%CI: 1.25, 3.40, P = 0.005) and Zhang et al[36] reported a significant association between cirrhosis and late intrahepatic recurrence (more than 24 mo after resection) (HR 1.99, 95%CI: 1.11, 3.56, P = 0.019). This may be due to the increased carcinogenic potential of remnant cirrhotic liver and biliary system which predisposes to neocarcinogenesis, resulting in de novo recurrence of cholangiocarcinoma[26]. While our meta-analysis showed that HCV+ group had worse OS compared to HCV- group, there was also increased incidence of liver cirrhosis in the HCV+ group (OR 5.78, 95%CI: 1.38, 24.14, P = 0.02). Liver cirrhosis may be a confounding factor for worse OS in HCV+ ICC as discussed above, rather than HCV alone.
An important consideration in surgical candidates is the risk of post hepatectomy liver failure (PHLF)[37]; Lei et al[38] showed that patients with PHLF diagnosed using the 50-50 criteria was independently associated with higher 90-d mortality (HR 8.63, 95%CI: 3.33-22.35, P < 0.001). Clinically relevant PHLF (grade B/C) has been reported to be associated with postoperative 90-d mortality (OR 7.26, 95%CI: 2.90, 18.17) and significantly worse long-term survival outcomes (HR 1.90, 95%CI:1.32, 2.71)[37]. Post-LR, adequate functional liver remnant (FLR) is required to sustain the body’s metabolic, synthetic and detoxifying requirements[39]. Due to chronic hepatocellular injury leading to scarring and fibrosis in cirrhotic livers, these functions are greatly reduced, predisposing to liver failure[40]. Current guidelines recommend FLR of > 30% in patients with liver steatosis and > 40% in patients with cirrhosis to reduce risk of PHLF[41,42]. One of the possible reasons for worse OS in HCV+ ICC may be due to PHLF in the HCV+ group due to higher incidence of cirrhosis. Unfortunately, in our review, none of the included studies described the incidence of PHLF; this remains a postulation to be validated, and correlation cannot be drawn. Nevertheless, other markers have been used to predict risk of PHLF, such as the use of indocyanine green retention rate at 15 minutes (ICGR15). Makuuchi’s criteria serve as a guide to assess the extent of hepatectomy based on ICGR15 to reduce risk of PHLF[43]. In our review, there was a mix of studies reporting either comparable ICGR15 between HCV+ and HCV- groups (such as the study by Hai et al[11] with comparable incidence of ICGR15 > 10% in HCV+ group (n = 11/17, 64.7%) compared to HCV- group (n = 4/21, 19.0%), P = 0.0656), or higher ICGR15 in HCV+ compared to HCV- (such as the study by Kaibori et al[19] with 71% with ICGR15 ≥10% in HCV+ compared to 48% in HCV-). Whether or not PHLF is a cause of worse OS in HCV+ ICC following LR remains to be answered.
Another possible reason for poorer prognosis in HCV+ patients may be attributed to synchronous or metachronous HCC in HCV+ patients. Chronic HCV infection is the leading cause of HCC in Western countries. HCV is also associated with a large proportion of HCC in certain Asian and African countries[44,45]. Carcinogenesis of HCC and cholangiocarcinoma in the background of chronic HCV-induced cirrhosis share similarities and has been postulated to be associated with the occurrence of synchronous or metachronous HCC and cholangiocarcinoma lesions[46]. A literature review of reported synchronous HCC and cholangiocarcinoma cases by Watanabe et al[47] found that 72.7% of cases were positive for HCV. Survival outcomes in patients with synchronous or metachronous HCC and cholangiocarcinoma are generally poorer and may distort survival outcomes in HCV+ group[48]. In our study, incidence of simultaneous HCC lesions found on pathologic studies is significantly higher in HCV+ group compared to HCV- group (OR 8.31, 95%CI: 2.36, 29.26, P = 0.001), which may confound and contribute to worse outcomes in the HCV+ group.
