Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.88164
Peer-review started: September 16, 2023
First decision: November 9, 2023
Revised: December 7, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: March 25, 2024
Processing time: 177 Days and 1.1 Hours
Hepatitis C virus (HCV), hepatitis B virus (HBV), and human immunodeficiency virus 1 (HIV-1) are the most epidemic blood-borne viruses, posing threats to human health and causing economic losses to nations for combating the infection transmission. The diagnostic methodologies that depend on the detection of viral nucleic acids are much more expensive, but they are more accurate than sero
To develop a rapid, cost-effective, and accurate diagnostic multiplex polymerase chain reaction (PCR) assay for simultaneous detection of HCV, HBV, and HIV-1.
The design of the proposed PCR assay targets the amplification of a short conserved region featured with a distinguishable melting profile and electrophoretic molecular weight inside each viral genome. Therefore, this diagnostic method will be appropriate for application in both conventional (combined with electrophoresis) and real-time PCR facilities. Confirmatory in silico investigations were conducted to prove the capability of the approached PCR assay to detect variants of each virus. Then, Egyptian isolates of each virus were subjected to the wet lab examination using the given diagnostic assay.
The in silico investigations confirmed that the PCR primers can match many viral variants in a multiplex PCR assay. The wet lab experiment proved the efficiency of the assay in distinguishing each viral type through high-resolution melting analysis. Compared to related published assays, the proposed assay in the current study is more sensitive and competitive with many expensive PCR assays.
This study provides a simple, cost-effective, and sensitive diagnostic PCR assay facilitating the detection of the most epidemic blood-borne viruses; this makes the proposed assay promising to be substitutive for the mistakable and cheap serological-based assays.
Core Tip: The current study approaches a cost-effective diagnostic assay to detect the most common blood-borne viruses (hepatitis C virus, hepatitis B virus, and human immunodeficiency virus 1) in a single test of multiplex polymerase chain reaction (PCR). This article includes the procedures in computational biology to achieve the PCR design and the practical examination of the given assay for detecting the targeted viruses with the interpretation of the results.
- Citation: Nemr WA, Nashwa RK. Development of a multiplex polymerase chain reaction assay for detection of hepatitis C virus, hepatitis B virus, and human immunodeficiency virus 1. World J Virol 2024; 13(1): 88164
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/88164.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.88164
The primary concerned blood-borne pathogens are hepatitis C virus (HCV), hepatitis B virus (HBV), and human immunodeficiency virus 1 (HIV-1). They can be transmitted by contacting the viral particles with wounded skin, mucous membranes, or blood during unsafe medical treatment, blood transfusion, or unprotected sex actions. The World Health Organization (WHO) reported that the inflammation of the liver (hepatitis) may lead to liver cirrhosis and cancer. Viral hepatitis is one of the major reasons of mortality worldwide; more than 350 million patients are affected by the infection with HCV or HBV. They cause death for more than 1.1 million people every year. If the spreading of the hepatitis viruses will not be addressed, it is expected that more than 3 million people will be newly infected every year. Accordingly, United States $150 million has been allocated to fund international programs specialized in infection control and treatment of hepatitis viruses (https://www.who.int/news/item/17-05-2023-high-level-resource-mobilization-conference-to-eliminate-viral-hepatitis).
On the other hand, HIV attacks the body’s immune system, making the body more vulnerable to microbial infection and cancer. The recent WHO records reported that HIV caused infection for more than 39 million people and death for 630000 people until 2022 (https://www.who.int/health-topics/hiv-aids#tab=tab_1).
Given that the first line to combat the infection transmission of these pathogens is the accurate screening for infected patients, this makes the invention of precise diagnostic assays highly interesting. Serological assays and nucleic acid amplification testing (NAT) are the most common methods for viral diagnosis. Serological methods can detect viral antigens or their specific antibodies in blood specimens, while NAT identifies viral genomes in any biological sample (such as tissues and body liquids).
The selection of a suitable diagnostic system depends on the ability of the assay to detect a wide range of viral variants with the same precision. This requires the knowledge of the viral biodiversity in the targeted geographic region. Many different subtypes diverged from HCV genotypes 1-3 are globally epidemic. They are sub-classified according to the divergence in the viral genome sequence. Africa shows the highest prevalence rate of HCV (5.3%), while Egypt recorded the highest burden of HCV genotype 4 (prevalence rate = 17.5%) until 2013[1]. However, recent records revealed a decrease in this percentage in Egypt after a national program for antiviral medications for HCV-infected patients[2].
Although serological tests are cheaper and faster, they are less accurate and may result in false positive or false negative reactions. The false negative may be exhibited due to the inability to detect the viral markers in samples with a low viral load (which may occur in recently infected patients)[3], or the difficulty of finding antibodies in samples derived from immune-suppressed patients and patients with current HIV infection[4]. On the other hand, the false positive may be exhibited in samples derived from patients who received interfering medications such as immunoglobulin[5], patients with rheumatoid arthritis[6], and patients who recovered from a previous infection[7], or due to the cross-reactivity of some antibodies with similar viral antigens[4]. Furthermore, antibody-targeted serological assays cannot differentiate between active- or past-infected patients. Also, these assays are inappropriate for examining recently infected patients because the detectable level of specific antibodies cannot be achieved before at least 2 mo of the viral infection[8].
Despite the cost, the NAT-based diagnostic methods [such as polymerase chain reaction (PCR)] are more effective and accurate than the mistakable serological methods[7]. Furthermore, PCR methodologies are easy to establish into highly throughput automation systems with the benefits of low risk of contamination, regardless of whether during reaction preparation or postoperative chemical disposal[9].
The current study aimed to develop a rapid, cost-effective, and accurate diagnostic multiplex PCR assay, which depends on the simultaneous detection of HCV, HBV, and HIV-1. Such cost-effective NAT-diagnostic assays will be promising to be a good substitute for cheap serological assays, especially in low-income countries.
