Published online Dec 25, 2023. doi: 10.5501/wjv.v12.i5.272
Peer-review started: July 26, 2023
First decision: September 4, 2023
Revised: September 13, 2023
Accepted: November 30, 2023
Article in press: November 30, 2023
Published online: December 25, 2023
Processing time: 152 Days and 4 Hours
The epidemic of severe acute respiratory syndrome coronavirus 2 infection, known as the coronavirus disease 2019 (COVID-19), has caused a global health concern. Since its emergence, numerous studies have focused on various clinical manifestations and outcomes in different populations. However, studies are ongoing as the consequences and impact of COVID-19 in children with chronic diseases such as asthma are controversial.
To fill this research gap by retrospectively evaluating the course, laboratory, and clinical findings of COVID-19 among 414 asthmatic children followed up from the pediatric allergy outpatient clinic and known to have had COVID-19.
The data of 5510 patients over the age of 5 diagnosed with asthma in our hospital's data were retrospectively scanned with specific parameters using protocol numbers from the hospital filing system. The data included retrospective evaluation of pulmonary function test results before and after COVID-19, routine hematological and biochemical parameters, sensitization states (total IgE, specific IgE, and skin prick test results), and radiological (computed tomography) findings. To inquire about the course and symptoms of COVID-19, asthma patients or their parents were then called and evaluated with a questionnaire.
As a result of retrospectively scanning the data of 5510 asthma patients over the age of 5, it was determined that 414 (7.5%) patients had COVID-19. The mean age of 414 patients was 17.18 ± 4.08 (min: 6; max: 28) years. Two hundred and three of our 414 patients are male, and 211 are female. When their vaccination status was questioned, 21.5% were vaccinated. When the symptoms of our 290 patients were questioned, it was stated that 59.0% had fever symptoms. The rate of using regular prophylactic asthma medications was 19%. The rate of using salbutamol in asthma was found to be 22%. The rate of patients using methylprednisolone was 1%. Emergency service admission was 17.2%, and hospitalization was found to be 4.8%. Leukopenia (< 4000) was found in 14.1% of patients, and 8.08% of our patients had neutropenia (< 1500). Lymphopenia (< 1500) was detected in 44.4% of patients, and lymphocytosis (> 4000) was found in 5.05% of patients. In 65% of our patients, the C-reactive protein value was elevated. A high aspartate aminotransferase and alanine aminotransferase value was detected in 3.2% and 5.4% of patients were found, respectively. 31% of patients had an elevated lactate dehydrogenase value. Typical radiological findings for COVID-19 were detected in 3/309 of patients.
According to our study, there is a correlation between the severity of COVID-19 and asthma symptoms and the course of the disease. However, it is worth noting that the retrospective nature of the study and the differences in sample size, age, and demographic characteristics between the two groups do not allow for an optimal comparison. Therefore, further investigation is needed to explore the relationship between COVID-19 and asthma, and it can be suggested that COVID-19 may trigger asthma attacks and asthma may impact the course of COVID-19.
Core Tip: In our comprehensive retrospective study, we have made a noteworthy observation indicating a correlation between the severity of coronavirus disease 2019 (COVID-19) and the presence of asthma symptoms, which also appear to influence the course of the disease. These findings offer valuable insights into the potential interaction between COVID-19 and asthma. Given the complexity of this relationship and its possible implications for patient management, further in-depth investigations are warranted to elucidate the precise mechanisms and associations at play, aiming to improve our understanding and management of both conditions.
- Citation: Özata MC, Dikici Ü, Özdemir Ö. COVID-19 frequency and clinical course in children with asthma. World J Virol 2023; 12(5): 272-285
- URL: https://www.wjgnet.com/2220-3249/full/v12/i5/272.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i5.272
The epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, known as the coronavirus disease 2019 (COVID-19), has caused a global health concern. Since its emergence, numerous studies have focused on various clinical manifestations and outcomes in different populations. However, studies are ongoing as the consequences and impact of COVID-19 in children with chronic diseases such as asthma are controversial.
Asthma, a chronic inflammatory disease of the airways, is one of the most common non-communicable chronic childhood diseases among children. According to the American Centers for Disease Control and Prevention's (CDC) data, approximately 6.5% of children under the age of 18 in the United States have asthma[1]. Globally, it is estimated that 262 million people have asthma in 2019[2]. Children with asthma are often more susceptible to viruses that infect the respiratory tract, including common coronaviruses[3]. However, the relationship between asthma and a novel coronavirus, SARS-CoV-2, in the pediatric population has not yet been fully established.
This study aims to fill this research gap by retrospectively evaluating the course, laboratory, and clinical findings of COVID-19 among 414 asthmatic children followed up from the pediatric allergy outpatient clinic and known to have had COVID-19. We hope to shed light on the clinical manifestations, severity, and prognosis of COVID-19 in this specific pediatric population, helping develop targeted treatment strategies.
In our study, the data of 5510 patients over the age of 5 diagnosed with asthma in our hospital's data were retrospectively scanned with certain parameters using protocol numbers from the hospital filing system. This study's approval was obtained from the Sakarya University Faculty of Medicine clinical research ethics committee (Decision No: E-71522473-050.01.04-128344-122).
The data included a retrospective evaluation of pulmonary function test results before and after COVID-19, sensitization states (total IgE, specific IgE, and skin prick test results), and radiological (computed tomography) findings.
Also, routine hematological and biochemical parameters of the patients including hemoglobin, leukocyte, neutrophil, lymphocyte, eosinophil, platelet counts, C-reactive protein (CRP), sedimentation rate, urea, aspartate aminotransferase (AST)-alanine aminotransferase (ALT), prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer, lactate dehydrogenase (LDH) and finally total IgE values were evaluated. These values were collected retrospectively from our hospital filing system and laboratory evaluations performed within 2 wk of having COVID-19.
In order to inquire about the course and symptoms of COVID-19, 414/5510 (7.5%) asthma patients who had COVID-19 or their parents were then called and evaluated with a questionnaire.
Descriptive analyses were performed to provide information on the general characteristics of the study population. The Kolmogorov-Smirnov test was used to evaluate whether the distributions of numerical variables were normal. Accordingly, one-way ANOVA or Kruskal Wallis test was used to compare the numeric variables among three groups (for multiple comparisons of ANOVA and Kruskal Wallis tests, Sheffe and Dunn’s test was used). Eta squared was calculated for the effect size of ANOVA or Kruskal Wallis test. The numeric variables were presented as mean ± standard deviation. The Chi-Square test compared categorical variables. Cramer V coefficient was calculated for the effect size of the Chi-Square test. Categorical variables were presented as a count and percentage. A P value < 0.05 was considered significant. Analyses were performed using SPSS statistical software (IBM SPSS Statistics, Version 22.0. Armonk, NY: IBM Corp.)
As a result of retrospectively scanning the data of 5510 asthma patients over the age of 5, it was determined that 414 (7.5%) patients had COVID-19. When their intra-familial contamination status was questioned, 36% stated that they had positive intra-familial transmission cases; the rest were not intra-familial transmission. The intra-familial transmission was highest in atopic patients. When their vaccination status was questioned, 21.5% were vaccinated. The mean age of 414 patients was 17.18 ± 4.08 (min: 6; max: 28) years. Two hundred and three of our 414 patients are male, and 211 are female. Three hundred and nine of our 414 patients could be reached by phone.