Tumor biology is another important consideration in survival. Higher tumor grade and poorly differentiated tumors confer a worse prognosis on survival. A retrospective study by Mao et al[49] identified tumor differentiation as an independent predictor of higher postoperative mortality in cholangiocarcinoma (relative risk 1.356, 95%CI: 1.081, 1.699, P = 0.008). Nickkholgh et al[50] reported that high grade tumor (defined as Grade 3-4) was an independent determinant of recurrence in ICC post-resection (HR 1.63, 95%CI: 1.04, 2.55, P = 0.034). HCV-induced development and progression of liver fibrosis involve epithelial-mesenchymal transition (EMT) of cholangiocytes, resulting in reduced expression of E-cadherin, which is associated with poor tumor differentiation in cholangiocarcinoma[26,51,52]. In our study, incidence of poorly differentiated ICC was significantly greater in HCV+ group compared to HCV- group (OR 2.55, 95%CI: 1.34, 4.82, P = 0.004). This may have consequently resulted in worse OS in the HCV+ group.
Advanced tumor stage and metastatic disease are poor prognostic factors in cholangiocarcinoma[53]. In advanced tumors, several factors contribute to more aggressive tumor behavior. Notably, presence of vascular invasion increases the risk of haematogenous spread of tumor cells, and tumor multiplicity provide additional nidus for tumor to grow and spread from[54-56]. Expectedly as well, nodal disease has been shown to be associated with worse survival (22.9 mo vs 30.1 mo, P = 0.03)[57]. The question lies in whether HCV+ increases the risk of more advanced disease or nodal metastases, since HCV infection results in EMT as described above[58]. This question remains unanswered based on our findings, but may be due to the low sample size of the included studies.
The advent of direct-acting antivirals (DAAs) have revolutionized the treatment of HCV, where it is possible to achieve a cure for hepatitis C[59]. The American Association for the Study of Liver Diseases recommends first-line therapy with glecaprevir/pibrentasvir and sofosbuvir/velpatasvir for 8 wk and 12 wk respectively for treatment-naïve adults[60]. However, there are no guidelines on antiviral therapy duration for patients with HCV+ HCC or ICC. In HCV-related HCC, HCV eradication therapy has been proven to improve long-term outcomes of HCC undergoing curative treatment[61-63]. Further meta-analyses suggest benefits of HCV treatment on long-term HCC survival outcomes[64,65]. While the literature on the utility of DAAs in HCV+ ICC is scarce, the oncogenesis of ICC is similar to that of HCC. Hence, we theorize similar benefits of HCV eradication therapy in the ICC population.
Adjuvant chemotherapy is recommended for patients with resected cholangiocarcinoma[66]. The American Society of Clinical Oncology recommends the use of adjuvant capecitabine as first-line therapy for 6 mo[67]. However, certain chemotherapy agents are hepatotoxic and may exacerbate or accelerate fibrosis in HCV+ patients with chronic liver inflammation. Studies have suggested that treatment of HCV infection may also reverse cirrhosis in some group of patients (e.g. those without decompensated liver cirrhosis), allowing for the use of adjunct treatment such as chemotherapy[68]. Unfortunately, the use of adjuvant chemotherapy and underlying liver function was not discussed in the included studies and this falls beyond the scope of our study. The combined role of DAAs and adjuvant chemotherapy on underlying liver function and long-term survival should be evaluated.
While our study excluded patients who underwent LT, the use of LT in treating ICC is worth exploring. LT was previously contraindicated in managing ICC due to poor outcomes and high recurrence post-LT. Initial studies reported 3-year OS post-LT ranging from 4.9%-39.0% without receiving pre-transplant treatment and 3-year RFS rate of 28.8-35.0%[69-71]. However, recent studies have reported reasonable outcomes in certain groups of patients with ICC who received LT, with 3-year OS rates post-LT ranging from 47.9%-83.3% and 5-year OS rates ranging from 31.3%-83.3%. 3-year RFS rates also ranged from 41.7%-52.0% in newer studies[72-74]. Transplant outcomes have improved drastically due to improved effectiveness of neoadjuvant therapy such as gemcitabine-based systemic chemotherapy and locoregional therapy including trans-arterial chemoembolization and radiofrequency ablation, in addition to protocols to determine eligibility for LT in patients who demonstrate disease stability or pathological response to these pre-transplant treatment modalities[73-75]. With these improvements in preoperative treatment and a more stringent organ recipient selection process, LT may provide an alternative treatment of cure as standard of care for ICC in the future. Additionally, LT also deals with the problem of cirrhosis and PHLF that comes with LR, which may be a contributing factor to worse survival. With ongoing trials assessing outcomes of LT in ICC currently underway, we anticipate treatment of cholangiocarcinoma to evolve in the future[76-78]. Although not explored in our study, subsequent studies could analyse the impact of HCV infection on outcomes following other treatment modalities in ICC.