Viral genomic data were retrieved for different isolates of HCV, HBV, and HIV-1 from the National Center for Biotechnology Information (NCBI) website. Previously published specific primers for each virus were computationally evaluated for the best matching with a wide range of different isolates. Therefore, ClustalW multiple sequence alignment was carried out to determine the lowest variability region among the whole genome sequences of different geographic strains or genotypes of each virus (Table 1). This is to verify that the selected primers are located and surround a universally conserved region that shares homologous sequences among different aligned sequences. The ClustalW alignment and the entropy analysis of aligned sequences were conducted using BioEdit software[10]. Then, amplicons located inside genomic regions that showed the lowest entropy values were selected for further analyses.
| Virus | Taxon ID | Classification | Country of isolation | NCBI accession number |
| HCV | 2847144 (genotype 1a) | Isolate ZS30 | China | KC844049 |
| 11103 (genotype 1b) | Isolate 2000621 | Israel | MT632133 | |
| 31649 (genotype 2) | Subtype 2a, isolate PR63 | China | KF676351 | |
| 356426 (genotype 3) | Subtype 3a | India | GQ275355 | |
| 356418 (genotype 4) | Subtype 4a, strain ED43 | Egypt | GU814265.1 | |
| 33746 (genotype 5) | Subtype 5a | United Kingdom | NC_009826 | |
| 356469 (genotype 6) | Subtype 6k, isolate KM41 | China | DQ278893 | |
| HBV | 489455 (genotype A) | Strain AON | Japan | LC488828 |
| 489460 (genotype B) | Isolate 4265-Viet12 | Viet Nam | LC064379 | |
| 2764122 (genotype C) | Isolate C173334 | Cambodia | LC535933 | |
| 2847137 (genotype D) | Isolate B-H10-Ban | Bangladesh | LC519824 | |
| 2847138 (genotype E) | Isolate Mart-B84 | Martinique | HE974384 | |
| 2847139 (genotype F) | Isolate VHB-PER036 | France | LT935669 | |
| 2847140 (genotype G) | Isolate MEX918M | Mexico | AB625342 | |
| 2847141 (genotype H) | Isolate Itabashi | Japan | LC491577 | |
| 2847142 (genotype I) | Isolate 8290 | Viet Nam | AF241411 | |
| HIV | 11676 (HIV-1) | Isolate 99SE-MP1299 (subtype O) | Senegal | AJ302646 |
| Isolate 5104_SEB_AIM_E3 | United States | MT190832 | ||
| Isolate 01AETH04BKM | Thailand | DQ314732 | ||
| Isolate RBF168 | France | GU111555 | ||
| Clone pCMO2.3 | Cameroon | AY618998 | ||
| Isolate 01ZATM45 (subtype: C) | South Africa | AY228557 | ||
| Isolate 193008 (subtype: AD) | Uganda | MW006063 | ||
| Isolate BI | Belgium | MN486005 | ||
| Isolate 99GR303 | Greece | AY046058 | ||
| Isolate HK002 (subtype: B) | Hong Kong | FJ460499 | ||
| Isolate SE8646 | Sweden | AY352654 | ||
| Isolate 01BRRJUD508 (subtype: F1) | Brazil | MG365771 | ||
| Isolate 04KBH8 (subtype: D) | South Korea | DQ054367 | ||
| Isolate D9451 (subtype: URF) | Japan | MN187301 | ||
| isolate C.IN.05.NIRT333.1 (subtype: C) | India | KF766540 | ||
| Isolate 98UA0116 (subtype: A; group: M) | Ukraine | AF413987 | ||
| Isolate M61 | Spain | DQ854714 | ||
| Isolate MtBs.18 | Russia | MK984159 | ||
| Isolate IIIB | United Kingdom | KJ925006 | ||
| Isolate MBC200 | Australia | AF042100 | ||
| Isolate 60000 (subtype: A1 variant) | Italy | EU861977 | ||
| Isolate TV721 | Canada | HM215249 | ||
| Isolate pXJDC6291-2-6 (subtype: CRF07_BC) | China | KC503852 |
Best-matched primers with the universally conserved regions, inside variables’ genomes of each virus, were examined for some specific criteria. The capability of primer pairs to work in a combined mixture was evaluated, as multiplex PCR primers, by predicting their ability to form dimers in silico, using AutoDimer software at a minimal score (= 4)[11].
Particularly, the selection of suitable primers was based on avoiding any primer that could form stable hairpins or dimers (whether by inter- or intra-oligos) having ΔG < -9 kcal/mol, or if their estimated melting temperature (Tm) is close to the expected primer annealing temperature (if the difference of Tm < 10 °C), according to primer design guidelines[12].
The harmonization of the selected primers was carried out to adjust the Tm of all primers at a suitable unique Tm value. This was done by adjusting the nucleotide length and the percentage of guanine and cytosine (%GC) inside each primer. Hence, these primers will be able to work at similar annealing temperatures during the triplex PCR run. Consequently, some selected primers were modified to equalize their melting temperature which was standardized at 60.095 ± 0.5 °C.
Therefore, primers’ melting temperature and their predicted amplicon sequences were determined using the NCBI Primer-BLAST web tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)[13]. The melting profile of each amplicon sequence was predicted by utilizing the uMELT web tool (https://www.dna-utah.org/umelt/quartz/um.php)[14].
Positive human plasma samples for HCV (genotype 4), HBV (genotype D), and HIV-1 were obtained from the Microbiology Reference Lab of the National Blood Transfusion Service, Ministry of Health, Egypt. These positive samples were tested to validate the theoretical design of the triplex PCR. Viral genomes were extracted using the PREP-NA-S DNA/RNA extraction kit (product# P-007-N, DNA-Technology LLC., Moscow) according to manufacturer’s instructions. The viral load of each sample was determined using commercial real-time PCR quantitative kits manufactured by DNA-Technology LLC (Moscow), including Hepatitis C Virus Quantitative Real-Time PCR Kit (#Q4-P603-24), Hepatitis B Virus Quantitative PCR Kit (#Q2-P602-24), and Human Immunodeficiency Virus Quantitative PCR Kit (#R3-P609-S3). Plasma samples were diluted with normal saline solution to serial logarithmic concentrations till achieving the viral load equivalent to 100 IU/mL. Then, further gradual dilutions were made, which ranged from 10 to 90 IU/mL. The amplification efficiency (E) and the limit of detection (LOD) of both monoplex and triplex PCRs were determined according to previously published guidelines[15]. The E values were calculated by the following equation: E = 10-(1/n).