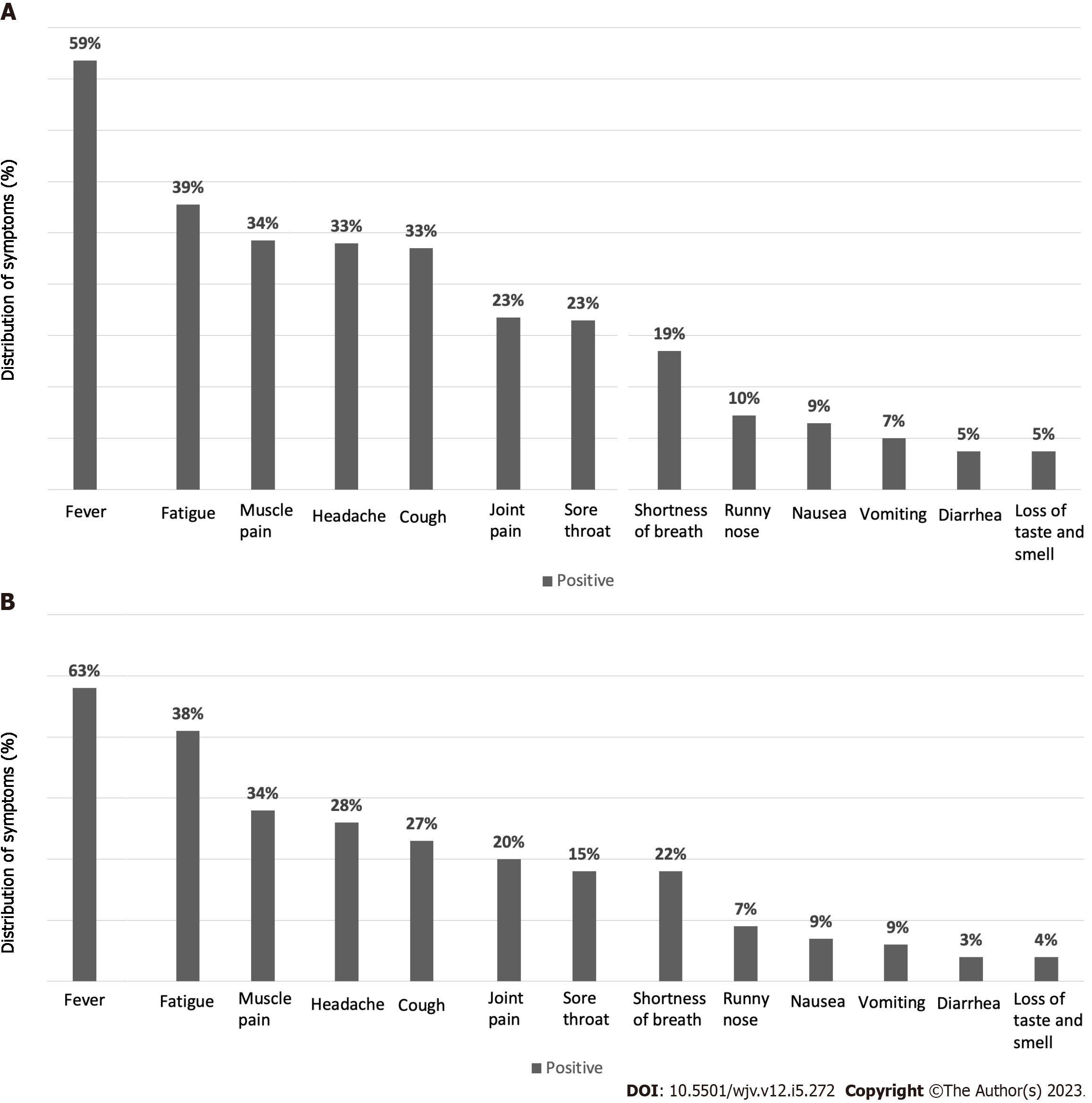
When the symptoms of our available 290 patients were questioned, it was stated that 59.0% had fever symptoms. Interestingly, one of our patients stated that he had a decrease in fever and was even admitted to the emergency department with the risk of hypothermia. Fatigue 39.0%; muscle pain 33.8%; headache 33.2%; cough 32.5%; sore throat 23%; joint pain 23%; shortness of breath 18.8%; runny nose 10.0%; nausea 9%; vomiting 6.8%; diarrhea 5%; loss of taste and smell 5.02% patients were reported to have symptoms. There was no significant difference among the rest of asthma group (others), vaccinated and atopic asthma patient groups, except for shortness of breath (Table 1; Figure 1).
| Others1 (n = 169) | Vaccinated patients (n = 57) | Atopic patients (n = 64) | P value | ES | |
| Gender (male) | 128/169 (47.6) | 26/57 (45.6) | 38/64 (59.4) | 0.199 | 0.091 |
| Fever | 100/163 (61.3) | 27/57 (47.4) | 40/63 (63.5) | 0.130 | 0.120 |
| Fatigue | 60/163 (36.8) | 27/57 (47.4) | 24/63 (38.1) | 0.365 | 0.084 |
| Headache | 51/163 (31.3) | 23/57 (40.4) | 22/63 (34.9) | 0.453 | 0.075 |
| Muscle pain | 62/163 (38) | 17/57 (29.8) | 18/63 (28.6) | 0.296 | 0.093 |
| Cough | 53/163 (32.5) | 24/57 (42.1) | 17/63 (27) | 0.205 | 0.106 |
| Sore throat | 41/163 (25.2) | 15/57 (26.3) | 10/63 (15.9) | 0.280 | 0.095 |
| Joint pain | 43/163 (26.4) | 11/57 (19.3) | 13/63 (20.6) | 0.452 | 0.075 |
| Shortness of breath | 23/163 (14.1) | 17/57 (29.8) | 14/63 (22.2) | 0.026 | 0.160 |
| Runny nose | 19/163 (11.7) | 5/57 (8.8) | 5/63 (7.9) | 0.653 | 0.055 |
| Nausea | 15/163 (9.2) | 5/57 (8.8) | 6/63 (9.5) | 0.990 | 0.008 |
| Vomiting | 10/163 (6.1) | 4/57 (7) | 6/63 (9.5) | 0.660 | 0.053 |
| Diarrhea | 9/163 (5.5) | 4/57 (7) | 2/63 (3.2) | 0.624 | 0.057 |
| Loss of taste and smell | 6/163 (3.7) | 6/57 (10.5) | 3/63 (4.8) | 0.148 | 0.119 |
| Intra-familial contamination | 59/164 (36) | 15/57 (26.3) | 31/64 (48.4) | 0.039 | 0.151 |
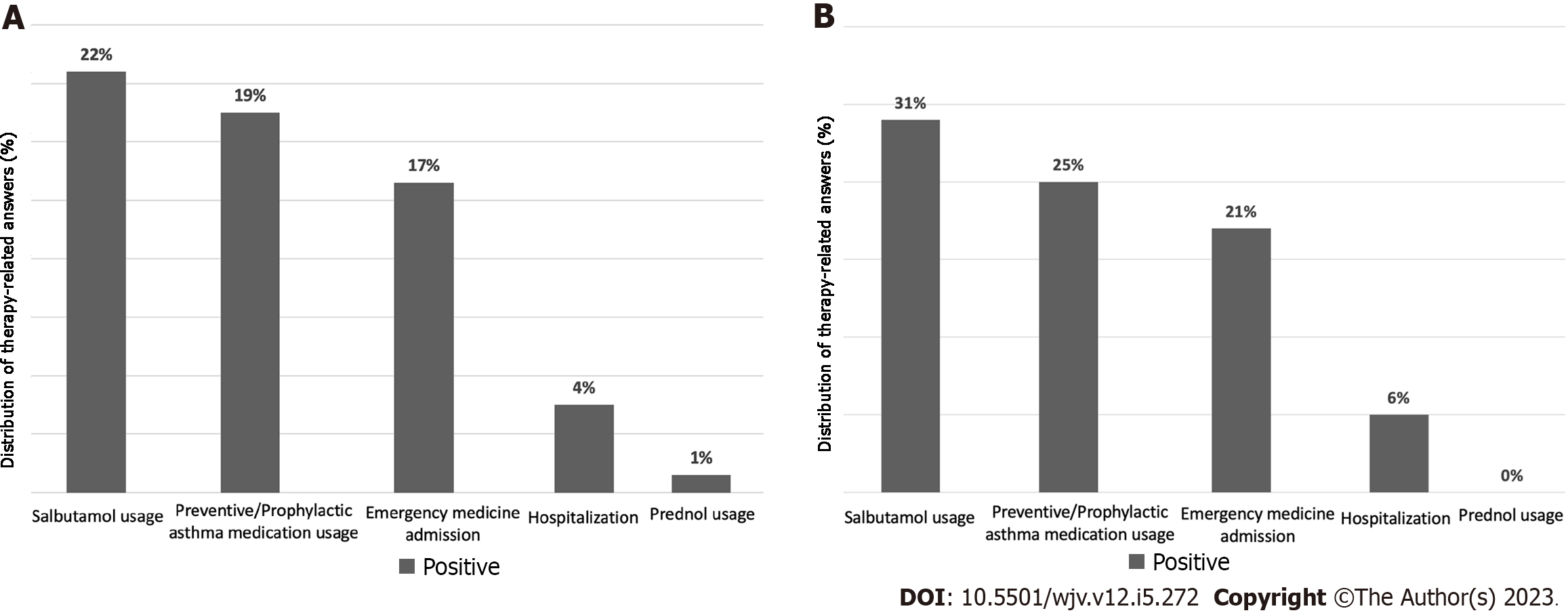
The rate of using regular preventive/prophylactic asthma medications was 19%. The rate of using salbutamol in asthma attacks was found to be 22%. The rate of patients using prednol (methylprednisolone) is 1%. Emergency service admission was 17.2%, and hospitalization was found to be 4%. The number of days hospitalized patients stay in the hospital varies between 3-10 d. In addition, it was determined that one of our patients was hospitalized in the intensive care unit. A table shows the evaluation of therapeutic features in different patient groups (Table 2; Figure 2).