There are a few limitations in our study. All the included studies were retrospective observational studies which have inherent selection bias. The absence of high-quality evidence from RCTs and prospective studies may limit interpretation of the outcomes from our analysis. Subsequent studies should employ methods such as PSM and RCTs to reduce bias for more conclusive results. Nevertheless, quality assessment was performed for the included studies and all the included studies had at least moderate quality evidence. The number of studies included in this meta-analysis is relatively small due to our strict inclusion criteria of studies comparing post-hepatectomy outcomes of ICC in HCV+ and HCV- subgroups. All included studies were conducted in Asia, namely Japan and China, despite including ICC globally, hence causing possible limitations in the generalizability of our results. Global incidence of cholangiocarcinoma is highest in Asia, especially Japan[79]. However, incidence of cholangiocarcinoma is rising in Western countries over the past decade, of which their population is underrepresented in our study[80]. Prevalence of chronic HCV infection share a different distribution globally, with middle-low-income countries in the Eastern Mediterranean and European regions suffering the highest burden of disease[81]. Ideally, a more heterogenous sample including these populations would produce results that may be more representative of the global population, hence future studies involving patients from regions of high HCV and cholangiocarcinoma prevalence will provide more insight. We could not perform meta-analysis on RFS and our secondary outcomes due to inadequate data from our included studies. Lastly, this study did not include subgroup analyses of tumors undergoing major hepatectomy. Performing major hepatectomy on a background cirrhotic liver or chronically HCV-infected liver has its additional risks (e.g. PHLF and post-operative mortality). Thus, a separate analysis focusing on this subgroup may provide valuable insight and guidance in management.
Our meta-analysis demonstrated that HCV infection is associated with significantly worse OS in ICC patients undergoing LR with curative intent. Further studies of the underlying mechanisms of oncogenesis of the biliary tree in HCV infection, including genetic and basic science studies are warranted to understand its disease process. More prospective studies with PSM-derived cohorts including analysis of other aspects of treatment such as PHLF and liver augmentation strategies should be conducted to validate our findings.
Incidence of intrahepatic cholangiocarcinoma (ICC) has been rising over the past decade. Hepatitis C virus (HCV) infection is an important risk factor in the development of ICC. Currently, liver resection (LR) remains the only curative treatment modality for ICC. Our study aims to study the outcomes of LR in ICC patients with HCV-positive (HCV+) compared to HCV-negative (HCV-) ICC patients.
Long-term outcomes of curative LR in ICC can be affected by patient and tumor characteristics. The impact of HCV infection on post-LR outcomes should be reviewed and quantitatively concluded.
We aim to identify HCV+ patients as a high-risk subgroup amongst ICC patients undergoing curative LR. Our analysis concluded that HCV+ patients had worse overall survival compared to HCV- patients following LR. Our findings act as a stepping stone for future studies to validate our findings, to determine a cause for this outcome, as well as to devise strategies to improve outcomes in HCV+ ICC patients undergoing curative LR.
Four databases (PubMed, EMBASE, Scopus and The Cochrane Library) were systematically searched for relevant studies, which were subsequently screened for inclusion in our study based on our inclusion criteria. We assessed the quality of included observational studies using the modified Newcastle-Ottawa Scale. There were no randomised controlled trials included in our study. Our primary outcomes were overall survival (OS) and recurrence-free survival. Secondary outcomes include perioperative mortality, operation duration, blood loss, intrahepatic and extrahepatic recurrence. Study variables, primary and secondary outcomes were extracted from included studies. Pooled hazard ratio (HR) was calculated through the inverse-variance method using the natural logarithm of HR [ln (HR)] and standard error. Dichotomous outcomes were pooled and calculated using the Mantel-Haenszel method and expressed as odds ratio (OR) with 95% confidence interval (CI). Continuous outcomes were pooled and calculated using the inverse variance method and expressed as mean difference with 95%CI.