Where n is the slope of the regression line of the calibration curve which is plotted by [log of concentration, quantification cycle (Cq value)] as (x, y) values. Then, the percentage of PCR amplification efficiency was determined by: %E = (E - 1) × 100.
The LOD was estimated as the lowest concentration of viral load that could be determined by the assay with a sensitivity and specificity both ≥ 90% (95% confidence interval). The sensitivity and specificity of the test were estimated according to Wang et al[16] by the following calculations: Sensititvity = Ture positivity/(Ture positivity + false negativity) × 100, Specificiy = Ture negativity/(False positivity + ture negativity) × 100.
The statistical evaluation of the diagnostic assay was conducted using MedCalc statistical software.
Practically, viral genomes were extracted from the diluted samples by the same extraction kit. Purified viral genomes (whether RNA or DNA) were added as templates with 10 pmol of each primer in reverse transcription (RT) reaction which was carried out using Invitroge SuperScript™ III Reverse Transcriptase according to the manufacturer’s instructions (Fisher Scientific Ltd., United Kingdom), to produce a complementary DNA (cDNA) amplicon from each viral RNA genome (HCV and HIV-1). An amount of 10 μL of the obtained RT reaction volume was transferred to a real-time PCR tube containing 10 pmol of each primer (Table 2) in iQ™ SYBR® Green Supermix reaction mix (Bio-Rad Laboratories, United States), according to the guide manual. The real-time PCR run was performed on a Rotor-gene Q 5plex high-resolution melting (HRM) machine (QIAGEN, United States) and was analyzed using its built-in software. The thermal profile of the PCR included 35 cycles of 95 °C for 30 s (denaturation), 58 °C for 15 s (annealing), and 72 °C for 30 s (extension) which was followed by optical fluorescent emission reading at the green channel. PCR product melting profile was obtained using end-point HRM analysis at 0.5 °C resolution. Additionally, PCR products were separated on 1.5% agarose gel electrophoresis to verify the accuracy of the experiment at the expected molecular weights.
| Virus | Primers | Primer Tm1 | Intended matches | Amplicon size (bp) | Amplicon Tm2 (oC) | Ref. | |
| HCV | Forward | GGTGCACGGTCTACGAGAC | 60.15 | HCV genotype 1 subtypes: 1a, 1b, 1c, 1g, and 1e. HCV genotype 2 subtypes: 2a, 2b, 2c, 2e, 2f, 2k, and 2m. HCV genotype 3 subtypes: 3a, 3b, 3g, 3i, and 3k. HCV genotype 4 subtypes: 4a, 4d, 4f, 4g, 4l, 4m, 4n, 4o, 4r, and 4v. HCV genotype 5 subtype: 5a. HCV genotype 6 subtypes: 6a, 6e, 6h, 6k, 6l, 6m, 6n, and 6r. HCV genotype 7 subtype: QC69. Unclassified HCV subtypes: 08.40.072, 08.80.075, 08.80.014, 08.80.070, 2b/1a, 2k/1b, and M2123. Recombinant HCV viruses | 64 | 86.5 | Chen et al[28], with modifications |
| Reverse | GCCTTGTGGTACTGCCTGAT | 60.04 | Chen et al[28] | ||||
| HBV | Forward | CTTCATCCTGCTGCTATGCCT | 60.20 | HBV genotypes A, A1, A2, and A3. HBV genotype B. HBV genotypes C and C1. HBV genotypes D and D4. HBV genotype E, including the relative Egyptian isolates AC# KU736891 and KU736892. HBV genotypes F, F2, and F4. HBV genotype G. HBV genotype H. HBV recombinant A/E. HBV recombinant B/C | 71 | 80.5 | Kishk et al[29] |
| Reverse | GACAAACGGGCAACATACCTT | 59.79 | Prakash et al[30], with modifications | ||||
| HIV | Forward | GCCTCAATAAAGCTTGCCTTGA | 59.51 | HIV type 1. Simian immunodeficiency virus | 121 | 85.5 | Rouet et al[31] |
| Reverse | GGCGCCACTGCTAGAGATTTT | 61.01 | |||||
The current study assigned four important in silico parameters as successful criteria to reach the suitable combination of triplex PCR primers. This is to improve the accuracy of the detection for all targeted viral genomes (HCV, HBV, and HIV-1) in a single PCR test. The first parameter is the suitability of the selected primer to match a wide range of subtypes of each virus. Thus, primers that surround the lowest entropy regions along the aligned whole genome sequences of each virus were selected as genus-specific universal primers (Figure 1). Therefore, the produced amplicon was predicted to be homologous among all genotypes of each virus. This is desirable for obtaining a universal PCR product specific to each type of virus. As shown in Table 2, the set of the selected primers could detect HCV genotypes 1-7 including 44 subtypes and their recombinant strains, HBV genotypes (A-G) including the recombinant strains A/E and B/C, and almost all HIV type 1 subtypes plus simian strains.
The second parameter is to obtain a similar annealing temperature for all primers. Therefore, some primers were modified to obtain a similar Tm value (60.095 ± 0.5 °C) among all the selected primers. The third parameter is to select primers that exhibit lower stable dimers or hairpin forms to increase their efficacy to match their targets during PCR. AutoDimer software outputs revealed that there are no hairpins and three low stable-primer dimers which may be formed at the maximum temperature < 22.3 °C. The first dimer is formed by HIV reverse primer with itself (ΔG = -6.23 kcal/mole), and there are two self dimers formed by HCV forward primer (ΔG = -5.37 and -1.76 kcal/mole) (Figure 2). However, these dimers will not be efficient at annealing temperatures higher than 33 °C which prevents their stability and dimerization.