| Others1 (n = 169) | Vaccinated patients (n = 57) | Atopic patients (n = 64) | P value | ES | |
| Preventive/prophylactic asthma medication usage | 31/164 (18.9) | 9/57 (16.1) | 16/62 (25.8) | 0.372 | 0.084 |
| Salbutamol usage | 34/164 (20.7) | 11/57 (19.6) | 20/63 (31.7) | 0.169 | 0.112 |
| Prednol usage | 2/164 (1.2) | 1/57 (1.8) | 0/64 (0) | 0.619 | 0.059 |
| Emergency service admission | 27/164 (16.5) | 8/57 (14) | 14/64 (21.9) | 0.485 | 0.071 |
| Hospitalization | 7/164 (4.3) | 3/57 (5.3) | 4/64 (6.3) | 0.866 | 0.038 |
Upon inquiry about the general medications employed by our patients during their COVID-19 illness, the investigation yielded the subsequent findings. Out of the participants, 126 individuals utilized antipyretic-analgesic group drugs, 22 antiviral drugs, 18 leukotriene receptor antagonists, and 11 were treated with antibiotic group drugs. Furthermore, six patients opted for 2nd generation antihistamine group drugs, and two patients utilized antiemetic derivative drugs. Among the sample of 272 patients, 173 individuals (63%) reported needing medication during the COVID-19 period.
After investigating the recovery times of our patients, the findings showed that out of 309 patients, 272 (88%) could heal within 1-15 d. Only a small percentage of patients, three (0.9%), required a longer recovery time of 30 days. Additionally, two patients needed more extensive recovery times of 45 and 150 d, respectively.
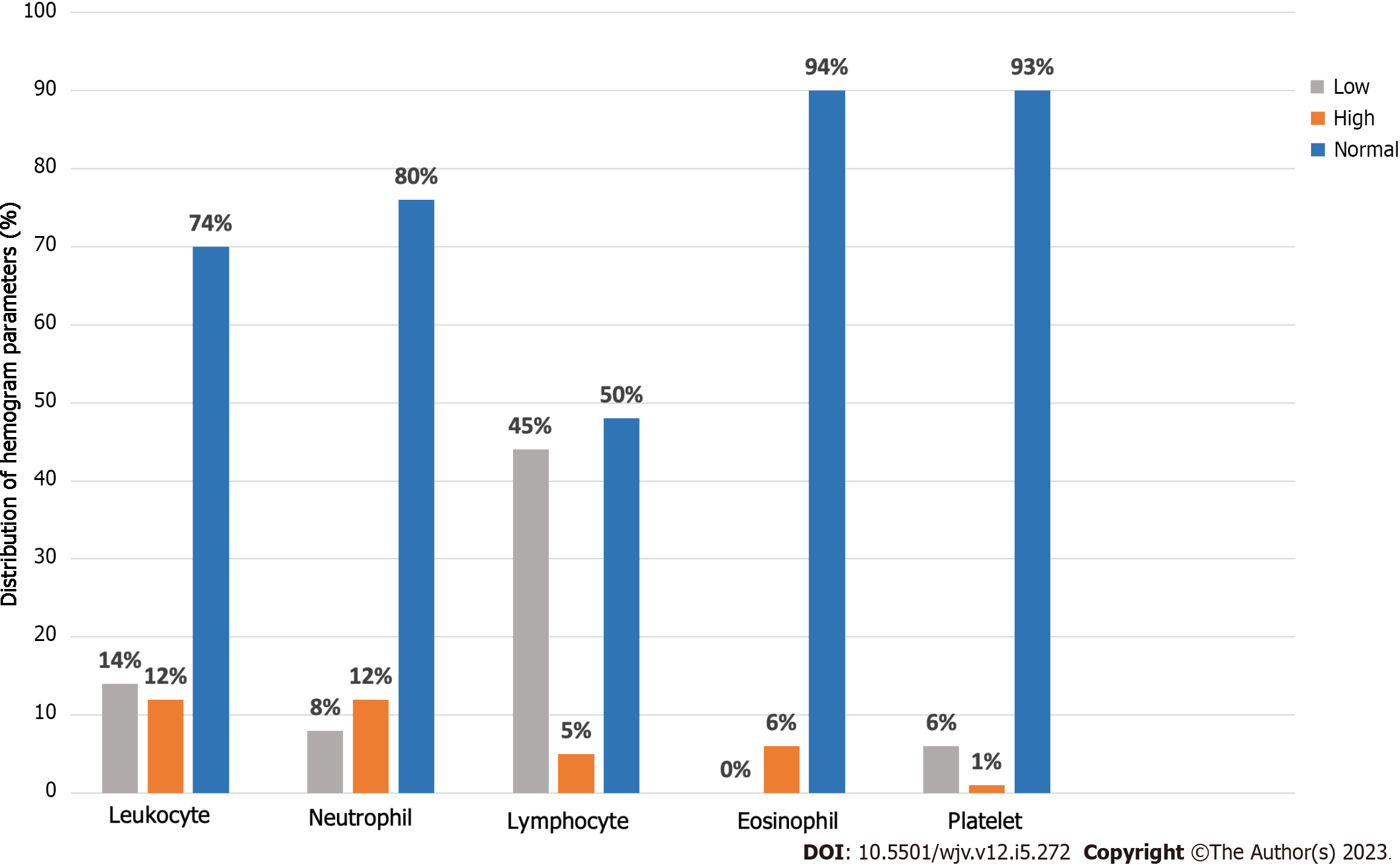
According to these parameters, the hemogram values of 97 of our patients could be reached (Table 3). Leukopenia was found in 14/96 (14.6%) patients, and leukocytosis was found in 12.5%. Eight point three percent of our patients had neutropenia (< 1500), and 12.5% of patients had neutrophilia (> 7500). Lymphopenia (< 1500) was detected in 45.4% of patients, and lymphocytosis (> 4000) was found in 5.2% of patients. In our 6.2% of patients, elevated eosinophil (> 500) values (eosinophilia) were detected. 6.2% of patients had thrombocytopenia (< 150000); 1.03% of patients had thrombocytosis (> 450.000) (Table 4; Figure 3).