Our meta-analysis demonstrated significantly worse OS in HCV+ patients with ICC that underwent curative resection compared to HCV- patients (HR 2.05, 95%CI: 1.46, 2.88, P < 0.0001). Our analysis also showed increased incidence of cirrhosis (OR 5.78, 95%CI: 1.38, 24.14, P = 0.02), poorly differentiated tumors (OR 2.55, 95%CI: 1.34, 4.82, P = 0.004), as well as simultaneous hepatocellular carcinoma (HCC) lesions in HCV+ patients (OR 8.31, 95%CI: 2.36, 29.26, P = 0.001) compared with HCV- patients. Our findings identify HCV infection as a significant poor prognostic factor in ICC patients undergoing curative LR and as a significant risk factor of liver cirrhosis, poor tumor differentiation and incidence of simultaneous HCC lesions. However, the presence of increased liver cirrhosis and poor tumor differentiation may be confounding factors for worse OS in HCV+ patients. No statistically significant differences were noted between HCV+ and tumor stage, tumor invasion and metastases in our study.
Our study concluded that HCV infection is associated with significantly worse OS outcomes in ICC post-LR. This may be confounded by increased incidence of cirrhosis and poorly differentiated tumors with HCV infection. The exact pathophysiology and confirmation of our findings ought to be explored in future well-designed prospective studies. The role of viral eradication therapy and chemotherapy in this subgroup of patients should also be explored.
Future research should be performed with randomized controlled trials or propensity score matched cohorts to validate our findings. Further studies should also explore the role of adjuncts such as anti-viral therapy and adjuvant chemotherapy in HCV+ ICC patients who underwent curative LR.
| 1. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1037] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 2. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 1787] [Article Influence: 297.8] [Reference Citation Analysis (0)] |
| 3. | Turati F, Bertuccio P, Negri E, Vecchia CL. Epidemiology of cholangiocarcinoma. Hepatoma Res. 2022;8:19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Lin CR, Lee YK, Chiang CJ, Yang YW, Chang HC, You SL. Secular trends of intrahepatic cholangiocarcinoma in a high endemic area: A population-based study. World J Gastroenterol. 2022;28:3695-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | O'Hagan K. Updates in Cholangiocarcinoma. J Adv Pract Oncol. 2022;13:320-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O'Reilly E, Abou-Alfa GK, Shimada H, Blumgart LH, Jarnagin WR. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 688] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 7. | Braconi C, Patel T. Cholangiocarcinoma: new insights into disease pathogenesis and biology. Infect Dis Clin North Am. 2010;24:871-884, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Liau MYQ, Toh EQ, Shelat VG. Opisthorchis viverrini-Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens. 2023;12:795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 9. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22:3038-3051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Hai S, Kubo S, Yamamoto S, Uenishi T, Tanaka H, Shuto T, Takemura S, Yamazaki O, Hirohashi K. Clinicopathologic characteristics of hepatitis C virus-associated intrahepatic cholangiocarcinoma. Dig Surg. 2005;22:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7062] [Cited by in RCA: 5665] [Article Influence: 1133.0] [Reference Citation Analysis (33)] |
| 13. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1852] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 14. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 8086] [Article Influence: 673.8] [Reference Citation Analysis (0)] |
| 15. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 16. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 5111] [Article Influence: 269.0] [Reference Citation Analysis (1)] |
| 17. | Ariizumi S, Kotera Y, Takahashi Y, Katagiri S, Chen IP, Ota T, Yamamoto M. Mass-forming intrahepatic cholangiocarcinoma with marked enhancement on arterial-phase computed tomography reflects favorable surgical outcomes. J Surg Oncol. 2011;104:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Cai Y, Zhang B, Li J, Li H, Liu H, Xie K, Du C, Wu H. A Novel Nomogram Based on Hepatic and Coagulation Function for Evaluating Outcomes of Intrahepatic Cholangiocarcinoma After Curative Hepatectomy: A Multi-Center Study of 653 Patients. Front Oncol. 2021;11:711061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kaibori M, Yoshii K, Kashiwabara K, Kokudo T, Hasegawa K, Izumi N, Murakami T, Kudo M, Shiina S, Sakamoto M, Nakashima O, Matsuyama Y, Eguchi S, Yamashita T, Takayama T, Kokudo N, Kubo S. Impact of hepatitis C virus on survival in patients undergoing resection of intrahepatic cholangiocarcinoma: Report of a Japanese nationwide survey. Hepatol Res. 2021;51:890-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Uenishi T, Nagano H, Marubashi S, Hayashi M, Hirokawa F, Kaibori M, Matsui K, Kubo S. The long-term outcomes after curative resection for mass-forming intrahepatic cholangiocarcinoma associated with hepatitis C viral infection: a multicenter analysis by Osaka Hepatic Surgery Study Group. J Surg Oncol. 