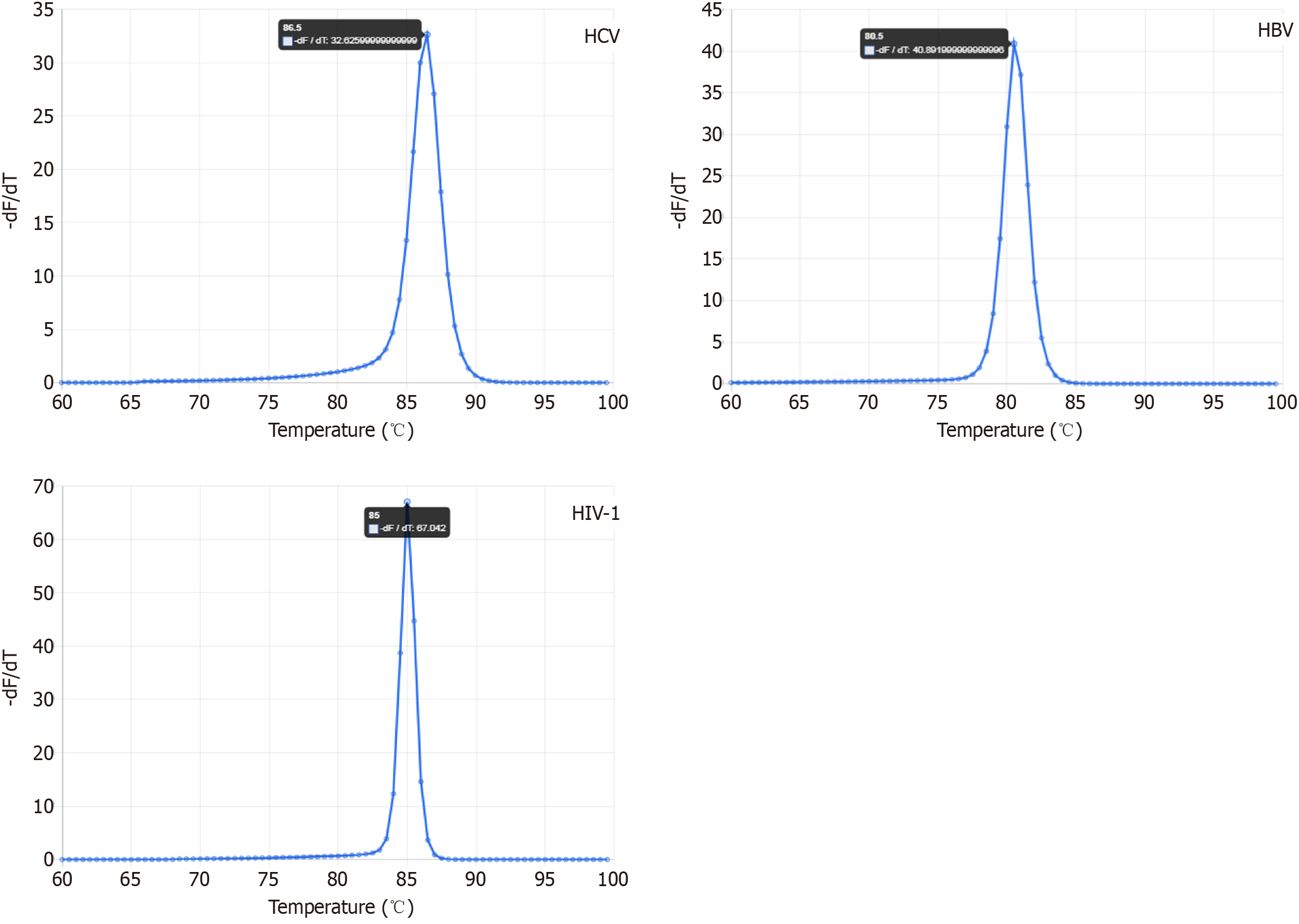
The fourth parameter is based on the ability to discriminate each amplicon of each virus by a distinguished melting profile using HRM. Therefore, the predicted melting curve of each amplicon revealed a distinguishable single melting point which is represented by a single peak, as shown in uMELT analysis data for each viral PCR product (Figure 3, Table 2).
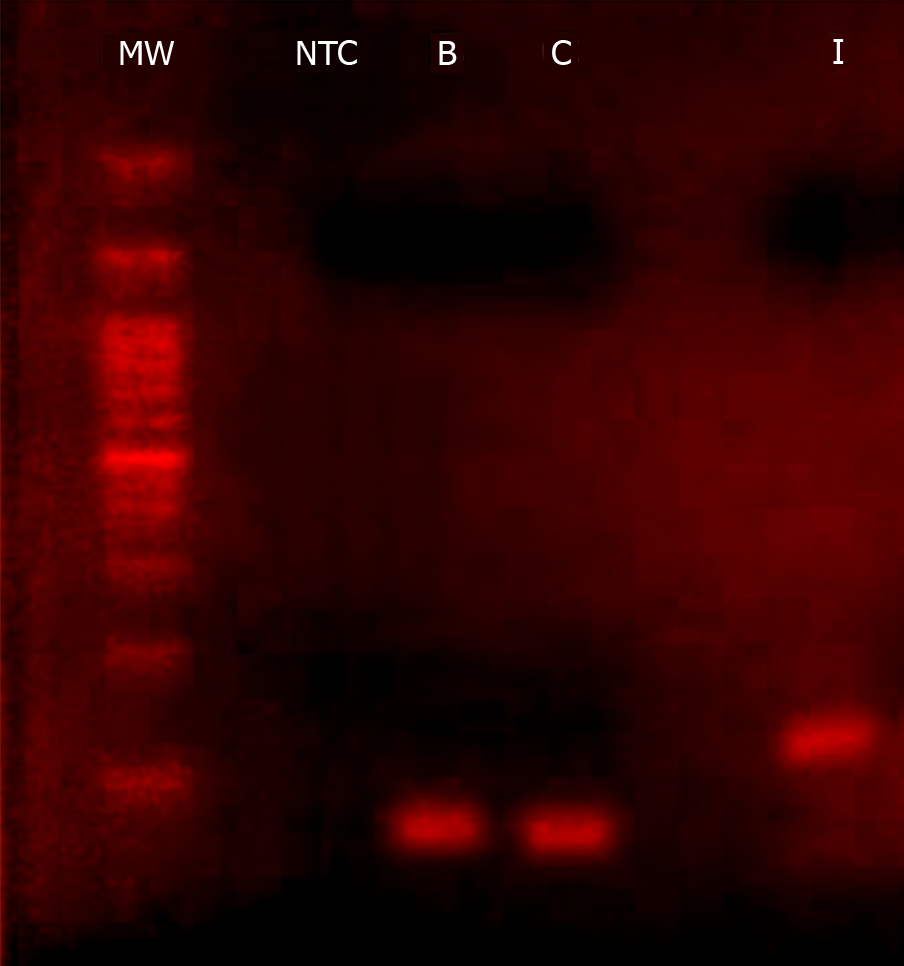
According to the in silico evaluations, a single PCR product will be obtained from both triplex and monoplex PCRs for each viral genome. This was verified by the visualization of a single band on gel electrophoresis at the expected molecular weight and a distinguished single melting curve peak for each virus (Figures 4 and 5). In detail, electrophoresis migration of PCR products resulted in a single band for HCV at 65 bp (base pairs), HBV at 71 bp, and HIV at 121 bp, without the appearance of non-specific amplifications or primer dimers in the gel.