| Others4, (n = 169) | Vaccinated patients, (n = 57) | Atopic patients, (n = 64) | P value | ES | ||||
| n | mean ± SD | n | mean ± SD | n | mean ± SD | |||
| Age | 269 | 16.57 ± 4.16a | 57 | 18.86 ± 3.43b | 64 | 15.77 ± 3.87a | < 0.0011,3 | 0.049 |
| COVID-19 age | 269 | 14.71 ± 4.09a | 57 | 17.28 ± 3.55b | 64 | 13.83 ± 3.75a | < 0.0011,3 | 0.063 |
| Hemoglobin | 68 | 13.13 ± 1.44 | 12 | 13.66 ± 1.57 | 17 | 12.96 ± 1.83 | 0.4561 | 0.017 |
| Leucocyte | 67 | 6803.79 ± 2825.88 | 12 | 6162.5 ± 2173.43 | 17 | 7068.24 ± 4155.47 | 0.9032 | 0.007 |
| Neutrophil | 67 | 4119.30 ± 2601.78 | 12 | 3563.33 ± 1721.86 | 17 | 3762.94 ± 2089.5 | 0.9202 | 0.008 |
| Lymphocyte | 68 | 1861.94 ± 939.88 | 12 | 1924.17 ± 1008.59 | 17 | 2503.53 ± 2908.44 | 0.9152 | 0.027 |
| Eosinophil | 67 | 141.91 ± 171.81 | 12 | 157 ± 149.52 | 17 | 146.29 ± 190.62 | 0.3272 | 0.001 |
| Platelet | 68 | 245864.71 ± 74049.74 | 12 | 273750 ± 77324.73 | 17 | 232352.94 ± 66343.75 | 0.3682 | 0.024 |
| CRP | 62 | 10.28 ± 20.13a | 8 | 7.84 ± 5.34a | 14 | 2.85 ± 2.41b | 0.0162,3 | 0.025 |
| Sedimentation | 5 | 11 ± 7.52 | 2 | 5 ± 1.41 | - | - | ||
| AST | 63 | 25.58 ± 11.28 | 12 | 22.92 ± 5.23 | 16 | 27.06 ± 12.68 | 0.8292 | 0.011 |
| ALT | 63 | 19.13 ± 13.72 | 12 | 20.17 ± 14.83 | 16 | 20.06 ± 17.47 | 0.9082 | 0.001 |
| Urea | 61 | 21.5 ± 6.2 | 12 | 21.35 ± 7.18 | 16 | 22.45 ± 6.81 | 0.7272 | 0.004 |
| PT | 33 | 12.07 ± 1.24 | 4 | 11.65 ± 0.95 | 8 | 12.49 ± 1.25 | 0.5121 | 0.031 |
| aPTT | 31 | 28.87 ± 3.4 | 4 | 28.53 ± 1.97 | 7 | 28.96 ± 2.17 | 0.9741 | 0.001 |
| D-Dimer | 48 | 508.98 ± 953.33 | 9 | 334.78 ± 206.13 | 11 | 265.18 ± 128.16 | 0.6472 | 0.015 |
| LDH | 47 | 243.44 ± 88.75 | 8 | 202.59 ± 54.89 | 12 | 225.16 ± 87.56 | 0.3122 | 0.027 |
| Total IgE | 37 | 176.05 ± 252.36a | 2 | 174.5 ± 144.96a,b | 13 | 1127.04 ± 1325.03b | 0.0092,3 | 0.274 |
| Others1, (n = 169) | Vaccinated patients (n = 57) | Atopic patients (n = 64) | P value | ES | |
| Leukopenia (< 4000) | 8/67 (11.9) | 2/12 (16.7) | 4/17 (23.5) | 0.541 | 0.125 |
| Leukocytosis (> 10000) | 11/67 (16.4) | 0/12 (0) | 1/17 (5.9) | 0.193 | 0.186 |
| Neutropenia (< 1500) | 6/67 (9) | 1/12 (8.3) | 1/17 (5.9) | 1.000 | 0.042 |
| Neutrophilia (> 7500) | 10/67 (14.9) | 0/12 (0) | 2/17 (11.8) | 0.447 | 0.147 |
| Lymphopenia (< 1500) | 29/68 (42.6) | 5/12 (41.7) | 10/17 (58.8) | 0.470 | 0.125 |
| Lymphocytosis (> 4000) | 3/68 (4.4) | 0/12 (0) | 2/17 (11.8) | 0.475 | 0.152 |
| Eosinophilia (> 500) | 4/67 (6) | 1/12 (8.3) | 1/17 (5.9) | 1.000 | 0.033 |
| Thrombocytopenia (< 150000) | 5/68 (7.4) | 0/12 (0) | 1/17 (5.9) | 0.832 | 0.099 |
| Thrombocytosis (> 450000) | 1/68 (1.5) | 0/12 (0) | 0/17 (0) | 1.000 | 0.067 |
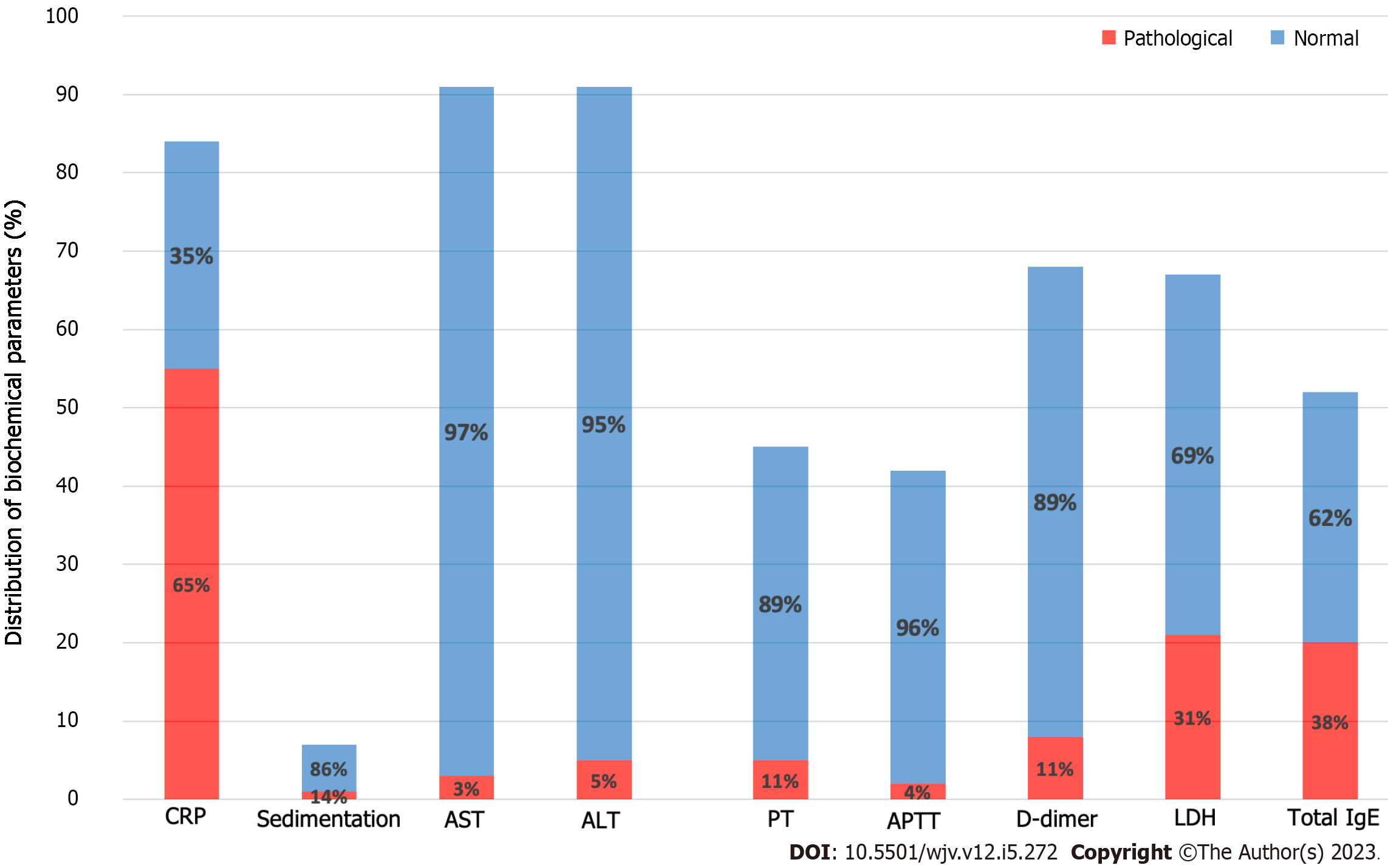
| Elevated CRP (> 3) | 42/62 (67.7) | 7/8 (87.5) | 6/14 (42.9) | 0.090 | 0.245 |
| Elevated sedimentation (> 20) | 1/5 (20) | 0/2 (0) | - | 1.000 | 0.258 |
| Elevated AST (> 50) | 2/63 (3.2) | 0/12 (0) | 1/16 (6.3) | 1.000 | 0.097 |
| Elevated ALT (> 50) | 3/63 (4.8) | 1/12 (8.3) | 1/16 (6.3) | 1.000 | 0.054 |
| Elevated urea (> 45) | 0/61 | 0/12 | 0/16 | - | - |
| Elevated PT (>13.2) | 3/33 (9.1) | 0/4 (0) | 2/8 (25) | 0.261 | 0.221 |
| Elevated aPTT (> 33.5) | 2/31 (6.5) | 0/4 (0) | 0/7 (0) | 1.000 | 0.133 |
| Elevated D-dimer (> 500) | 6/48 (12.5) | 1/9 (11.1) | 1/11 (9.1) | 1.000 | 0.039 |
| Elevated LDH (> 248) | 15/47 (31.9) | 2/8 (25) | 4/12 (33.3) | 1.000 | 0.052 |
| Elevated total IgE (> 150) | 10/37 (27) | 1/2 (50) | 9/13 (69.2) | 0.019 | 0.376 |
From the parameters of the coagulation system, the number of patients whose PT values were reached is 45. A high PT value (> 13.2) was detected in 11.1% of patients. The number of patients whose aPTT value was reached is 42. aPTT values were elevated (> 33.5) in 4.7% of our patients. D-dimer value was elevated (> 500) in 11.7% of our patients (Table 4; Figure 4).