2014;110:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Yang H, Wang J, Li Z, Yang Y, Yang L, Zhang Y, Shi Y, Cao Y, Zhou J, Wang Z, Chen Q. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Front Oncol. 2019;9:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Terakawa NS, Satoi H, Yanagimoto H, Yamamoto T, Yamamoto S, Takai AH, Kwon S, Yamamoto Y, Kubota Y, Ueyama Y. The annual changes and clinicopathologic features of cholangiocellular carcinoma in patients with hepatitis C virus in the Kansai Medical University Hospital. JPN J Gastroent Surg. 2004;37:1813-1818. [DOI] [Full Text] |
| 23. | Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Navas MC, Glaser S, Dhruv H, Celinski S, Alpini G, Meng F. Hepatitis C Virus Infection and Cholangiocarcinoma: An Insight into Epidemiologic Evidences and Hypothetical Mechanisms of Oncogenesis. Am J Pathol. 2019;189:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 29. | Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Li YY, Li H, Lv P, Liu G, Li XR, Tian BN, Chen DJ. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol. 2018;18:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 33. | Zaydfudim VM, Turrentine FE, Smolkin ME, Bauer TB, Adams RB, McMurry TL. The impact of cirrhosis and MELD score on postoperative morbidity and mortality among patients selected for liver resection. Am J Surg. 2020;220:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Sasaki K, Shindoh J, Margonis GA, Nishioka Y, Andreatos N, Sekine A, Hashimoto M, Pawlik TM. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg. 2017;152:e165059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 35. | Tsilimigras DI, Sahara K, Wu L, Moris D, Bagante F, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Moro A, Sasaki K, Aucejo F, Zhang XF, Matsuyama R, Endo I, Pawlik TM. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg. 2020;155:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 36. | Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Pawlik TM. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 37. | Baumgartner R, Gilg S, Björnsson B, Hasselgren K, Ghorbani P, Sauter C, Stål P, Sandstöm P, Sparrelid E, Engstrand J. Impact of post-hepatectomy liver failure on morbidity and short- and long-term survival after major hepatectomy. BJS Open. 2022;6:zrac097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Lei GY, Shen L, Junnarkar SP, Huey CT, Low J, Shelat VG. Predictors of 90-Day Mortality following Hepatic Resection for Hepatocellular Carcinoma. Visc Med. 2021;37:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Chapelle T, Op De Beeck B, Huyghe I, Francque S, Driessen A, Roeyen G, Ysebaert D, De Greef K. Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on (99m)Tc-mebrofenin hepatobiliary scintigraphy: can this tool predict post-hepatectomy liver failure? HPB (Oxford). 2016;18:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 41. | Asencio JM, García Sabrido JL, Olmedilla L. How to expand the safe limits in hepatic resections? J Hepatobiliary Pancreat Sci. 2014;21:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 747] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 43. | Kobayashi Y, Kiya Y, Sugawara T, Nishioka Y, Hashimoto M, Shindoh J. Expanded Makuuchi's criteria using estimated indocyanine green clearance rate of future liver remnant as a safety limit for maximum extent of liver resection. HPB (Oxford). 2019;21:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Fassio E. Hepatitis C and hepatocellular carcinoma. Ann Hepatol. 2010;9 Suppl:119-122. [PubMed] [DOI] [Full Text] |
| 45. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 395] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 46. | Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Watanabe T, Sakata J, Ishikawa T, Shirai Y, Suda T, Hirono H, Hasegawa K, Soga K, Shibasaki K, Saito Y, Umezu H. Synchronous development of HCC and CCC in the same subsegment of the liver in a patient with type C liver cirrhosis. World J Hepatol. 2009;1:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Nakamura S, Suzuki S, Sakaguchi T, Serizawa A, Konno H, Baba S, Muro H. Surgical treatment of patients with mixed hepatocellular carcinoma and cholangiocarcinoma. Cancer. 1996;78:1671-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Mao ZY, Guo XC, Su D, Wang LJ, Zhang TT, Bai L. Prognostic Factors of Cholangiocarcinoma After Surgical Resection: A Retrospective Study of 293 Patients. Med Sci Monit. 