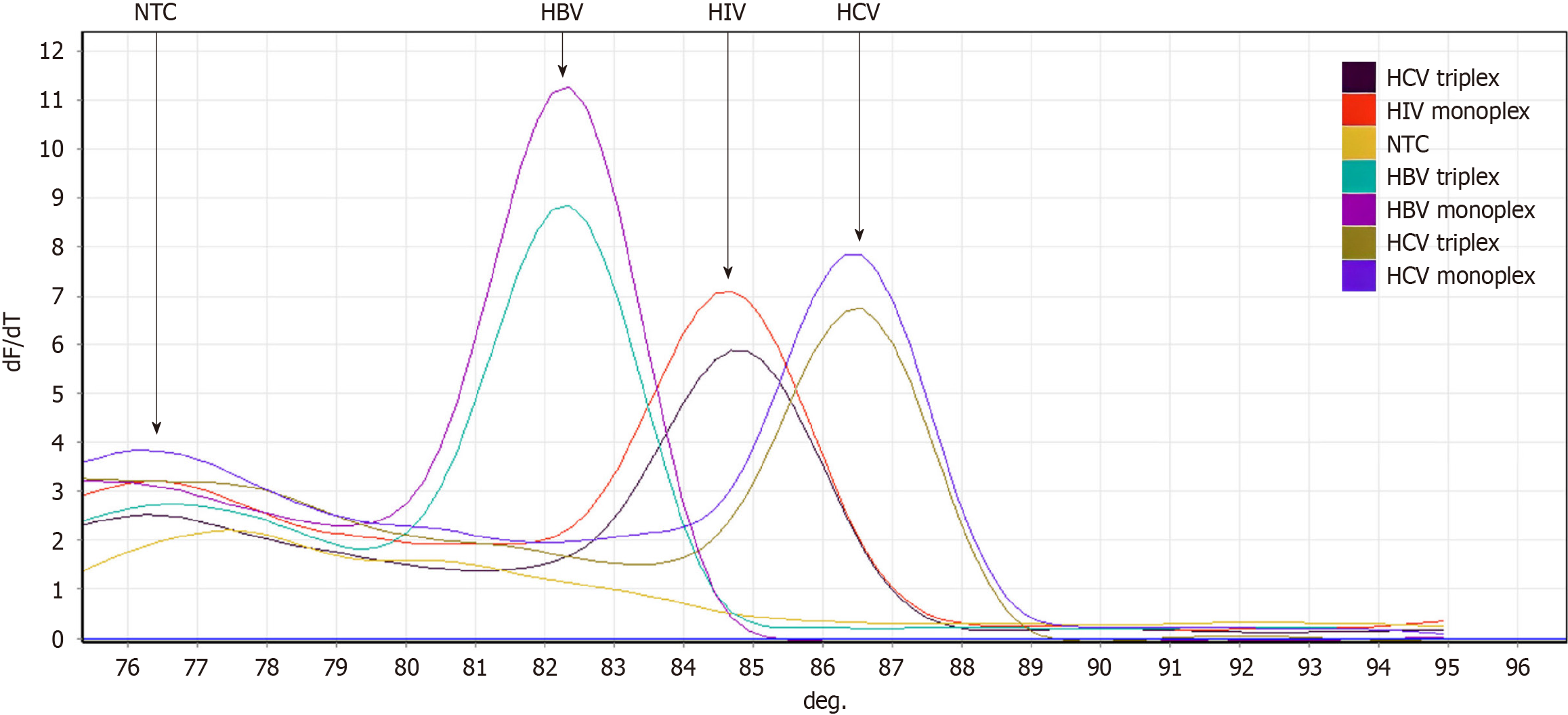
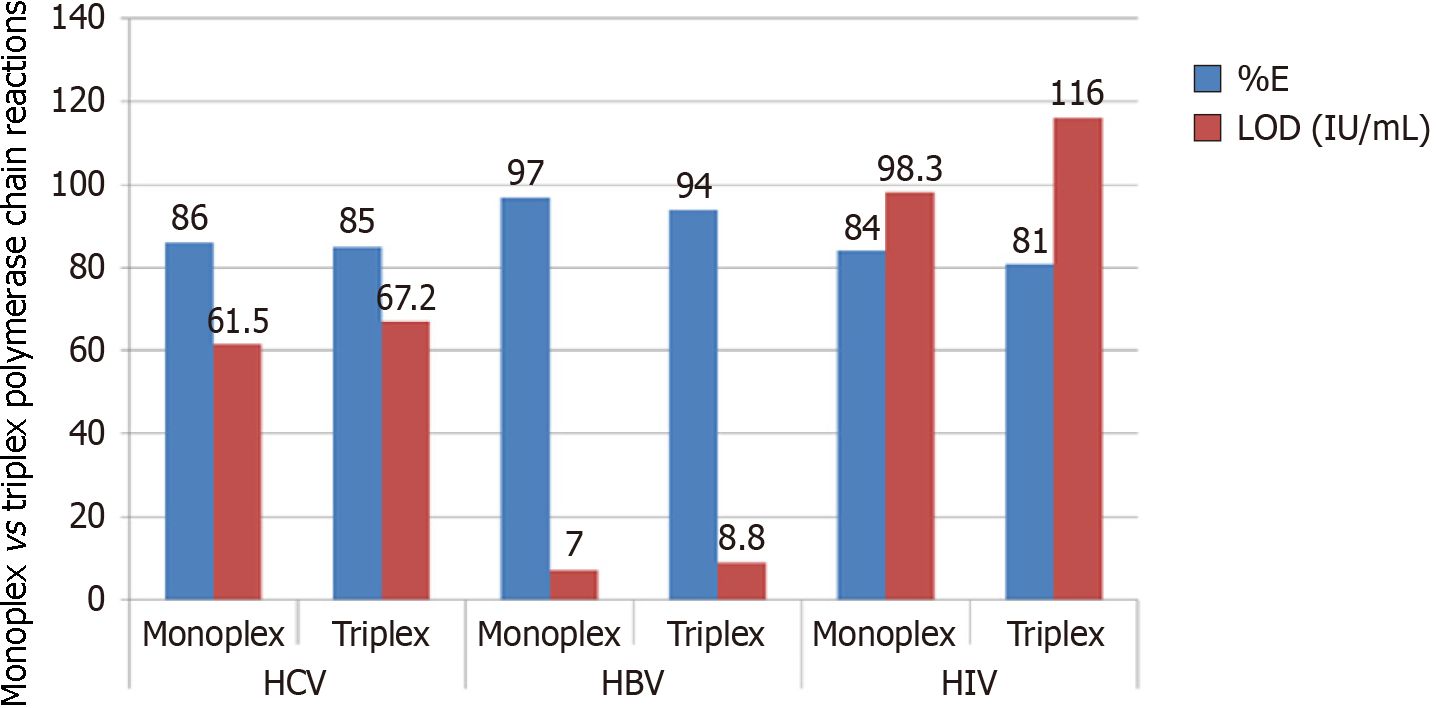
Accordingly, HRM analysis revealed that the formation of oligonucleotides dimers or non-specific amplicons was negligible; a tiny melt-curve peak emerged at a lower melting temperature (76.2 °C), and this signal rose from unintended noises of non-reacted nucleic acids. These noises did not interfere with the peaks of each viral amplicon (82 °C for HBV, 84.8 °C for HIV, and 86.7 °C for HCV). Accurately, each viral amplicon showed similar and reliable melting profiles, even by using monoplex or triplex primer sets. When both of these PCR sets were run with the LOD viral copies, HRM showed that the peak height of monoplex PCRs was somewhat greater than the analogous triplex ones (Figure 5). This agreed with the estimated rational median of %E which was only higher as 3.19% and the LOD was decreased to -15% for monoplex PCRs over the opposite triplex PCRs (Figure 6). Overall, the obtained LOD values satisfy 100% for specificity and more than 90% for sensitivity at 95% confidence interval. Statistically, the correlation between %E and LOD equals -0.966 which is significant (P < 0.002) at the 0.01 level (2-tailed Pearson correlation).
The development of accurate and cost-effective detection methods for diagnosing epidemic viruses attracts the concern of many scientists and manufacturers. Commercially, serological-based diagnostic kits are lower priced than NAT-based ones. However, many previous studies praised the accuracy of NAT over serological assay[17-20]. Such diagnostic methodologies are important for screening blood samples to confirm the absence of blood-borne viruses before transplant or blood donation. This is important to ensure the safety of biological sample transplantation and to besiege the spreading of epidemic viruses.
Because RNA viruses have a better ability for mutation than DNA viruses, many variants were generated with multiple mutated locations in their RNA genomes. Thus, this may result in false negative PCR detection when the primer or the probe cannot match mutated targets[21]. Consequently, many in silico investigations in this study were conducted to evaluate the conservation of primer matching sites; this is to decrease the possibility of mismatching with their targeted genomes and to ensure the capability of the given PCR test to detect a wide range of variants for each virus. In contrast, many similar published studies lack enough evidence in the same regard[3,16,22]. Additionally, there is a lack of guiding information in the literature on how to design new universal primers for many variants of a particular organism. This information should also include a demonstration of how to use computer simulations to evaluate the specificity of the primers in multiplexed PCRs.