When we look at the other biochemical parameters, the CRP value of 84 of our patients was reached. In 65% of our patients, the CRP value was elevated. High sedimentation value was detected in 14% of our patients. The number of patients whose AST and ALT values were reached is 92. An increased AST and ALT value was detected in 3.2% and 5.4% of patients, respectively. The number of patients whose LDH value was reached was 67, and 31.3% had an elevated LDH value. Total IgE values of 52 patients were reached. A high (> 150) total IgE value was found in 38.4% of patients (Table 4; Figure 4).
Computed tomography (CT) reports of our 7/309 patients that may be associated with COVID-19 have been obtained. When we evaluated our patients' very few CT findings regarding COVID-19, findings that were shown as typical findings for COVID-19[4] were detected in 3 of our patients. The findings detected in the remaining patients were classified as findings in other viral pneumonia types that are not specific to COVID-19.
When we look at patients with COVID-19, it was determined that 110/414 (26%) patients had atopy/sensitivity. In the filing system, inhalant allergy was found in 100 patients, food allergy in 4 patients, and inhalant and food allergy in 6 patients.
Considering that 64 patients could be reached by phone; in 48.4% of patients, intra-familial transmission was detected. In 41.6% of patients, it was transmitted from outside the family. 27% of our patients were vaccinated before contracting COVID-19. A higher incidence of intra-familial transmission was detected in these patients compared to the others.
Of 64 patients, 63.5% reported fever as a symptom, while 38.1% experienced fatigue and 34% had headaches. Muscle pain was reported by 28% of the patients, while 27% had a cough. Joint pain and shortness of breath were reported by 20% and 22% of patients, respectively; while sore throat and runny nose were reported by 15% and 7%, respectively. Vomiting was reported by 9% of patients, while 9% experienced nausea and 3.2% had diarrhea. Additionally, 4% of patients experienced loss of taste and smell (Figure 1B).
When questioning the regular use of preventive/prophylactic asthma medication during COVID-19; 25% of our patients felt the need to use asthma medications. While 31% of patients needed salbutamol, none of our patients required prednol (methylprednisolone). The number of patients admitted to the emergency department was 20.6%. 5.7% of our patients were hospitalized (Figure 2B).
Our total vaccinated patients were 57/290 (19.6%). Among the vaccinated patients, fever symptoms were found at 47.4%, fatigue at 47.4%, and the number of patients with shortness of breath was 29.8%. Among the unvaccinated patients, 59% of patients described fever symptoms. While 36% of our patients complained of fatigue, 16% complained of shortness of breath (Table 1).
The rate of using salbutamol among vaccinated patients was 19.6%. The number of patients admitted to the emergency department is 14%. We had a total of 5.3% vaccinated patients hospitalized, and the need to use prednol was necessary in only 1/57 of our patients (Table 2). The average recovery time of the vaccinated patients was calculated as 9.1 d.
The rate of using salbutamol among unvaccinated patients was 26%. The number of patients admitted to the emergency department was 19%. A total of 5% of patients were hospitalized among unvaccinated patients, and the need for prednol was necessary for 3/228 of our patients (Table 2). In addition, the average recovery time of unvaccinated patients was calculated as 6.2 d.
In a review that included eight retrospective studies and 2914 children with COVID-19, asthma is one of the most common causes of comorbidity[5]. In the report published by the American CDC in 2020, the prevalence of asthma among those who had COVID-19 was reported as 5.8%[1]. In another study, which included 1802 patients with COVID-19, the rate of asthmatic patients was 7.8%[6]. It is known that the prevalence of asthma among COVID-19 patients is less common than in the general population[7,8].
After scanning the data of 5510 asthma patients over the age of 5, it was determined that 414 (7.5%) patients had COVID-19. In another study involving 43000 SARS-CoV-2 positive patients, asthma was reported as the most common comorbidity, with a prevalence of 10.2%[9]. In a study that involved 91 centers caring for approximately 133000 children with asthma, only 13/91 (14%) of the participating centers reported suspected cases of COVID-19 in children with asthma[10].
When we look at the vaccination rate of our patients, the vaccination rates among our patient group for individuals aged 12 and above and those aged 18 and above were determined to be 21.5% and 12.7% respectively. The vaccination rates in our study were lower than the current data because asthmatic patients in the childhood age group (17.18 ± 4.08 years) were included in the study, and the age limit for vaccination was lowered to 12 years in our country much later. In addition, as another factor, vaccine hesitancy at the time of the first use of vaccines is a known phenomenon that is still effective today. In a study that included 637 parents with children between 12 and 15 in the United States, vaccine hesitancy against COVID-19 vaccines was found in almost 1/3 of the parents[11]. A study conducted in our country stated that parents who were hesitant towards childhood vaccines also had a negative attitude toward COVID-19 vaccines[12]. In addition, in a study conducted with the parents of children with asthma in 2020, 19% of the participants
In our study, fever complaints were found at a rate of 59%, while this rate was lower in the literature compared to ours. In a study including 54 pediatric COVID-19 patients with asthma, complaints of fever were reported at a rate of 27.5%[5]. In a study comparing asthmatic COVID-19 patients and non-asthmatic COVID-19 patients, the rate of fever symptoms was 37.3% in the asthmatic group. Still, no significant difference was found between the two groups (P = 0.55)[7]. These data suggest that the rate of fever complaints in our study is higher than in other studies documented in the literature. When comparing vaccinated patients and unvaccinated patients regarding fever symptoms, no significant difference was observed between the two groups (P = 0.012).
The number of patients with cough complaints was found to be 33%. In a study including 54 COVID-19-positive asthma patients, the rate of cough (59.3%) was higher than in our study. In addition, the same study reported a significant difference in cough symptoms between the two groups with and without asthma (P = 0.002)[14]. In a study including 60 hospitalized asthma patients, it was reported that all asthma patients with COVID-19 (n = 10) had cough symptoms[15]. In our study, no significant difference was detected between the vaccinated and unvaccinated patient groups regarding cough complaints (P = 0.205).
When we look at the complaint of fatigue, the rate of 39% in our study was found to be higher compared to the literature. In one study, the rate of fatigue among COVID-19-positive patients with asthma was 16.9%[7]. Another study reported this rate as 16.7%[5].
When we evaluated our vaccinated and unvaccinated patients in terms of symptom severity, it was found that 47% of vaccinated and 63% of unvaccinated patients had fever. It can be said that being vaccinated provides a 16% decrease in fever complaints, which seems significant (P = 0.008). When we look at fatigue complaints, vaccinated patients complained of fatigue 10% more often than non-vaccinated patients, a difference that can be considered significant (P = 0.002). In addition, dyspnea is 12% more common in vaccinated patients compared to unvaccinated patients (P = 0.041). When we look at the studies published in the literature, it has been reported that being vaccinated leads to a significant decrease in the frequency of symptoms, unlike the results we found. So, in a report published by the CDC, it was reported that vaccinated children had 60% fewer symptoms than those who were not vaccinated[16].