2015;21:2375-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Nickkholgh A, Ghamarnejad O, Khajeh E, Tinoush P, Bruckner T, Kulu Y, Mieth M, Goeppert B, Roessler S, Weiss KH, Hoffmann K, Büchler MW, Mehrabi A. Outcome after liver resection for primary and recurrent intrahepatic cholangiocarcinoma. BJS Open. 2019;3:793-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Vaquero J, Guedj N, Clapéron A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J Hepatol. 2017;66:424-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 52. | Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Epithelial-to-Mesenchymal Transition and Cancer Invasiveness: What Can We Learn from Cholangiocarcinoma? J Clin Med. 2015;4:2028-2041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Yu TH, Chen X, Zhang XH, Zhang EC, Sun CX. Clinicopathological characteristics and prognostic factors for intrahepatic cholangiocarcinoma: a population-based study. Sci Rep. 2021;11:3990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 54. | Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, Cappellani A, Malfermoni G, Iacono C. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 56. | Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World J Surg. 2011;35:2501-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 572] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 58. | Li T, Li D, Cheng L, Wu H, Gao Z, Liu Z, Jiang W, Gao YH, Tian F, Zhao L, Wang S. Epithelial-mesenchymal transition induced by hepatitis C virus core protein in cholangiocarcinoma. Ann Surg Oncol. 2010;17:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Zeuzem S. Treatment Options in Hepatitis C. Dtsch Arztebl Int. 2017;114:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 561] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 61. | Turgeon MK, Lee RM, Gamboa AC, Yopp A, Ryon EL, Goel N, Wang A, Lee AY, Luu S, Hsu C, Silberfein E, Maithel SK, Russell MC. Impact of hepatitis C treatment on long-term outcomes for patients with hepatocellular carcinoma: a United States Safety Net Collaborative Study. HPB (Oxford). 2021;23:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Luo Y, Zhang Y, Wang D, Shen D, Che YQ. Eradication of Hepatitis C Virus (HCV) Improves Survival of Hepatocellular Carcinoma Patients with Active HCV Infection - A Real-World Cohort Study. Cancer Manag Res. 2020;12:5323-5330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Baumert TF, Jühling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Müllhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Li XH, Zhao CY, Zhou EL, Lin XJ. Efficacy and safety of adjuvant chemotherapy in T1N0M0 intrahepatic cholangiocarcinoma after radical resection. BMC Cancer. 2022;22:1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 68. | Torres HA, Shigle TL, Hammoudi N, Link JT, Samaniego F, Kaseb A, Mallet V. The oncologic burden of hepatitis C virus infection: A clinical perspective. CA Cancer J Clin. 2017;67:411-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 69. | O'Grady JG, Polson RJ, Rolles K, Calne RY, Williams R. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 330] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Wiencken AE, Casagrande VA. Endothelial nitric oxide synthetase (eNOS) in astrocytes: another source of nitric oxide in neocortex. Glia. 1999;26:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Shimoda M, Farmer DG, Colquhoun SD, Rosove M, Ghobrial RM, Yersiz H, Chen P, Busuttil RW. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 73. | Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 74. | McMillan RR, Javle M, Kodali S, Saharia A, Mobley C, Heyne K, Hobeika MJ, Lunsford KE, Victor DW 3rd, Shetty A, McFadden RS, Abdelrahim M, Kaseb A, Divatia M, Yu N, Nolte Fong J, Moore LW, Nguyen DT, Graviss EA, Gaber AO, Vauthey JN, Ghobrial RM. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am J Transplant. 2022;22:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 75. | Huang G, Song W, Zhang Y, Yu J, Lv Y, Liu K. Liver transplantation for intrahepatic cholangiocarcinoma: a propensity score-matched analysis. Sci Rep. 2023;13:10630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Liver Transplantation for Early Intrahepatic Cholangiocarcinoma (LT for iCCA). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT02878473ClinicalTrials.gov Identifier: NCT02878473. |
| 77. | Liver Transplant for Stable, Advanced Intrahepatic Cholangiocarcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04195503 ClinicalTrials.gov Identifier: NCT04195503. |
| 78. | Liver Transplantation for Non-Resectable Intrahepatic Cholangiocarcinoma: a Prospective Exploratory Trial (TESLA Trial). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04556214 ClinicalTrials.gov Identifier: NCT04556214. |
| 79. | Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126:2666-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 80. | Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 81. | World Health Organization. Hepatitis C. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu HB, China; Wang MK, China S-Editor: Qu XL L-Editor: A P-Editor: Guo X