Therefore, in the current study, the methodology of the primer design was clarified with the interpretation of primer analysis. Particularly, the PCR assay utilizes a fluorescent intercalating dye (Sybr-green) to recognize the new synthetic double-stranded PCR products in the reaction pool. Accordingly, the addition of TaqMan probes, as fluorescent reporters, is not needed; this reduces the cost per run and reduces the possibility of variable oligonucleotide cross-reactivity which may cause interference in the multiplex PCR pool. Given that the PCR efficiency in Sybr-green PCRs and TaqMan is comparable[23], this increases the benefit of using Sybr-green PCRs. However, the possible formation of primer dimers may cause overlapped Syber-green emission signals. Therefore, it is important to calculate the Tm value of the expected amplicon sequence to specify its fluorescent signals through the HRM analysis[24]. In addition, the sequence of some primers was modified in the current study to enhance the accuracy of primers annealing to their intended targets at a uniformed Tm lower than each amplicon’s Tm, as proved in the in silico evaluations. These evaluations also proved the suitability of the selected primers to detect a wide range of variants for each virus in a multiplex PCR without detectable cross-reactivity.
The evaluations of the practical laboratory experiment revealed that the %E value was greater in HBV (having DNA genome) than in HCV and HIV (having RNA genome). This may be due to the limited sensitivity of the cDNA synthesis, which is an important step to provide PCR with a DNA template synthesized from a template of RNA sequence. Consequently, the LOD value in HBV-PCRs was lower than that in PCRs detecting RNA viruses. Therefore, this correlation between %E and LOD reflects the importance of improving the %E of the PCR to increase the sensitivity of the diagnostic assay. Accordingly, further developmental trials are required to enhance the sensitivity of cDNA synthesis by trying more sensitive reverse transcriptases or using single-step RT-PCR systems.