In our study, when the rate of asthma medication use was examined, we observed a rate of 21.2%. This rate was found to be lower compared to other studies in the literature. In a study conducted by Metbulut et al[5], the rate of asthma medication use was reported as 42.7%. When considering the rates of salbutamol usage, we found a rate of 22%, which was higher than that reported in other studies in the literature. In a study conducted by Gaietto et al[7], the rate of Salbutamol usage among asthmatic COVID-19 patients was 17.6%. As expected, when regular maintenance medications are not used, asthma attacks tend to occur more frequently, leading to an increased usage of rescue medication, such as beta-agonists. No significant difference was observed in the asthma medication usage rate between vaccinated and unvaccinated patients (P = 0.957).
In our study, the rate of emergency department visits was determined to be 17.2%. Indeed, this rate appears to be similar to other studies in the literature. In one study, the rate of emergency department visits among asthmatic COVID-19 patients was 13.4%[7]. Another study demonstrated that implementing lockdown measures during COVID-19 significantly reduced the rate of emergency department visits in asthmatic patients[17]. The rate of methylprednisolone usage among our patients was determined to be 1%. As emergency department visits decrease, the usage rate of oral steroids, usually required during attacks, is also found to decrease. Vaccinated patients had 6% fewer emergency department visits than unvaccinated ones, but this difference was not statistically significant.
Hospitalization rates were found to be 4% in our study. Indeed, this rate was similar to the rate (4.9%) in the study conducted by Gaietto et al[7]. Furthermore, in this study and other studies, asthma is a risk factor for hospitalization in SARS-CoV-2 infection[7,9]. In one study, the prevalence of asthma was reported as 34.2% in 34 hospitalized COVID-19 patients, and it was stated that COVID-19 did not show a serious course in asthma patients and required a lower level of care compared to other patients[18]. No significant difference was detected in terms of hospitalization between the vaccinated and unvaccinated patients (P = 0.692).
When examining the laboratory values of our patients, it was found that 14% exhibited leukopenia. While 10/14 (71%) of these patients had values in the normal range before COVID-19, a significant decrease was found in leukocyte counts during the period they had COVID-19. High leukocyte values were detected in one of our patients before contracting COVID-19. Based on these data, it appears that SARS-CoV-2 infection causes changes in patients' leukocyte counts. Leukopenia may be a common finding among patients with COVID-19, and a decrease in leukocyte count may inform the severity and course of the infection. However, it should be evaluated together with other clinical and laboratory data. In addition, in an article including 184 patients, leukopenia was found in 58 (34%) of the patients, but it is reported that only children with COVID-19 were screened in this article[14]. In a study comparing ten asthmatic patients with COVID-19 and 25 non-asthmatic patients, no significant difference was found in leukocyte counts[18]. No significant difference was observed in leukopenia frequency between our vaccinated patients and the unvaccinated patient group (P = 0.92).
When we look at our patients with leukocytosis (12%), the leukocyte count of only 23% of our patients was within the normal range before SARS-CoV-2 infection. There was no decrease in the rest of our patients, and an increase in leukocyte counts was detected before the infection. As a result, it was determined that the leukocyte count was not within the normal range and was already high in the majority of patients with leukocytosis in the period before SARS-CoV-2 infection. This situation requires careful evaluation of the effect of SARS-CoV-2 infection on leukocyte count, and the general clinical status and laboratory results of these patients should be evaluated together. In another article, it was reported that leukocytosis was detected in 194/610 (32%) pediatric moderately severe COVID-19 patients and 4/16 (25%) patients with severe COVID-19[19]. In another study comparing asthmatic patients with COVID-19 and patients without asthma, it was found that there was no significant difference between the two groups in terms of leukocyte values (P = 0.675)[20].
The number of patients with lymphopenia in our study was 44 out of 97 (45.3%), which is higher than other studies in the literature. In a study evaluating 66 COVID-19 patients aged between 6 and 17, lymphopenia was only observed in 2 out of 66 patients (3%)[21]. Another study, which included 486 hospitalized patients with confirmed SARS-CoV-2 infection, reported a prevalence of lymphopenia in 21% of the patients[22]. In a study comparing SARS-CoV-2 positive asthmatic patients (n = 54) with non-asthmatic patients (n = 162), no significant difference in serum lymphocyte levels was reported between the two groups (P = 0.263)[5].
When evaluating patients with neutropenia in our study, we observed neutropenia in only 8% of patients. Our findings appear to be consistent with the study conducted by Üzel et al[23]. In this study involving 59 patients, neutropenia was reported in 5 out of 59 patients (8.5%)[23]. However, it is worth noting that this study, unlike ours, specifically included only children with COVID-19. Similar to our study, in a study comparing SARS-CoV-2 positive patients with and without asthma, no significant difference was found between the two groups in terms of serum neutrophil counts (P = 0.379)[5]. Another study comparing COVID-19 patients with and without asthma did not observe a significant difference between the two groups (P = 0.810)[20].
It can be stated that the platelet values of our patients yielded similar results compared to other studies in the literature. In our study, thrombocytopenia was observed in 6% of our 97 patients, while in a study conducted in Türkiye with 633 included patients, this rate was reported as 2%[24]. Furthermore, in the same article, the number of patients with thrombocytosis was 56 out of 633 (8.8%), whereas in our study, this rate was found to be 1% and represented a much smaller number of patients (n = 1)[24]. In another study comparing SARS-CoV-2 positive patients with and without asthma, no significant difference was found in platelet count (P = 0.480)[5].
When examining the CRP values of our patients, a significant elevation was observed in CRP levels in 54 out of 84 patients (65%). This rate is higher compared to other studies in the literature. In a study involving 633 patients, this rate was reported as 20%. The authors also noted that elevated CRP and other inflammatory markers may be associated with the severity of COVID-19[24]. In another study comparing SARS-CoV-2 positive patients with and without asthma, no significant difference was found in CRP values between the two groups (P = 0.523).
When evaluating LDH values, we found an elevation in LDH levels in 31% of our patients. This result is similar to the findings reported by Üzel et al[23]. Their study reported LDH elevation in 37.3% (n = 22) of the 59 symptomatic patients[23]. Another study compared LDH values between COVID-19 patients with asthma (n = 27) and without asthma (n = 42). This study found a significant difference in LDH values between the two groups (P = 0.035)[20].
When examining the D-dimer values of our patients, an elevation in D-dimer levels was observed in 8 out of 68 patients (11%). In a study involving 470 patients, D-dimer elevation was observed in 84 individuals (17.9%)[24]. Additionally, another article has linked elevated D-dimer levels and fibrinogen degradation products with COVID-19 mortality[25].
When comparing the severity of COVID-19 in our patients with atopy and the other (nonatopic) group, we found that cough was observed in 27% of patients with atopy, while it increased to 32.5% in the other group; however, this difference was not statistically significant (P = 0.205). Fatigue was reported to be 1% more in patients with atopy compared to the other group. However, muscle pain was 10% more in other patients without atopy. Nevertheless, these two symptoms did not significantly differ (P = 0.365 and P = 0.296). Based on these findings, it is challenging to claim that systemic symptoms are a significant marker in patients with atopy. However, according to a study, the milder symptoms of COVID-19 in patients with atopy compared to those without atopy may be attributed to the hyperactivation of T cells in individuals with allergies[26]. Additionally, it is known that individuals with allergies who contract COVID-19 tend to have a milder course of the disease compared to those without allergies[27].