Referring to the global hepatitis program report of the WHO (https://www.who.int/hepatitis/publications/annex_4-7.pdf), the most sensitive PCR-based qualitative detection kits for HCV (as a representative example for the other targeted viruses) have lower LOD values (up to 60 IU/mL) than serological kits which have LOD values greater than 1000 IU/mL. However, the cost price per test of such PCR kits ranges from $20 to $100, depending on whether the kit is manual- or automated-based technology. Furthermore, the PCR cycling time to perform a single run for each virus ranges from 2-3 h, in addition to more than 1 h for the viral nucleic acid extraction procedure. Hence, to determine three types of viruses by separately specific PCR tests, the cost and the running time will increase three times, besides the effort, extra time labor cost, and instrument maintenance cost. However, this study revealed that triplex PCRs have fewer amplification efficiencies than analogous monoplexes, but they still maintain the benefit of the competitive sensitivity with the compensation of their less cost and time. This reflects the good impact of the proposed multiplex PCR assay for potential application especially in low-income countries.
The approached methodology in the present study reduces the cost of determining the three viruses simultaneously to $15 (price in January 2022) in a single tube, and this also reduces the work and time to achieve the final results within 2.5 h. In addition, the ability to detect a wide range of subtypes of each targeted virus increases the benefits of this assay.
Moreover, the LOD in the present assay is greater than the LOD of the serological tests and is competitive with the expensive TaqMan-based assays. In detail, as described above, the LOD of most HCV-serological kits is greater than 1000 IU/mL (according to the previously mentioned WHO report), while the present assay of the current study exhibited LOD values at 61.5 IU/mL for HCV, 7 IU/mL for HBV, and 98.3 IU/mL for HIV-1 in the monoplex reaction. These values were increased to 67.2, 8.8, and 116 IU/mL, respectively, in the multiplex reaction.
The achieved LODs in a related study, which aimed at developing Sybr-green-based duplex PCR to detect HCV and HIV-1, were equivalent to 568 and 232.6 IU/mL, respectively[25]. In another study, the achieved LODs reached 114 IU/mL for HCV and 291 IU/mL for HIV-1[26]. Compared to a TaqMan-based assay conducted by Meng et al[27], the automated-complete system (performs the nucleic acid extraction and multiplex PCR run in a closed system) for detecting the HCV, HBV, and HIV-1 exhibited LOD values equivalent to 77 IU/mL, 5.4 IU/mL, and 24.7 IU/mL, respectively.
Accordingly, the assay of the present study showed greater sensitivity than the published Sybr-green-based assays and is a good competitive to the expensive automated TaqMan systems. Generally, the development of combined automated diagnostic systems improves the sensitivity of the assay; this is due to the reduction of the loss to viral templates-copy number through the extraction and cDNA synthesis steps. This indicates that the further development of the proposed PCR assay in this study to apply it in an automated-complete system will result in promising outcomes and further save time and cost. This is because the using of fluorescent intercalating dyes (such as Sybr-green) as reporter dyes in real-time PCR systems, eliminates the need to add expensive fluorescent-labeled probes. However, the specifying of Sybr-green-fluorescent signals to a particular amplicon in the multiplex PCR is urgently required through the HRM analysis to distinguish the detected virus.
The current study approached a rapid, cost-effective, and sensitive PCR-based diagnostic assay for the simultaneous detection of HCV, HBV, and HIV-1 in a single tube. In silico and in vitro evaluations proved the eligibility of this assay for application in large-scale screening of blood samples. Further developmental studies are recommended to translate this work into a commercial product.
The most epidemic blood-borne viruses are hepatitis C virus (HCV), hepatitis B virus (HBV), and human immunodeficiency virus 1 (HIV-1). They cause mortality for millions of people worldwide. Although serology diagnostic methods are less accurate than nucleic acid amplification testing (NAT) for detecting blood-borne viruses in blood samples, they are commonly used to save money and time.
The innovation of rapid and cost-effective NAT assays will allow us to substitute the mistakable serologoical assays, and this can be achieved through a multiplex polymerase chain reaction (PCR) assay for the simultaneous detection of blood-borne viruses in a single test.
The present study focused on developing a new multiplex PCR assay for simultaneous detection of HCV, HBV, and HIV-1 in a single tube.
The in silico design of the PCR assay targets conserved sequences in each viral genome among all variants. This was evaluated by multiple sequence alignment and finding the lowest entropy regions. The selected primers were evaluated also to avoid the possibility of forming stable dimers or hairpins. All primers were harmonized in the melting temperature to anneal at the same temperature during PCR. A practical experiment was conducted to prove the feasibility of the present assay.
The in silico evaluations proved the worthiness of the selected primers to match with many variants of each virus with negligible ability to form dimers, this ensures the efficiency of the proposed PCR assay. Consequently, the sensitivity of this assay showed the ability to detect HCV at a limit of detection (LOD) of 61.5 IU/mL. Furthermore, the LOD value is 7 IU/mL for HBV and 98.3 IU/mL for HIV-1.
The proposed cost-effective PCR assay of the current study achieved a competitive sensitivity with the analogous multiplex PCR assays.
The findings of the current study encourage further developmental studies to apply this assay in an automated system for large-scale virology screening of blood samples.
The authors would like to thank Dr. Ihab Serag, Microbiology Reference Lab of National Blood Transfusion Service, Ministry of Health, Egypt, for supplying infected human plasma samples.
| 1. | Karoney MJ, Siika AM. Hepatitis C virus (HCV) infection in Africa: a review. Pan Afr Med J. 2013;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Abdel-Gawad M, Nour M, El-Raey F, Nagdy H, Almansoury Y, El-Kassas M. Gender differences in prevalence of hepatitis C virus infection in Egypt: a systematic review and meta-analysis. Sci Rep. 2023;13:2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Ohnuma H, Tanaka T, Yoshikawa A, Murokawa H, Minegishi K, Yamanaka R, Lizuka HY, Miyamoto M, Satoh S, Nakahira S, Tomono T, Murozuka T, Takeda Y, Doi Y, Mine H, Yokoyama S, Hirose T, Nishioka K; Japanese Red Cross NAT Screening Research Group. The first large-scale nucleic acid amplification testing (NAT) of donated blood using multiplex reagent for simultaneous detection of HBV, HCV, and HIV-1 and significance of NAT for HBV. Microbiol Immunol. 2001;45:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kheirabad AK, Farshidfar G, Nasrollaheian S, Gouklani H. Prevalence and Characteristics of Precore Mutation in Iran and Its Correlation with Genotypes of Hepatitis B. Electron Physician. 2017;9:4114-4123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Sánchez Herrero A, Nieto Benito LN, Rosell Díaz AM, Pulido-Pérez A. False-Positive Serology for Hepatitis B After Intravenous Immunoglobulin Therapy for Toxic Epidermal Necrolysis. Actas Dermo-Sifiliograficas. 2020;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Batten RL, Payne B, McPherson S, Thompson B. 001. False-Positive Hepatitis B Serology Due to Suspected Cross-Reactivity in a Patient with Rheumatoid Arthritis. Rheumatology. 2015;54:i50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Faraz M, Mansoori H, Ali M, Ali SA. Different Shapes Of Megakaryocytes In Essential Thrombocythemia. J Ayub Med Coll Abbottabad. 2022;34:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wang L, Lv H, Zhang G. Hepatitis C virus core antigen assay: an alternative method for hepatitis C diagnosis. Ann Clin Biochem. 2017;54:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Roth WK. History and Future of Nucleic Acid Amplification Technology Blood Donor Testing. Transfus Med Hemother. 2019;46:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 1999. [DOI] [Full Text] |
| 11. | Vallone PM, Butler JM. AutoDimer: a screening tool for primer-dimer and hairpin structures. Biotechniques. 2004;37:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 376] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Nybo K. DNA and general PCR methods: PCR primer design. Biotechniques. 2009;46:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 13. | Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3185] [Cited by in RCA: 4125] [Article Influence: 294.6] [Reference Citation Analysis (0)] |
| 14. | Dwight Z, Palais R, Wittwer CT. uMELT: prediction of high-resolution melting curves and dynamic melting profiles of PCR products in a rich web application. Bioinformatics. 2011;27:1019-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Kralik P, Ricchi M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front Microbiol. 2017;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 572] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 16. | Wang N, Gao XQ, Han JX. Simultaneous detection of HBV and HCV by multiplex PCR normalization. World J Gastroenterol. 2004;10:2439-2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | El Sanousi SM, Osman ZA, Mohamed A, Al Awfi MS, Babair YH, Babair MH. Comparison of real-time PCR versus ELISA in the diagnosis of cytomegalovirus infection in pregnant women. Clin Microbiol Infect Dis. 2016;. [DOI] [Full Text] |
| 18. | Vigne E, Garcia S, Komar V, Lemaire O, Hily JM. Comparison of Serological and Molecular Methods With High-Throughput Sequencing for the Detection and Quantification of Grapevine Fanleaf Virus in Vineyard Samples. Front Microbiol. 2018;9:2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Hwang KA, Ahn JH, Nam JH. Diagnosis of viral infection using real-time polymerase chain reaction. J Bacteriol Virol. 2018;48:1. [DOI] [Full Text] |
| 20. | Ibrahim AM, Abo-El-Azaem NG, Mohamed MA, Ghaith AA, Ahmed SH, Zaki MM. Evaluation of some available HCV antibody detection tests (ELISA, Chemiluminescence, Immune Assay) and RT-PCR assay in the diagnosis of Hepatitis C virus infection. Egyptian J Hospital Med. 2018;72:4874-4879. |
| 21. | Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 710] [Article Influence: 118.3] [Reference Citation Analysis (3)] |
| 22. | Candotti D, Temple J, Owusu-Ofori S, Allain JP. Multiplex real-time quantitative RT-PCR assay for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. J Virol Methods. 2004;118:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Tajadini M, Panjehpour M, Javanmard SH. Comparison of SYBR Green and TaqMan methods in quantitative real-time polymerase chain reaction analysis of four adenosine receptor subtypes. Adv Biomed Res. 2014;3:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Pereira-Gómez M, Fajardo Á, Echeverría N, López-Tort F, Perbolianachis P, Costábile A, Aldunate F, Moreno P, Moratorio G. Evaluation of SYBR Green real time PCR for detecting SARS-CoV-2 from clinical samples. J Virol Methods. 2021;289:114035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | De Crignis E, Re MC, Cimatti L, Zecchi L, Gibellini D. HIV-1 and HCV detection in dried blood spots by SYBR Green multiplex real-time RT-PCR. J Virol Methods. 2010;165:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Gibellini D, Gardini F, Vitone F, Schiavone P, Furlini G, Re MC. Simultaneous detection of HCV and HIV-1 by SYBR Green real time multiplex RT-PCR technique in plasma samples. Mol Cell Probes. 2006;20:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 27. | Meng Q, Wong C, Rangachari A, Tamatsukuri S, Sasaki M, Fiss E, Cheng L, Ramankutty T, Clarke D, Yawata H, Sakakura Y, Hirose T, Impraim C. Automated multiplex assay system for simultaneous detection of hepatitis B virus DNA, hepatitis C virus RNA, and human immunodeficiency virus type 1 RNA. J Clin Microbiol. 2001;39:2937-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Chen L, Li W, Zhang K, Zhang R, Lu T, Hao M, Jia T, Sun Y, Lin G, Wang L, Li J. Hepatitis C Virus RNA Real-Time Quantitative RT-PCR Method Based on a New Primer Design Strategy. J Mol Diagn. 2016;18:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kishk R, Nemr N, Elkady A, Mandour M, Aboelmagd M, Ramsis N, Hassan M, Soliman N, Iijima S, Murakami S, Tanaka Y, Ragheb M. Hepatitis B surface gene variants isolated from blood donors with overt and occult HBV infection in north eastern Egypt. Virol J. 2015;12:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Prakash S, Jain A, Jain B. Development of novel triplex single-step real-time PCR assay for detection of Hepatitis Virus B and C simultaneously. Virology. 2016;492:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, Danel C, Anglaret X, Leroy V, Msellati P, Dabis F, Rouzioux C. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soni S, United States S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zheng XM