When considering the need for salbutamol, patients with atopy felt 11% more need than the other group; this difference was not statistically significant (P = 0.169). In our study, children with atopic asthma used asthma medications [inhaled corticosteroid (ICS)] 7% more than other group. However, this difference was not statistically significant (P = 0.372). In light of this information, it can be stated that patients with atopy experience an increase in asthma attacks when they contract SARS-CoV-2 infection. However, some studies in the literature have reported findings in favor of a decrease, rather than an increase, in asthma attacks during COVID-19, attributing the decrease in attack frequency to reduced exposure to allergens and outdoor inhaler risk factors for asthmatic children nationwide due to the precautions taken during the COVID-19 pandemic[26,28]. In our study, being atopic was suggested as a risk factor for triggering asthma attacks when children with atopy contracted SARS-CoV-2. When examining hospital admissions, those with atopy had 5% more emergency department visits compared to other group; however, this difference was not statistically significant (P = 0.485). Table 2 shows that the rates of emergency department visits and hospitalizations were higher but statistically insignificant in the atopic patients compared to the other group. Furthermore, it has been reported that COVID-19 does not progress to severe illness in patients with atopic asthma[29]. According to another study, overall hospital admission rates during the COVID-19 pandemic have dramatically decreased, but specific information about patients with atopic asthma was not shared in that study[29]. Another study reported that the rate of emergency department visits due to asthma decreased by 80% in 2020 compared to 2019 and 2018. Still, no patient atopic status data was provided[17].
When examining the biochemical and hematological parameters during the period of COVID-19 in patients with atopy and the other (nonatopic) group, it was found that patients with atopy had 10% less leukocytosis compared to the other group; however, this difference was not statistically significant (P = 0.193). In patients with atopy, a 12% higher rate of leukopenia was observed compared to the other group; however, we believe this difference is not significant (P = 0.541). In atopic children, elevated CRP values were found to be 25% less compared to the other group, and this difference was not statistically significant (P = 0.090). While lymphopenia, leukopenia, and elevated CRP values are known to be present in children with COVID-19, studies emphasize that these laboratory values are not specific to COVID-19[19].
When examining the symptoms of our patients who required hospitalization and experienced severe COVID-19, the most common complaints were fever and fatigue, with a prevalence of 57% and 40.6%; respectively. This was followed by headache and cough, with a prevalence of 35% and 33.6%; respectively. These data become more significant when comparing the group of hospitalized patients with those who were not. It was found that hospitalized patients had 23% more cough symptoms, but it was not statistically significant (P = 0.07). Hospitalized patients were seen to use 60% more salbutamol than non-hospitalized patients, and this difference was statistically significant. A positive correlation exists between hospitalization and salbutamol use (P = 0.001). Hospitalized patients presented to the emergency department 34% more frequently; this difference was significant (P = 0.001). Additionally, a positive correlation exists between hospitalization and admission to the emergency department (P < 0.05). Hospitalized patients used methylprednisolone 13% more and asthma medications (ICS) 55% more compared to non-hospitalized patients, and these differences were statistically significant (P = 0.03 and 0.002, respectively).
When examining the acute phase reactants of our patients, elevated CRP levels were detected in 66%. These CRP values appear to be higher than in another study that included children with severe COVID-19 but are consistent[19]. Furthermore, leukopenia was observed in 17.3%, while leukocytosis in 7.3%. Neutropenia was found in 7.6%, and lymphopenia was found in 47.6%. Additionally, one patient had lymphocytosis and one patient had eosinophilia. Moreover, D-dimer elevation was observed in 11%, and LDH elevation was observed in 30%. D-dimer levels in the context of asthma and COVID-19 in children are crucial due to their association with coagulation activity and potential thrombotic complications. Increased D-dimer levels can function as indicators of heightened coagulation and fibrinolysis processes, potentially increasing the susceptibility of individuals to cerebrovascular events, including stroke[30]. Furthermore, research findings have indicated a notable correlation between D-dimer levels and the severity of the disease[31].
As many studies have indicated, asthma exacerbations did not increase in children during the COVID-19 period, and the implemented precautions and reduced allergen exposure have been reported to lead to a decrease in asthma symptoms[28,32]. According to our study, there is a correlation between the severity of COVID-19 and asthma symptoms and the course of the disease. However, it is worth noting that the retrospective nature of the study and the differences in sample size, age, and demographic characteristics between the two groups do not allow for an optimal comparison. Hospitalizations in children due to COVID-19 may increase asthma exacerbations, and asthma, which is shown as a risk factor in some data in the literature, is of importance[33]. Therefore, further investigation is needed to explore the relationship between COVID-19 and asthma, and it can be suggested that COVID-19 may trigger asthma attacks and asthma may impact the course of COVID-19.
When evaluating the symptom severity of the vaccinated and other (unvaccinated) group, it was found that 47% of vaccinated patients experienced fever. In comparison, the rate of fever complaints among the other group was 61%. It can be said that getting vaccinated resulted in a 14% decrease in fever complaints, which appears insignificant (P = 0.130). Regarding fatigue, vaccinated patients reported 11% more fatigue compared to the other group, but this difference is not significant (P = 0.365). Shortness of breath appears to be 15% more frequent in vaccinated patients compared to the other group. When examining the studies published in the literature, it is reported that vaccination leads to a significant decrease in symptom frequency, contrary to our findings. In fact, according to a report published by the CDC, vaccinated children experience the disease with 60% fewer symptoms compared to the unvaccinated[16].
As observed in Table 2, it can be seen that the rate of emergency department visits was lower in the vaccinated patients compared to atopic and other groups. Moreover, when we look at the control of asthma symptoms and hospitalization, no significant difference was observed between our study's groups of patients (vaccinated, atopic, and other [neither vaccinated nor atopic] group). Consistent with the findings of our study, a study conducted by Grandinetti et al[9] did not find any evidence supporting the hypothesis that COVID-19 vaccination exacerbates asthma attacks in asthmatic children. In our study, consistent with the previous findings, vaccinated patients experienced asthma exacerbations at a similar rate compared to the other group, and this difference did not appear to be statistically significant. The same study recommended deferring COVID-19 vaccination in patients with uncontrolled asthma until their clinical condition improves. A case study reported that an asthma patient who received the BNT162b2 (Pfizer-BioNTech) vaccine experienced an asthma exacerbation[34]. However, the authors noted that this study represents a single case, and further research is needed to conclude the general population.
For a better understanding of the results of the study and the discussion, it would be appropriate to emphasize a few points about the pathophysiology of COVID-19 and allergic/non-allergic asthma diseases.
It is well known that asthma patients show reduced production of the antiviral interferon and lower ACE-2 expression. This is probably because ACE-2 expression is inversely correlated with type 2 (Th2: T helper 2) cytokine levels in atopic/allergic asthmatics. However, severe COVID-19 shows strong type I interferon expression early on. Consequently, this is inconsistent with the pathophysiology of COVID-19 disease development in asthma patients with a worse prognosis[8,27,29].
Although the impact of non-allergic and allergic asthma on the course of COVID-19 is often discussed in detail in the literature. It is reported that the prognosis of COVID-19 cases with common allergic diseases (atopic asthma, allergic rhinitis, atopic eczema, etc.) are not severe. Thus, the shift of the immune system to the Th2 phenotype in these patients may indicate a favorable balance in the pathogenesis of COVID-19 development[8,27,29].
According to our study, there is a correlation between the severity of COVID-19 and asthma symptoms and the course of the disease. However, it is worth noting that both the retrospective nature of the study and the differences in sample size, age, and demographic characteristics between the two groups do not allow for an optimal comparison. Therefore, further investigation is needed to explore the relationship between COVID-19 and asthma, and it can be suggested that COVID-19 may trigger asthma attacks and asthma may impact the course of COVID-19.
Studies are ongoing as the consequences and impact of coronavirus disease 2019 (COVID-19) in children with chronic diseases such as asthma are controversial.
The consequences of COVID-19 in children with asthma are controversial.
This study aims to fill this research gap by retrospectively evaluating the course, laboratory, and clinical findings of COVID-19 among 414 asthmatic children followed up from the pediatric allergy outpatient clinic and known to have had COVID-19.
The data of 5510 patients over the age of 5 diagnosed with asthma in our hospital's data were retrospectively scanned with certain parameters using protocol numbers from the hospital filing system.
As a result of retrospectively scanning the data of 5510 asthma patients over the age of 5, it was determined that 414 (7.5%) patients had COVID-19. The mean age of 414 patients was 17.18 ± 4.08 (min: 6; max: 28) years. 203 of our 414 patients are male, and 211 are female.
According to our study, there is a correlation between the severity of COVID-19 and asthma symptoms and the course of the disease.
Further investigation is needed to explore the relationship between COVID-19 and asthma, and it can be suggested that COVID-19 may trigger asthma attacks, and asthma may impact the course of COVID-19.
We would like to thank Prof. Ünal Erkorkmaz for his support in performing statistical analyses.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Türkiye
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lucke-Wold B, United States; Mukhopadhyay A, India; Gao S, China; Wang K, China S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Centers for Disease Control and Prevention. Most Recent National Asthma Data. May 10, 2023. [cited 6 November 2023]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. |
| 2. | World Health Organization. Asthma. May 4, 2023. [cited 6 November 2023]. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma. |
| 3. | Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF Jr. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1011] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 4. | Penha D, Pinto EG, Matos F, Hochhegger B, Monaghan C, Taborda-Barata L, Irion K, Marchiori E. CO-RADS: Coronavirus Classification Review. J Clin Imaging Sci. 2021;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Metbulut AP, Mustafaoğlu Ö, Şen G, Kanık Yüksek S, Külhaş Çelik İ, Akça H, Dibek Mısırlıoğlu E. Evaluation of the Clinical and Laboratory Findings of Asthmatic Children with SARS-CoV-2 Infection. Int Arch Allergy Immunol. 2021;182:989-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Gaietto K, Freeman MC, DiCicco LA, Rauenswinter S, Squire JR, Aldewereld Z, Iagnemma J, Campfield BT, Wolfson D, Kazmerski TM, Forno E. Asthma as a risk factor for hospitalization in children with COVID-19: A nested case-control study. Pediatr Allergy Immunol. 2022;33:e13696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Adir Y, Saliba W, Beurnier A, Humbert M. Asthma and COVID-19: an update. Eur Respir Rev. 2021;30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Özdemir Ö, Nezir Engin MM, Yılmaz EA. COVID-19-Related Pneumonia in an Adolescent Patient with Allergic Asthma. Case Rep Med. 2021;2021:6706218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Grandinetti R, Palazzolo E, Rizzo L, Carbone R, Pisi G, Fainardi V, Esposito S. Impact of SARS-CoV-2 Infection in Children with Asthma and Impact of COVID-19 Vaccination: Current Evidence and Review of the Literature. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Papadopoulos NG, Custovic A, Deschildre A, Mathioudakis AG, Phipatanakul W, Wong G, Xepapadaki P, Agache I, Bacharier L, Bonini M, Castro-Rodriguez JA, Chen Z, Craig T, Ducharme FM, El-Sayed ZA, Feleszko W, Fiocchi A, Garcia-Marcos L, Gern JE, Goh A, Gómez RM, Hamelmann EH, Hedlin G, Hossny EM, Jartti T, Kalayci O, Kaplan A, Konradsen J, Kuna P, Lau S, Le Souef P, Lemanske RF, Mäkelä MJ, Morais-Almeida M, Murray C, Nagaraju K, Namazova-Baranova L, Garcia AN, Yusuf OM, Pitrez PMC, Pohunek P, Pozo Beltrán CF, Roberts GC, Valiulis A, Zar HJ; Pediatric Asthma in Real Life Collaborators. Impact of COVID-19 on Pediatric Asthma: Practice Adjustments and Disease Burden. J Allergy Clin Immunol Pract. 2020;8:2592-2599.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 11. | Ruiz JB, Bell RA. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022;137:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Akgül E, Ergün A. Ebeveynlerin Çocukluk Çağı Aşıları ile COVID-19 Aşısına Yönelik Tutumları Arasındaki İlişki. Halk Sağlığı Hemşireliği Dergisi. 2023;5:64-75. |
| 13. | Drouin O, Fontaine P, Arnaud Y, Montmarquette C, Prud'homme A, Da Silva RB. Parental decision and intent towards COVID-19 vaccination in children with asthma: an econometric analysis. BMC Public Health. 2022;22:1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Patel NA. Pediatric COVID-19: Systematic review of the literature. Am J Otolaryngol. 2020;41:102573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 15. | Asseri AA. Pediatric Asthma Exacerbation in Children with Suspected and Confirmed Coronavirus Disease 2019 (COVID-19): An Observational Study from Saudi Arabia. J Asthma Allergy. 2021;14:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Centers for Disease Control and Prevention. COVID-19 Study Shows mRNA Vaccines Reduce Risk of Infection by 91 Percent for Fully Vaccinated People. June 7, 2021. [cited 21 June 2023]. Available from: https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html. |
| 17. | Simoneau T, Greco KF, Hammond A, Nelson K, Gaffin JM. Impact of the COVID-19 Pandemic on Pediatric Emergency Department Use for Asthma. Ann Am Thorac Soc. 2021;18:717-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Farzan S, Rai S, Cerise J, Bernstein S, Coscia G, Hirsch JS, Jeanty J, Makaryus M, McGeechan S, McInerney A, Quizon A, Santiago MT. Asthma and COVID-19: An early inpatient and outpatient experience at a US children's hospital. Pediatr Pulmonol. 2021;56:2522-2529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Henry BM, Benoit SW, de Oliveira MHS, Hsieh WC, Benoit J, Ballout RA, Plebani M, Lippi G. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin Biochem. 2020;81:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Esmaeilzadeh H, Sanaei Dashti A, Mortazavi N, Fatemian H, Vali M. Persistent cough and asthma-like symptoms post COVID-19 hospitalization in children. BMC Infect Dis. 2022;22:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 22. | Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: A meta-analysis and systematic review. J Med Virol. 2021;93:234-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Üzel VH, Yılmaz K, Şen V, Aktar F, Karabel M, Yolbaş İ, Gözü Pirinççioğlu A, Söker M. Evaluation of Hematological Parameters of Children Diagnosed with COVID-19: Single-Center Experience. Turk Arch Pediatr. 2021;56:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Alkan G, Sert A, Emiroglu M, Tuter Oz SK, Vatansev H. Evaluation of hematological parameters and inflammatory markers in children with COVID-19. Ir J Med Sci. 2022;191:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kosmeri C, Koumpis E, Tsabouri S, Siomou E, Makis A. Hematological manifestations of SARS-CoV-2 in children. Pediatr Blood Cancer. 2020;67:e28745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Castro-Rodriguez JA, Forno E. Asthma and COVID-19 in children: A systematic review and call for data. Pediatr Pulmonol. 2020;55:2412-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Özdemir Ö. Letter to the Editor: Regarding COVID-19 in Children with Asthma. Lung. 2021;199:435-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Yang Z, Wang X, Wan XG, Wang ML, Qiu ZH, Chen JL, Shi MH, Zhang SY, Xia YL. Pediatric asthma control during the COVID-19 pandemic: A systematic review and meta-analysis. Pediatr Pulmonol. 2022;57:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Özdemir Ö. Asthma and prognosis of coronavirus disease 2019. World Allergy Organ J. 2022;15:100656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Panther EJ, Lucke-Wold B. Subarachnoid hemorrhage: management considerations for COVID-19. Explor Neuroprotective Ther. 2022;2:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Small C, Mehkri Y, Panther E, Felisma P, Lucke-Wold B. Coronavirus Disease-2019 and Stroke: Pathophysiology and Management. Can J Neurol Sci. 2023;50:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Pelletier JH, Rakkar J, Au AK, Fuhrman D, Clark RSB, Horvat CM. Trends in US Pediatric Hospital Admissions in 2020 Compared With the Decade Before the COVID-19 Pandemic. JAMA Netw Open. 2021;4:e2037227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 33. | Graff K, Smith C, Silveira L, Jung S, Curran-Hays S, Jarjour J, Carpenter L, Pickard K, Mattiucci M, Fresia J, McFarland EJ, Dominguez SR, Abuogi L. Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J. 2021;40:e137-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 34. | Colaneri M, De Filippo M, Licari A, Marseglia A, Maiocchi L, Ricciardi A, Corsico A, Marseglia G, Mondelli MU, Bruno R. COVID vaccination and asthma exacerbation: might there be a link? Int J Infect Dis. 2021;112:243-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |