Published online Jun 25, 2023. doi: 10.5501/wjv.v12.i3.172
Peer-review started: February 15, 2023
First decision: March 15, 2023
Revised: March 16, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: June 25, 2023
Processing time: 125 Days and 22.7 Hours
Autism spectrum disorder (ASD) is a group of heterogeneous, multi-factorial, neurodevelopmental disorders resulting from genetic and environmental factors interplay. Infection is a significant trigger of autism, especially during the critical developmental period. There is a strong interplay between the viral infection as a trigger and a result of ASD. We aim to highlight the mutual relationship between autism and viruses. We performed a thorough literature review and included 158 research in this review. Most of the literature agreed on the possible effects of the viral infection during the critical period of development on the risk of developing autism, especially for specific viral infections such as Rubella, Cytomegalovirus, Herpes Simplex virus, Varicella Zoster Virus, Influenza virus, Zika virus, and severe acute respiratory syndrome coronavirus 2. Viral infection directly infects the brain, triggers immune activation, induces epigenetic changes, and raises the risks of having a child with autism. At the same time, there is some evidence of increased risk of infection, including viral infections in children with autism, due to lots of factors. There is an increased risk of developing autism with a specific viral infection during the early developmental period and an increased risk of viral infections in children with autism. In addition, children with autism are at increased risk of infection, including viruses. Every effort should be made to prevent maternal and early-life infections and reduce the risk of autism. Immune modulation of children with autism should be considered to reduce the risk of infection.
Core Tip: There is a mutual relationship between viral infections and autism. There is an increased risk of developing autism when contracting a viral infection during pregnancy or early postnatal life during the critical period of brain development. At the same time, children with autism have many co-morbidities that expose them to more risk of contracting infections, including viruses. Therefore, every effort should be made to prevent infections, especially during this critical period of neurodevelopment. Parents should also be educated about the importance of vaccination and immune modulation in children with autism to avoid further infections.
- Citation: Al-Beltagi M, Saeed NK, Elbeltagi R, Bediwy AS, Aftab SAS, Alhawamdeh R. Viruses and autism: A Bi-mutual cause and effect. World J Virol 2023; 12(3): 172-192
- URL: https://www.wjgnet.com/2220-3249/full/v12/i3/172.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i3.172
Autism is a group of heterogeneous, multi-factorial, neurodevelopmental disorders that occur during infancy and toddlerhood, best described as a spectrum rather than a disease. It is characterized by language, social communication, interaction problems with restricted, repetitive, or stereotyped behaviours or interests, and the inability to generate biologically determined, regular, emotional interaction with others[1]. Autism spectrum disorders (ASD) involve autism, pervasive developmental disorder not otherwise specified, and Asperger's disorder. In addition, ASD, childhood disintegrative disorder, Rett's disorder, and the overactive disorder accompanied by mental retardation and stereotyped movements form pervasive developmental disorders. The diagnosis of autism is made by identifying at least two domains (out of three) of impaired social interaction and/or communication and restricted, repetitive, or stereotyped behaviour, interests, and activities[2,3].
There is no one definite cause of autism. Several factors play together to increase the risk of de-velopment of autism, including genetic, epigenetic, and environmental factors that may work in combination to affect the brain during the crucial phases of early development. The genetic causes of autism could result from single-gene mutations or abnormal copy number variations (such as large deletions, duplications, inversions, or translocations of chromosomes)[4]. As autism is likely to run in families, specific genetic changes may increase the risk of autism development in children. Therefore, the risk of recurrence of pervasive developmental disorders ranges between 2% and 8% in siblings of children with autism[5]. Lichtenstein et al[6] showed that monozygotic twins had higher rates of ASD and other neuropsychiatric disorders than dizygotic twins. However, this increase in the risk is not only due to the shared genes but also could be related to the shared environment, as identified by several twin studies[6,7].
Therefore, specific environmental conditions may increase or decrease the risk of autism in genetically predisposed patients through epigenetic modification by affecting gene expression quantity and quality without altering the DNA sequence. The epigenetic modification of gene expression occurs through altering DNA methylation, changing histone proteins, or modifying the expression of noncoding RNAs[8]. According to Barbeau[9], three pathways trigger the development of autism. The first pathway is activated by in-utero insult or injury, while obstetric complications at birth initiate the second pathway. The third pathway is triggered by various environmental triggers affecting infants in the first three years of life. The environmental factors include antennal factors (e.g., parental age, especially the father's age, the mother's physical and mental health, prenatal drug use, and family socioeconomic status), prenatal factors (preterm delivery, abnormal presentation, cesarean section, fetal complications, neonatal hypoxia, respiratory distress, natal bleeding, low-birth weight, seizures at birth), and postnatal factors (e.g., neonatal jaundice, early infection, sepsis, meningitis, encephalopathy, postnatal vitamin D deficiency, etc.)[10].
The sharp rise in ASD incidence observed worldwide is due to the increased prevalence of risk factors such as genetic predisposition, adverse environmental circumstances, and increased awareness about this disorder[11]. Infection is a common triggering factor in the three environmental pathways that affect autism development. Viral infection is widespread in all ages, including during the antenatal period. Viral infection during critical periods of early in-utero neurodevelopment may lead to an increased risk of autism in the offspring. On the other side, autism may be associated with impaired cellular and humoral immunity, which predisposes children with autism to encounter different types of infections, including viruses[12].
Meanwhile, some viruses may be used as a vector during gene therapy which could be a promising therapy for many diseases, including autism[13]. This review highlighted the mutual relationship between autism and viruses. The study focused on how viral infections may affect brain development and how this could be at play in some traits commonly associated with autism, such as difficulty communicating verbally or recognizing familiar faces. It also focused on how children with autism may have an increased frequency of infections, especially viral infections.
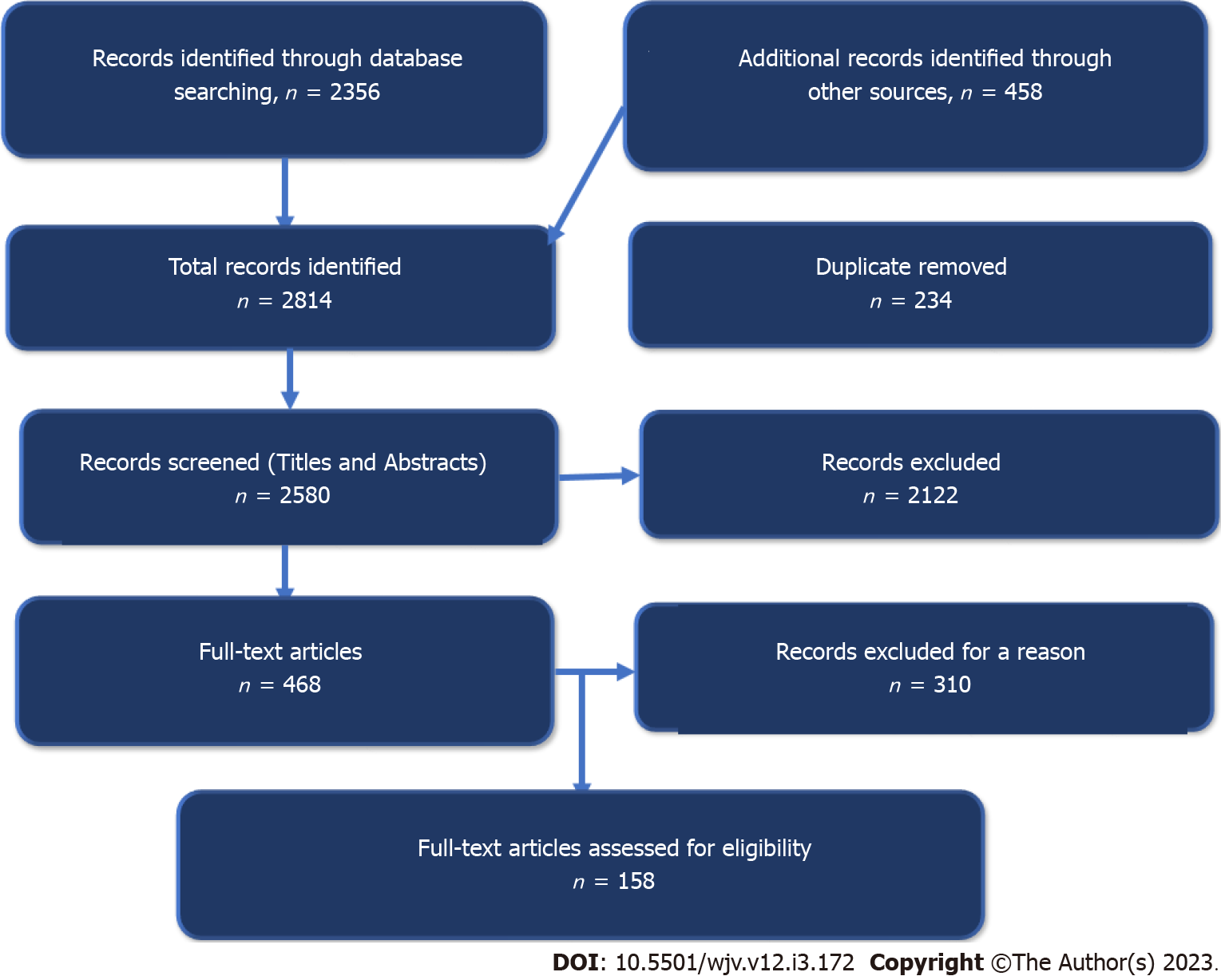
We conducted an inclusive literature review by searching different electronic databases, including Embase, PubMed, Cumulative Index to Nursing and Allied Health Literature, Cochrane Library, Scopus, Web of Science, Library and Information Science Abstracts, the National Library of Medicine catalogue, Ovid/Medline, and google search until January 31, 2023, related to viral infections and autism using the terms autism, autism spectrum disorders, viral infections, rubella, cytomegalovirus, influenza virus, Zika virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), vibrobiota, neurodevelopment, perinatal, antenatal, natal, and postnatal. Reference lists were inspected, and citation searches were done on the included studies. We included papers written in English and with open access. Figure 1 shows the flow chart of the reviewed articles. We reviewed 458 articles concerned with the viral infection and autism in children; 158 were included in the study.
ASD pathogenesis is not fully recognized. Autism has different pathophysiological neuroanatomical, and neuropsychological changes in the affected patients' brains that affect many brain functions resulting in the characteristic cognitive and behavioural changes of autism. Different genetic and environmental factors activate pathological pathways that disrupt brain development[14,15]. Behaviour and social impairment are among the hallmarks of autism. ASD is diagnosed based on behavioural impairments in social communication, interest fixation, and repetitive behaviours. These social impairments may be related to the improper interpretation of social signals[16]. Evidence from healthy individuals suggests that potentially threatening situations, such as others' proximity, can trigger several physiological responses that help regulate the distance between themselves and others during social interaction. Vicarious fear learning critically impacts cognitive abilities, receiving a neutral image as threatening and frightening as phylogenetically innate negative and dangerous stimuli, consequently affecting the person's behavioural control[17,18]. Individuals with ASD have social impairments, potentially due to the lack of or improper social signal interpretation, resulting in the inability to interpret these signals to guide appropriate behaviours[19]. In utero or early life, disruption of normal brain development triggers the subsequent development of neuropsychiatric disorders during later life[20].
The brain volume and weight are usually more prominent in children with autism with larger head circumferences than in typically developed children. The brain overgrowth observed in children with autism is not precisely understood. However, it could be related to excess neurons that induce local overconnectivity in specific brain regions. It also could be related to abnormal neuronal migration during early pregnancy with abnormal synaptogenesis and formation of dendritic spines and disturbed excitatory-inhibitory networks[21-23]. Changes in the division rates of germinal cells induce abnormal daughter cells migration to their target regions, causing autism-associated sensory and motor deficits[24].
Neuroinflammation is common in ASD with low-grade chronic inflammatory reactions and increased pro-inflammatory cytokines in cerebrospinal fluids (CSF) and specific brain areas due to microglial cells and innate neuroimmune system activation[25]. Altered immune function induces neuroinflammation, affecting many neurological processes, including neural development, brain structures, synapse plasticity, cognition, and behaviour[26]. This neuroinflammation may begin in early embryogenesis during the critical periods of neurodevelopment, resulting in unsuccessful neurodevelopment. Various antenatal factors, such as maternal vitamin D deficiency, medication use, such as valproic acids, pre-natal infection, and neonatal hypoxia, can trigger autism-related neuroinflammation. Brain development during the perinatal period is critical and particularly vulnerable to the effects of abnormal immune activation with detrimental consequences on neurodevelopment and alterations in neural connectivity[27]. Gastrointestinal abnormalities, repeated infections with gut dysbiosis, and impairment of the gut-brain axis cause immune imbalance and trigger neuroinflammation[28]. The role of neuroinflammation in autism pathogenesis is proved by observing the raised reactive microglial and astrocyte numbers in postmortem tissue from patients with ASD and animal models[29]. Other evidence of neuroinflammation is frequent reporting of dysregulated immune responses, anti-brain antibodies in CSF and blood, and several neurotransmitter abnormalities, such as increased serotonin levels in children with autism. Neuroinflammation identification and other markers of immune profile abnormalities can hypothetically lead to more consistent diagnostic measures and options for treating ASD[30,31].
The mirror neuron system (MNS) is essential in developing social and communication skills by helping to understand other people via imitating their behaviour through personified simulation, intentions, action perceptions, and emotions. Children with autism were found to have structural abnormalities in MNS regions, causing impaired activation of the imitation core circuit in children with autism. Remarkably, the activity of MNS regions is inversely related to the severity of the deterioration of social domain communication, which can explain the social impairment in children with autism[32,33]. However, children with autism can still mimic goal-directed behaviours[34]. In addition to the impaired MNS observed in children with autism, they have impaired activation of many other brain circuits outside the MNS[35]. For example, patients with autism may have an altered functional organization of their large-scale task-negative brain network engaged in social and emotional processing. However, they have an intact organization of the task-positive brain network, which maintains attention and goal-directed thinking[36]. Therefore, it was unsurprising when Chiu et al[37] found different signalling patterns in the cingulate cortex using functional neuroimaging with severely reduced cingulate self-response when playing games in patients with autism from that observed in typically developed partners. Some studies showed that patients with autism might have local overconnectivity in the cortex with reduced functional connectivity between the frontal lobe cortex and the rest of the cerebral cortices with poor high-level neural connectivity and synchronization and disordered association cortex[38,39].
Neuroinflammation of the brainstem causes brainstem dysfunction, including sensory processing abnormalities[40]. Since the thalamus is close to the brainstem, neuroinflammation could also affect it, augmenting autonomic nervous system dysfunction. Therefore, children with autism have intermittent autonomic nervous system dysfunction with enhanced sympathetic excitation and parasympathetic hypofunction causing chronic sensory hyperarousal state in children and sleep disorders. Autonomic dysfunction also affects heart rate, blood pressure, respiratory rhythm, gastrointestinal motility, gastric acid, and intestinal enzyme secretion[41,42]. Immune dysregulation and autoimmunity can induce neuroinflammation, cytokine dysregulation, and inducing anti-brain antibodies, significantly impacting brain development, causing neurodevelopmental deficits and playing a role in autism progression[43]. Vargas et al[44] showed the presence of neuroinflammatory markers and elevated cytokines, and enhanced activity of microglia and astrocytes in postmortem autopsies from patients with autism between 5 to 44 years, indicating permanently activated immune status in patients with autism. Table 1 summarizes the possible underlying mechanisms and triggering factors for autism.
| Possible mechanism | Effects |
| Immune dysregulation and autoimmunity | Neuroinflammation, cytokine dysregulation, and inducing anti-brain antibodies |
| Neuroinflammation | Neuroinflammation of the Cortex: Raised reactive microglial and astrocyte numbers → excess neurons → local overconnectivity in specific brain regions, abnormal neuronal migration during early pregnancy → abnormal synaptogenesis and formation of dendritic spines and disturbed excitatory-inhibitory networks, prominent brain volume and weight volume |
| Neuroinflammation of brainstem → brainstem dysfunction → sensory processing abnormalities → enhanced sympathetic excitation and parasympathetic hypofunction | |
| Neuroinflammation of thalamus → autonomic nervous system dysfunction | |
| Abnormalities in mirror neuron system regions | → Impaired activation of the imitation core circuit → social impairment |
| Impaired signaling patterns in the cingulate cortex | → Severely reduced cingulate self-response → social impairments |
| Autonomic nervous system dysfunction | Chronic sensory hyperarousal state in children and sleep disorders |
| Affects heart rate, blood pressure, and respiratory rhythm | |
| Impaired gastrointestinal motility, gastric acid, and intestinal enzyme secretion | |
| Underlying triggering factors | Maternal vitamin D deficiency, use of medication such as valproic acids during pregnancy, prenatal infection, neonatal hypoxia, preterm delivery, abnormal presentation, cesarean section, fetal complications, neonatal hypoxia, respiratory distress, natal bleeding, low-birth weight, seizures at birth, neonatal jaundice, early postnatal infection, sepsis, meningitis, encephalopathy, postnatal vitamin D deficiency |
| Augmenting factors | Gastrointestinal abnormalities, repeated infections with gut dysbiosis, and impairment of the gut-brain axis cause immune imbalance and trigger neuroinflammation |
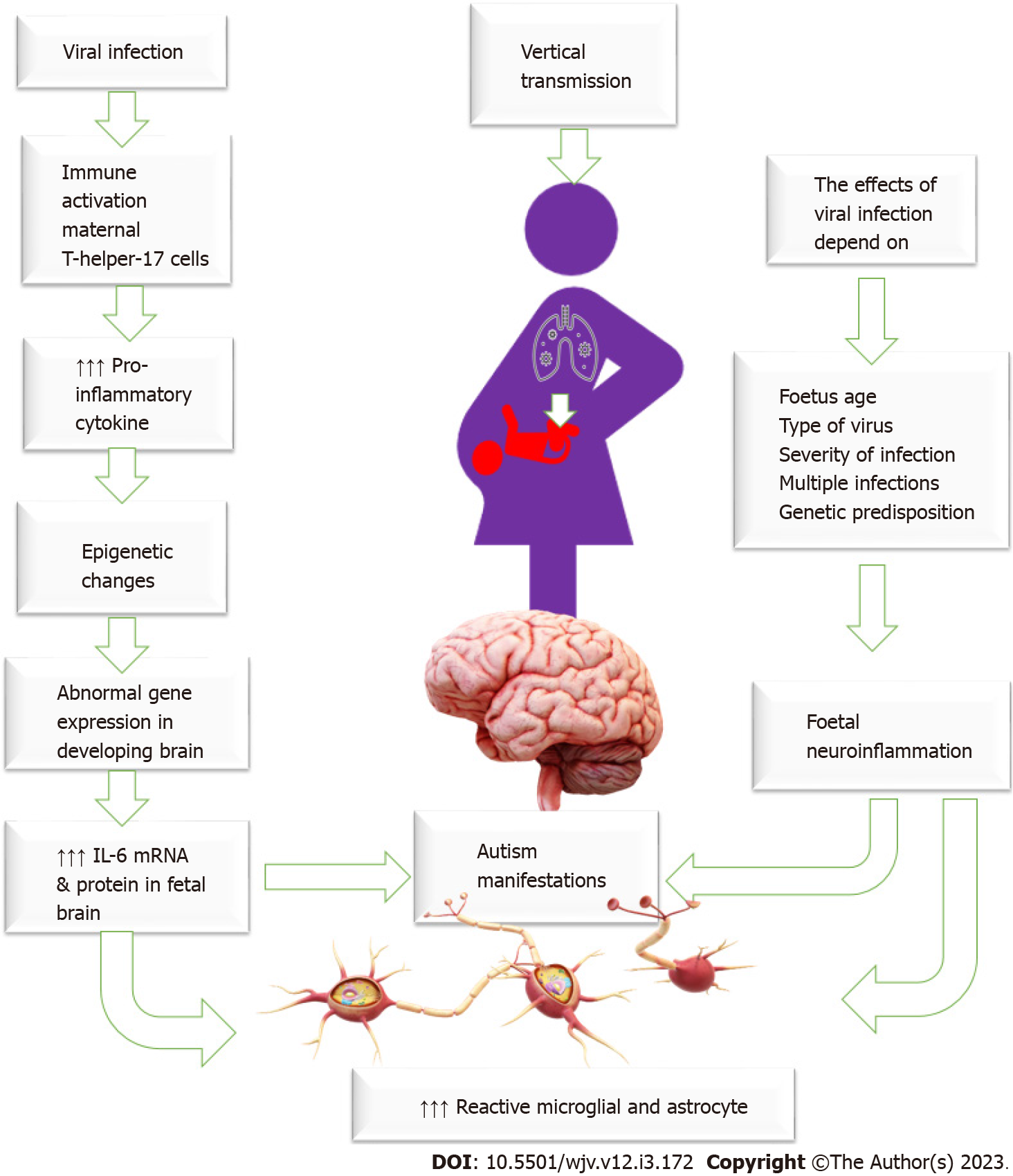
Increased susceptibility to infection is a common problem during pregnancy due to various pathophysiological and mechanical changes and immune system adaptation necessary to keep the fetus in utero and prevent expulsion. Therefore, there is an increased chance of asymptomatic and symptomatic viral infection during pregnancy[45]. Some viruses can cross the placental barrier, reaching the fetus and causing devastating developmental fetal effects[46]. Infection is an important trigger for immune activation. Viruses can directly infect the brain, causing neuron cell death by cell lysis, the release of free radicals, or apoptosis induction, inducing a systemic inflammatory response that affects the brain or alters the maternal or their offspring's immune status, which could influence autism development. Viral-induced immune activation causes elevated levels of pro-inflammatory cytokine IL-6, which changes brain gene expression in the offspring, driving abnormal behaviour development[47]. Maternal immune activation increases maternal pro-inflammatory cytokines, activates maternal T-helper-17 cells, and increases IL-6 mRNA and protein in the fetal brain and the placenta making specific placental tissue changes commonly observed in patients who developed ASD[48-51]. Some viruses, such as rubella and cytomegalovirus, may have a teratogenic effect on the fetus, impairing brain functions and causing autism[46]. The brain and immune system are not fully developed in fetuses and young infants, so they are at high risk of viral-induced brain damage. Therefore, maternal viral infection and inflammation during critical periods of pregnancy could result in an unfavourable intrauterine environment and alter the brain structure and function, raising the risks of having a child with autism[52]. The effects of maternal infections depend on many factors, including genetic susceptibility, the time of infection in pregnancy (early or late), and the severity of the infection[53]. Figure 2: General mechanism of viral infection in the induction of autism.
More than 50 years had elapsed since the link between viral infection and autism development appeared on the surface when Chess et al[54] observed the development of autism in 7% of children suffering from congenital rubella. One year later, Deykin and MacMahon[55] hypothesized that direct exposure to or getting infected with prenatal rubella, measles, mumps, or postnatal mumps might have a causal role in developing autism. After that, many studies documented the link between other viral infections, such as polyomaviruses, cytomegalovirus, and influenza, with the increased risk of autism[56,57]. Many animal studies have shown the role of prenatal or early postnatal exposure to infections, including viruses, in developing permanent neurological and behavioural abnormalities in offspring involving autism features[57,58]. Shi et al[57] showed that pregnant mice infected with the human influenza virus give up offspring with significant behavioural abnormalities as adults, including schizophrenia and autism, with deficits in prepulse inhibition in the acoustic startle response. Moreno et al[59] showed that antenatal mice-adapted influenza virus infection could induce behavioural changes by altering serotonin and glutamate systems via upregulating serotonin 2 A receptors and downregulating metabotropic glutamate receptor 2 in the frontal cortex of the born mice.
There is increasing evidence that a prenatal infection can lead to autism. Maternal infection by DNA or RNA viruses that can cross the maternal-fetal interface may induce neurodevelopmental problems in the developing fetus[60]. A study published in the American Journal of Perinatology 2017 Looked at data from more than 100000 children who were a part of the Kaiser Permanente health system in California from 1991 to 2009. It showed that women who developed an infection in the third trimester were at an increased risk for developing autism in their children[61]. Another study by Croen et al[62] showed that the risk of autism is double in mothers with second-trimester infections and fever, suggesting that the infection-induced inflammation during a specific temporal window of pregnancy may be an etiological factor. Fang et al[63] studied 4184 children with ASD, and 16734 healthy, controlled-matched children over seven years between 2000 and 2007 to evaluate the link between prenatal infections and the risk of developing autism across three trimesters. They showed that contracting infections in the third trimester was slightly associated with a raised risk of ASDs. However, the effects of infection in the third trimester on the risk of autism development demands further exploration. Zerbo et al[64] showed that maternal infections during pregnancy, especially bacterial and multiple infections that need hospitalization, are associated with an increased risk of having a child with ASD. There is some evidence that boys are at a more increased risk of antenatal viral infection and, consequently, autism, primarily due to the noted sex bias seen in the placental immune response related to the viral infection[65]. The placenta in female babies can induce random X- chromosome reactivation, potentially increasing the gene dosage of specific X-linked genes at a very early or late pregnancy period, inducing enhanced protection against viral infection[66].
It should be noted that most of the included studies give evidence from an epidemiological perspective. It is essential to consider possible confounding factors and other disorders during preg-nancy, such as preterm birth, low birth weight, maternal smoking, and seasonal variation. An interesting study showed that the risk of autism was highest in babies conceived in the winter and born in the fall, while the rate was lowest in babies conceived in the summer and born in the spring[67]. Could the rate be related to an increased incidence of infection during winter time or low exposure to sunlight during a period of critical neurodevelopment? This finding needs further exploration.
Not only does infection during pregnancy increases the risk of developing ASD, but infection in early postnatal life is also associated with increased risk. A study by Getahun et al[68] showed that preterm and term babies who encountered neonatal sepsis had a significantly increased probability of autism for both boys and girls and in all race-ethnic groups except Asian/Pacific Islanders compared with unexposed children. A case-control Danish study involving 414 children with ASD cases and 820 children as controls showed that children with autism had a higher risk of infection-related hospital admission during the first year of life than the controls[69]. However, another study by Rosen et al[70] showed an increased risk of autism with infection during the first 30 days postnatal and not the first two years of life.
On the other hand, some previous studies showed no relation between antenatal viral infection and the risk of autism. Anlar et al[71] found no association between antibody levels against Human parvovirus B19 and the risk of autism in 22 children with autism and 50 children with other neurological disorders as control. However, they did not compare children with autism with typically developed children, which is a substantial limitation of the study. In addition, Sylvester Jørgensen et al[72] found no significant differences in the prevalence of herpes simplex virus antibodies in 123 children with different psychiatric disorders, including autism, and 86 typically developed children. Meanwhile, Deykin and MacMahon[55] suggested that antenatal or early postnatal exposure to chickenpox, mumps, measles, or rubella did not increase the likelihood of developing autism. However, they proposed that combined infection of two or more of these viruses could increase the risk of autism, especially for antenatal mumps, measles, rubella, and postnatal mumps. However, there may be a limitation in these studies' methodology that could explain the contradictory results with recent studies.
Certain viruses are known to be more commonly associated with the development of autism.
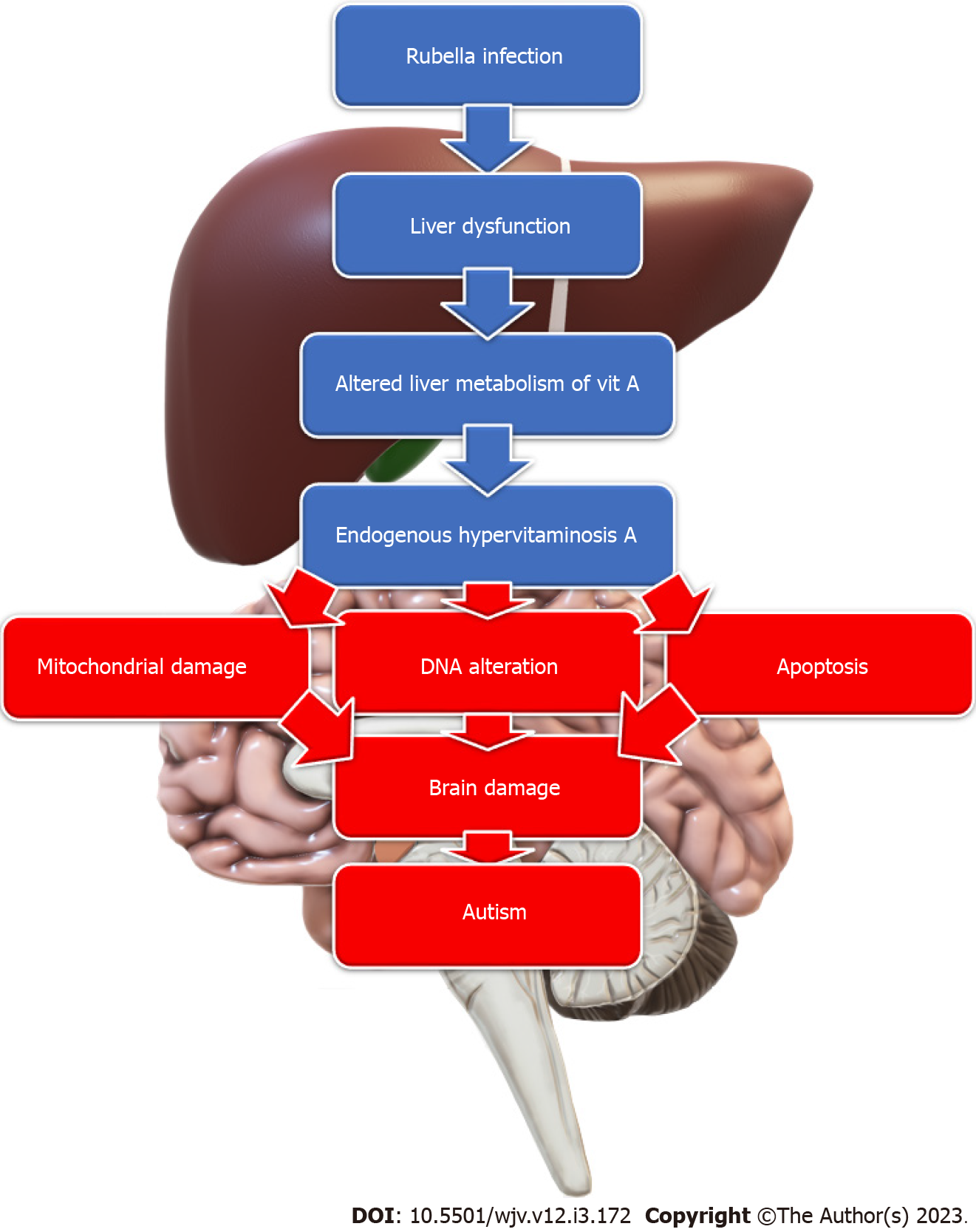
Rubella: Rubella is a well-known RNA virus that can cause various congenital malformations when contracted, especially during the first trimester of pregnancy. Intellectual disabilities, including autism, are common among children with congenital rubella infection. The autism rate is 200 times more in these children than in the general population, reaching a rate of 8%-13% of children with congenital rubella syndrome[73]. Different mechanisms were proposed, including infection-induced hypervitaminosis A. Acute rubella infection induces mild liver dysfunction altering the liver metabolism of vitamin A with the spilling of the stored vitamin A complexes into the circulation, resulting in an endogenous type of hypervitaminosis A, which serves as a teratogen causing mitochondrial damage, DNA alteration, and apoptosis; inducing autism development[74] (Figure 3). In addition, the rubella virus can invade and replicate inside the brain cells. It also can cause cerebral vascular lesions and haemorrhages. It can also cause fulminant degeneration of leptomeninges with significant brain volume loss and destruction of white matter[73]. Therefore, many manifestations of congenital rubella syndrome, such as congenital heart defects, spasticity, deafness, and visual impairment, are common in children with autism. In addition, radiological findings, such as dilated perivascular spaces, periventricular leukomalacia, calcifications, and decreased perfusion to specific brain areas, are common for children with congenital rubella syndrome and those with autism[73]. Hence, rubella might still be a possible cause of autism, even in countries with well-vaccination policies.
Due to the high incidence of autism in children with congenital rubella infection, they should be followed strictly for signs of autism. Symptoms of autism may not appear till adolescence. Conversely, clinicians should screen children with autism for possible signs of congenital rubella infection[75]. This delay in developing congenital rubella infection manifestations could be related to persistent rubella virus infection in the affected organs or developing autoimmune responses to an old infection[76]. Even though the United States of America was declared free from congenital rubella syndrome in 2004, the rate of autism is increasing in the USA, reaching a rate of 1:59[77]. The increased autism rate may indicate that subclinical rubella still plays a role, or at least rubella infection is just one of the players.
Cytomegalovirus: Human cytomegalovirus (CMV) is a double-stranded DNA neurotropic beta-herpes virus. It is the most prevalent member of the human herpesvirus family with high species-specificity as a human is the only host. Therefore, it is recently known as human herpes virus 5 (HHV-5)[78]. Congenital CMV infection is one of the leading infectious causes of neurological impairment in the new coming baby, with an infection rate between 0.5% and 1%. Congenital CMV infection can produce various clinical manifestations at birth in 10%–15% of the infected babies. However, asymptomatic infections can be reactivated anytime throughout life[79]. Engman et al[80] showed that congenital CMV infection is present in 3% of children with autism. CMV infection blocks interferon production and impairs CD8 T-cell function, thus inhibiting the antiviral defense mechanism of the host and inducing inflammation both in the maternal circulation and the placenta. In addition, it causes local infiltration of macrophage and T-cells at the site of infection in vivo, altering immune function and playing a significant role in perinatal brain injury[81,82]. CMV infection-associated alteration of the immune response causes disrupted development of particular regions or structures in the brain that lead to the development of autism[83].
CMV infection increases the circulating maternal pro-inflammatory cytokine levels, which is associated with an increased risk of autism and other mental disorders[84]. Children with congenital CMV infection may have many typical features of autism, including poor development of adequate interpersonal relationships, weak eye-to-eye contact, impaired language development, and non-thematic use of objects[85]. The presence of high CMV-IgM levels may indicate maternal infection with CMV[86]. Neonates with suspected congenital CMV could be diagnosed by detecting CMV-DNA in the cord blood or their urine in the first postnatal two weeks using real-time polymerase chain reaction[87,88]. Children with autism suspected of having congenital CMV infection may also have positive CMV-DNA in the urine and positive CMV- IgM antibodies in their serum[89,90]. Cranial ultrasound may show calcifications, increased periventricular echogenicity, ventriculomegaly, and intraventricular adhesions[91]. Magnetic resonance imaging (MRI) of their brain may reveal the presence of abnormal periventricular white matter intense areas, suggestive of disturbed myelination. MRI brain may also show an increased rate of hippocampal abnormality in children with congenital CMV infection. MRI may be normal in the first few weeks of life in children with congenital CMV; however, It may show the characteristic changes in white matter from temporal to posterior regions by one year of age[92,93]. Therefore, at birth, asymptomatic children might develop autism within the first few years of life[94].
In addition to congenital infection, CMV infection in infancy may raise the risk of developing autism. Postnatal CMV infection can impair overall cognitive functions and reduce intelligence during childhood and adolescence[58]. Lin et al[95] showed that CMV infection in infancy might raise the risk of consequent autism and epilepsy but not attention deficit hyperactivity disorder, particularly in infants under two years old. Yang et al[96] also showed that postnatal CMV infection might raise the predisposition to tuberous sclerosis and autism spectrum disorders. Both epilepsy and tuberous sclerosis are associated with an increased risk of autism[97].
Herpes simplex virus: The herpes simplex virus (HSV) is divided into two main types; HSV-1 and HSV-2. HSV-1 is mainly transferred through the oral route, while HSV-2 is principally transmitted through sex. About 67% of the global population has HSV-1 infection, while 13% have HSV-2 infection worldwide. About 15% of women of childbearing age are seropositive against HSV-2 globally[98]. HSV induces the release of inflammatory molecules and immune system activation that alter the brain structure with abnormal growth of the cerebral neocortex, a common finding in children with autism, especially during the first 6-12 mo of life[99]. A Norway study between 1999 and 2008 showed that active infection with HSV-2 in early pregnancy doubles the risk of developing autism in the male fetus. They found that increasing HSV-2 IgG levels in maternal mid-pregnancy plasma from 240 to 640 arbitrary units/mL doubles the risk of developing autism in male fetuses after adjusting for parity and the child's birth year. There were few female fetuses included in the study to conclude similar results. The presence of high mid-pregnancy antibody levels against HSV-2 might indicate an active maternal infection during early pregnancy[100]. However, a study by Gentile et al[101] showed no significant differences in the seropositivity rates and levels of anti-HSV-1 or anti-HSV-2 between children with autism and the controls. Another study by Slawinski et al[88] showed that the antenatal infection with CMV and not HSV-2 increases the risk of autism in the coming children. We still need more research to prove or disprove the effect of antenatal infection with HSV-2 on the risk of developing autism in the offspring.
Varicella-zoster virus: Varicella-zoster virus is s an alpha herpes DNA virus known as human herpesvirus 3 that causes chickenpox and herpes zoster (shingles). It is a neurotropic virus that can cause latent infection in the sensory nerve ganglia. It also induces neuroinflammation and is implicated in many central nervous system disorders, such as multiple sclerosis, that can share some common pathophysiologic features with ASD[102]. There is not much evidence about its role in the pathogenesis of autism. Gentile et al[103] showed an increased seropositivity rate for Varicella Zoster Virus in children with autism than in controls. They hypothesized that exposure to Varicella Zoster Virus and high titers of specific anti- Varicella Zoster Virus antibodies had a significant association with ASD. However, this association could be a cause or, on the other side, be due to increased susceptibility to infection with Varicella Zoster Virus due to vaccinophobia to MMR. Therefore, we need more studies to prove or disprove its causal relation. Some anti-varicella medications that are used to prevent or treat latent varicella infection can improve the symptoms of autism, establishing the causal relation of varicella infection with autism[104].
Influenza viruses: Influenza is caused by a group of enveloped negative-sense RNA Orthomyxoviridae viruses categorized into four main subgroups; Influenza types A, B, C, and D; among them, types A and B cause the well-known respiratory influenza disease. Influenza affects about 5%-15% of the world's population yearly, with more than three million people encountering severe infections and large numbers of hospitalization and deaths[105]. Influenza is associated with increased morbidity and mortality in high-risk groups, including pregnant women, and during the first two postpartum weeks, reliant on pre-existing immunity[106]. Some studies showed an increased risk of adverse neurodevelopmental outcomes such as autism or schizophrenia when pregnant ladies encounter influenza during pregnancy[107]. Mahic et al[108] found a statistically non-significant increase in the risk of autism in the offspring of seropositive mothers with symptoms of influenza during mid-pregnancy compared to seronegative mothers. The chance may be responsible for this difference. Other biological factors, such as maternal immune system activation, could also be responsible. In addition, the influenza virus can trigger a cascade of acute-phase reactions, including fever which by itself increases the risk of autism, together with a systemic increase in cytokine expression[109].
However, many recent studies showed no increased risk of developing autism with influenza infection or vaccination during pregnancy in the offspring. In addition, Zerbo et al[110] showed no significant association between influenza infection and the risk of autism in the offspring. Meanwhile, they found a statistically non-significant increased risk of autism in the offspring when pregnant ladies received influenza vaccination during the first trimester. However, they emphasized that this observation should not call for any change in vaccination policy or practice. A more recent study by Becerra-Culqui et al[111] reported the same finding when they found no association between prenatal influenza infection or vaccination and increased risk of autism in offspring. They strongly recom
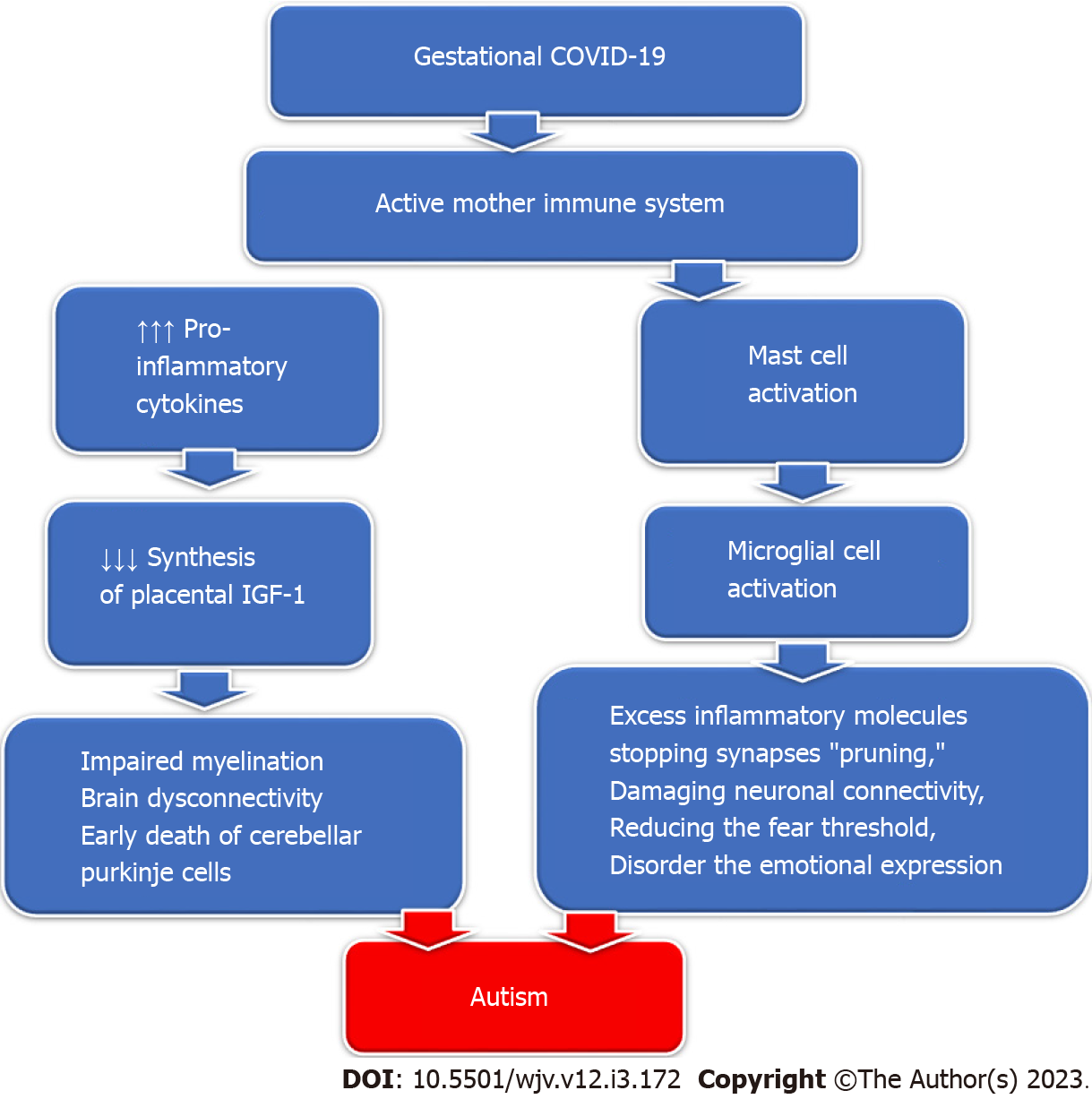
SARS-CoV-2 viruses: COVID-19 is caused by infection by SARS-CoV-2, one of the beta coronaviruses, which caused the pandemic coronavirus COVID-19, posing a severe threat worldwide. SARS-CoV-2 can spread from the respiratory tract to the central nervous system through the olfactory bulb. COVID-19 induces brain structural changes that cause various neurologic complications that could last long[112]. There is a rising concern about the potentially harmful effects of SARS-CoV-2 on pregnancy that could affect the pregnant lady and her fetus[113]. Currently, there is no proof of SARS-CoV-2 vertical transmission from the mother to her fetus, which could be due to the preventive effect of placental lactoferrin. However, the virus could be transferred postnatally via the mother's air droplets or during breastfeeding[114]. Severe gestational COVID-19 induces uncontrolled inflammatory cytokine storm release and maternal immune activation, causing possible fetal organ damage, including the brain, that could manifest later with autism symptoms[28]. The induced inflammation causes amygdala neurodegenerative changes by "short-circuiting the electrical system," producing emotional feeling ability impairment and abnormal fear regulation due to an abnormal hypothalamic-pituitary-adrenal axis system[115].
Gestational COVID-19 activates the maternal immune system, increasing the pro-inflammatory cytokine production that inhibits the synthesis of placental Insulin-like growth factor-1 (IGF-1). Decreased IGF-1 production impairs perinatal myelination and induces dysconnectivity of the developing brain with permanent neurological deficits[116]. IGF-1 deficiency causes reduced perinatal neo-neuronal myelination mediated by oligodendrocytes in the developing nervous system, which in turn causes the early death of cerebellar Purkinje cells and the development of autism[117]. Additionally, SARS-CoV-2 infection can activate mast cells which in sequence activate microglial cells, releasing excessive inflammatory molecules, stopping synapses "pruning," damaging neuronal connectivity, and reducing the fear threshold, disorderly the emotional expression detected in children with autism[118] (Figure 4).
These changes can explain the increase in the rate of autism during the COVID-19 pandemic. Edlow et al[119] showed that in-utero exposure to SARS-CoV-2 infection might be associated with increased neurodevelopmental disorder rates in some offspring. However, Brynge et al[120] showed that the increased association of autism and other intellectual disabilities in the offsprings of mothers infected with SARS-CoV-2 during pregnancy does not necessarily reflect the causal relationship but is more probable to be related to common familial conditions such as shared genetic and environmental factors. On the other hand, Dutheil et al[121] claimed that the COVID-19 pandemic might be a reason for a decrease in the incidence of autism in a paradoxical phenomenon due to decreased global air pollution, which is a significant environmental trigger for autism.
To lessen the influence of gestational COVID-19, pregnant women should have adequate amounts of n-3 polyunsaturated fatty acids, vitamin D, folic acid, and a high choline and luteolin supplement. These supplements benefit brain development and function in the offspring of women who encounter viral infections during early pregnancy. Luteolin is a potent natural flavonoid inhibitor of microglia and mast cell activation and prevents SARS-CoV-2 binding to ACE2 receptors[118,122].
Zika virus: Zika virus (ZIKV) is an anthropoid-borne positive-sense RNA Flavivirus that spreads mainly by biting infected Aedes species mosquitos (Ae. aegypti and Ae. albopictus). It poses a significant threat to human health worldwide[123]. The severity of ZIKV infection ranges from mild influenza-like infection to severe conditions with neurological complications such as seizures and Guillain–Barré syndrome. Some strains of ZIKV can cross the placental barrier and infect cortical progenitor cells to promote cell death via enhancement of apoptosis and autophagy, producing severe congenital malformations (ZIKV Congenital Syndrome)[124]. ZIKV Congenital Syndrome includes microcephaly, hypoplasia or atrophy of the cerebral cortex, cerebellum, brainstem, abnormal cortical formation, corpus callosum anomaly, other neurological abnormalities, cerebral palsy, severe developmental delay, and eye defects, which are one of the severe complications that occur when the mother gets infected during pregnancy[125]. Unfortunately, no specific antiviral medications or vaccines are available against ZIKV infection[126].
In a large population-based mother-child cohort study during the 2016-ZIKV outbreak in the United States, about 15.3% of toddlers with in-utero exposure to ZIKV had abnormal neurodevelopment findings at two years of age[127]. The inflammatory process generated by ZIKV during pregnancy has bi-mutual pathways. In one aspect, it helps to eradicate the virus, and in another aspect, it causes damage to the developing brain. The released inflammatory mediators, such as interleukin-6 and tumour necrosis factor-alpha, affect brain development, delay neuronal maturation, alter brain connectivity, and trigger autism symptoms[128]. There were some reports of increasing reported cases of autism in the offspring of mothers infected with ZIKV during pregnancy. Nielsen-Saines et al[129] observed 18 children who developed neurological symptoms out of 216 offspring from mothers infected with ZIKV during pregnancy. Six children out of 18 had autism manifestations. In addition, three children born asymptomatic developed autism manifestations at one year of age. In addition, Abtibol-Bernardino et al[130] showed that five children out of 26 who had antenatal exposure to ZIKV developed severe neurological disorders; two of them had autism. Therefore, children with a positive history of in-utero ZIKV exposure should have a serial follow-up for any neurological impairment, including autism, even when born asymptomatic.
Henderson[131] described a four-year-old child with a bipolar disorder associated with violent aggression and manifestations of autism and attention deficit hyperactivity disorder resistant to conventional treatment, including anticonvulsants, methylphenidate, guanfacine, lithium, and neuroleptics. After the failure of multiple regimens to stabilize the mood, the patient received a trial of valacyclovir 1000 mg twice till improved, then with half the dose to complete three years. The patient improved dramatically in irritability, volatile mood, social reciprocity, concentration, and overall personality. The potential benefit of valacyclovir could be related to improving subclinical herpes simplex. Meanwhile, Kimberlin et al[132] found that oral acyclovir (300 mg/square meter/dose taken three times daily for six months a) can improve the neurodevelopmental outcomes in children who survived neonatal herpes simplex disease with central nervous system involvement. In addition, naltrexone therapy may benefit children with autism, particularly in the presence of self-injurious behaviour, failure of other treatments, and "high opioid tone" autism[133]. Naltrexone has immune-modulating effects and prevents the activation of grey and white matter astrocytes in specific brain areas, especially frontal cortical tissues, causing a reduction of injurious behaviour[134]. Intravenous immunoglobulins (IVIG) are one of the well-established treatment methods for severe systemic infections, including viral infections[135]. IVIG showed promising efficacy in treating autoimmune encephalopathy in children with autism, especially with high anti-dopamine D2L receptor antibodies[136]. Connery et al[137] showed that IVIG effectively alleviated the symptoms of irritability in children with autism who developed autoimmune encephalopathy. The presence of anti-dopamine D2L receptor antibodies is associated with improved responsiveness to IVIG therapy with modulation of behaviour. Therefore, we can use these antibodies to predict the responsiveness to IVIC therapy in these children.
N-acetylcysteine is derived from the amino acid L-cysteine and helps to regenerate glutathione with the release of glutamate into the extracellular space, reducing glutamatergic neurotransmission at synapses, correcting brain glutaminergic dysregulation, and consequently improving the autistic manifestations. It helps to reduce irritability and hyperactivity and improves social awareness in patients with autism[138,139]. Boris et al[140] found in a small cohort of children with autism that oral using 30 or 60 mg of Pioglitazone for three to four months might induce apparent clinical improvement of the behavioural symptoms in those children without significant side effects. Pioglitazone is an anti-diabetic medication that modulates the effect of insulin. Pioglitazone can reduce IL4 production in CD4 cells and block IL10 and IL4 production by T cells. It also may help shift the T-cell response from Th2 to Th1 or decrease Th2 cytokine expression. Therefore, oral Pioglitazone may be of therapeutic advantage in children with autism[141].
Children with autism have more chances of getting infected. Sabourin et al[142] showed that children with autism are more susceptible to infections in the first four postnatal weeks and the first three years of life than the typically developed children. In addition, they found that children with autism also have more infections in the first four weeks of life than children with other developmental disorders. Children with autism have multiple co-morbidities and behavioural problems that increase the risk of contracting various infections, including viruses[97]. Table 2 shows some important risk factors and co-morbidities that increase the risk of contracting diseases in children with autism.
| Risk factors | Description |
| Immune system disorders | High rate of autoimmune diseases |
| Immune dysregulation of T cell functions | |
| Impaired levels of immune mediators | |
| lower plasma IgG, and IgM | |
| Continuing immune dysfunction | |
| High rate of mitochondrial dysfunction | |
| Oxidative stress | |
| Neutrophils dysfunction | |
| Medical co-morbidities | Genetic disorders: e.g., fragile X syndrome, Down syndrome, Duchenne muscular dystrophy, tuberous sclerosis complex, and neurofibromatosis type I |
| Neurological disorders: e.g., cerebral palsy and congenital abnormalities | |
| Gastrointestinal disorders: Gastroesophageal reflux and inflammatory bowel disease | |
| Metabolic disorders: mitochondrial disorders, disorders of creatine metabolism, selected amino acid disorders, disorders of folate or vitamin B12 metabolism, and selected lysosomal storage disorders | |
| Allergic disorders: Such as asthma, nasal allergies, atopic diseases (IgE-mediated) | |
| Behavioral problems | Stereotyping behavior: Affecting mouth and general hygiene |
| Mouthing and pica behavior | |
| Faecal smearing | |
| Feeding and nutritional disorders | Biological food intolerance |
| Restrictive and selective behavior | |
| Sensory-based feeding problems | |
| Relational sphere disorders | |
| Medically-related feeding problems | |
| restrictive dietary management | |
| Nutritional deficiencies: e.g., Vitamin A, D, C, and zinc | |
| Gastrointestinal dysfunctions | Gastroesophageal reflux |
| Autonomic dysfunction and Impaired intestinal motility | |
| Gastric hypoacidity and | |
| Impaired digestive enzyme production | |
| Gut dysbiosis | |
| Inflammatory bowel disease | |
| Vaccinophobia | Lack of parental education and awareness |
| Anti-vaccine movement |
Children with autism have a high rate of autoimmune diseases, immune dysregulation, and impaired levels of immune mediators, indicating continuing immune dysfunction[51]. Some genetic causes of autism are associated with an immune deficiency that increases the risk of infections, including viral disease, e.g., Timothy syndrome[143]. Heuer et al[144] showed that children with autism have lower plasma IgG and IgM levels than typically developed children and children with developmental delays. The levels of these immunoglobulins were negatively correlated with the autism severity, as indicated by the Aberrant Behavior Checklist score. Children with autism commonly have mitochondrial dysfunction. Mitochondrial dysfunction is associated with reduced oxidative phosphorylation and burst in granulocytes which causes an impaired immune response and weak antioxidant defence[145].
Children with autism are subject to restrictive nutrition supplements either due to the restrictive and selective behaviour associated with autism, sensory-based feeding problems, relational sphere dis-orders, biological food intolerance, medically related feeding problems, and restrictive dietary management, that expose them to various nutritional deficiencies which further impair their immunity and susceptibility to infection[97]. Babaknejad et al[146] found statistically significantly lower plasma zinc levels in patients with autism than in healthy controls. Zinc deficiency negatively impairs children's mental development and physical growth and might damage their immune systems[147]. Vitamin C deficiency was also reported in some patients with autism due to the severely restricted diet or the associated gastrointestinal disorders that prevent adequate vitamin C intake or absorption[148,149]. Vitamin C is essential for the integrity of the mucous membrane and general immunity[147]. In addition, children with autism have the highest rate of vitamin A deficiency compared to other micronutrients, and the degree of deficiency is correlated with autism severity[150]. Vitamin A significantly impacts gastrointestinal functions and the skin's innate immunity. Therefore, vitamin A deficiency increases the risk of skin infections[151]. Vitamins A and D regulate microbial complexity, mucosal barrier function, and immune responses, ensuring intestinal homeostasis. Therefore, its deficiency induces gut dysbiosis and enhances the dysbiotic microbial communities, increasing susceptibility to infection and the risk of gastrointestinal tract injury[152].
Normal intestinal motility is required for healthy gut microbiota by washing out any abnormal bacterial growth in the body. Children with autism have impaired intestinal peristalsis and digestive abilities due to autonomic dysfunction. They frequently get constipation resulting in abnormal proliferation and impaired clearance of the pathogenic intestinal bacteria. In addition, children with autism have gastric hypoacidity and impaired digestive enzyme production, which augments the resulting dysbiosis[153]. Conversely, gut dysbiosis correlates with the severity of autism, cytokine quantities, and tryptophan homeostasis. Therefore, gut microbiota modulation may alleviate the symptoms of autism[154].
Another critical risk factor that increases the risk of viral infections in children with autism is the reluctance of some parents to vaccinate their children with autism. Despite the marked progress in studying the epidemiology, aetiology, pathophysiology, and genetics of autism; many misguided scientists; politicians, and frustrated parent groups still resist vaccinating children with autism[155]. Since Andrew Wakefield and his 12 colleagues published their frauded paper in British Medical Journal in 1998, the vaccination rate of children with autism significantly declined, especially for measles, mumps, and rubella[156]. The resultant antivaccine movements considered three main pillars for their action. They hypothesized that combining the measles-mumps-rubella vaccine can induce intestinal mucosal damage, allowing the entry of encephalopathic proteins that trigger autism. In addition, they suppose that the ethylmercury-containing vaccine preservative thimerosal is neurotoxic. They also suggest that concurrently administering multiple vaccines devastates or weakens the immune system[157]. Zerbo et al[158] found that the vaccination rate in children with autism and their younger siblings was significantly lower than in the typically developed children. Parents of children with autism were more reluctant to vaccinate at least one recommended vaccine for the child's younger sibling and to limit the number of vaccines given during the first year of life of the younger siblings.
Many included studies were of a limited number of patients or were done in animal models. We need to have long-term, multicentered studies that include different races and populations to better judge the interplay between infections and autism as a cause or effect. Pregnant women should ensure their vaccination against influenza, as this can prevent them from getting the flu while pregnant, which can cause complications for the mother and the baby[105-108]. When women get vaccinated against influenza, they protect their unborn babies from getting this illness while in the womb. Studies have shown that influenza virus infection can cause severe problems for babies if infected during the first trimester of pregnancy, leading to preterm birth or stillbirth in some cases[109-111]. By getting vaccinated, pregnant women can protect themselves and their unborn babies from the flu while helping to reduce the spread of the disease in the community. Pregnant women could have enough vitamin D, folic acid, n-3 polyunsaturated fatty acids, and high choline and luteolin[117,122].
Early screening and possibly diagnosis to detect and may prevent autism are crucial for reducing the burden of this condition. Long-term follow-up is necessary for infants whose mothers report an inflammatory event due to viral infection at any time during pregnancy to monitor for signs of autism. In addition, children with autism should be screened for congenital rubella and cytomegalovirus infections[76,96]. Further research is needed to investigate whether specific vaccines or other measures taken during pregnancy can prevent the development of autism in children born to mothers whose certain viruses have infected them.
Autism is a group of heterogeneous, multi-factorial, neurodevelopmental disorders. Several factors play together to increase the risk of development of autism, including genetic, epigenetic, and environmental factors, together with antenatal and early-life infections. Viral infection during critical periods of early in-utero neurodevelopment may lead to an increased risk of autism. Maternal infection by DNA or RNA viruses that can cross the maternal-fetal interface may induce neurodevelopmental problems in the developing fetus. Viral infection induces neuroinflammation that affects many neurological processes, including neural development, brain structures, synapse plasticity, cognition, and behaviour; therefore, it may end in the development of autism. There are many shreds of evidence that rubella, cytomegalovirus, herpes simplex virus, influenza viruses, SARS-CoV-2 viruses, and zika virus infections during pregnancy and early life can trigger autism development. Conversely, children with autism are at increased risk of infections, including viruses. Every effort should be made to prevent maternal and early-life infections and reduce the risk of autism. Vaccination against common viral agents may help to reduce the prevalence of autism. Immune modulation of children with autism should be considered to reduce the risk of infection.
We thank the anonymous referees for their valuable suggestions.
| 1. | Al-Biltagi M, Saeed NK, Qaraghuli S. Gastrointestinal disorders in children with autism: Could artificial intelligence help? Artif Intell Gastroenterol. 2022;3:1-1. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Joon P, Kumar A, Parle M. What is autism? Pharmacol Rep. 2021;73:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Rapin I. The autistic-spectrum disorders. N Engl J Med. 2002;347:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 4. | Garcia-Forn M, Boitnott A, Akpinar Z, De Rubeis S. Linking Autism Risk Genes to Disruption of Cortical Development. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14:281-292. [PubMed] |
| 6. | Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 7. | Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 710] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 8. | Eshraghi AA, Liu G, Kay SS, Eshraghi RS, Mittal J, Moshiree B, Mittal R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front Cell Neurosci. 2018;12:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Barbeau WE. Neonatal and regressive forms of autism: Diseases with similar symptoms but a different etiology. Med Hypotheses. 2017;109:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Karimi P, Kamali E, Mousavi SM, Karahmadi M. Environmental factors influencing the risk of autism. J Res Med Sci. 2017;22:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Rice CE, Rosanoff M, Dawson G, Durkin MS, Croen LA, Singer A, Yeargin-Allsopp M. Evaluating Changes in the Prevalence of the Autism Spectrum Disorders (ASDs). Public Health Rev. 2012;34:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Shuid AN, Jayusman PA, Shuid N, Ismail J, Kamal Nor N, Mohamed IN. Association between Viral Infections and Risk of Autistic Disorder: An Overview. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Gładysz D, Krzywdzińska A, Hozyasz KK. Immune Abnormalities in Autism Spectrum Disorder-Could They Hold Promise for Causative Treatment? Mol Neurobiol. 2018;55:6387-6435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Faras H, Al Ateeqi N, Tidmarsh L. Autism spectrum disorders. Ann Saudi Med. 2010;30:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S. The Influence of Vicarious Fear-Learning in "Infecting" Reactive Action Inhibition. Front Behav Neurosci. 2022;16:946263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Candini M, Battaglia S, Benassi M, di Pellegrino G, Frassinetti F. The physiological correlates of interpersonal space. Sci Rep. 2021;11:2611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front Behav Neurosci. 2022;16:998714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Tanaka M, Spekker E, Szabó Á, Polyák H, Vécsei L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J Neural Transm (Vienna). 2022;129:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897-6906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 22. | Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Casanova MF. Autism as a sequence: from heterochronic germinal cell divisions to abnormalities of cell migration and cortical dysplasias. Med Hypotheses. 2014;83:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Toscano CVA, Barros L, Lima AB, Nunes T, Carvalho HM, Gaspar JM. Neuroinflammation in autism spectrum disorders: Exercise as a "pharmacological" tool. Neurosci Biobehav Rev. 2021;129:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 27. | Matta SM, Hill-Yardin EL, Crack PJ. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav Immun. 2019;79:75-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 28. | Al-Beltagi M, Saeed NK, Bediwy AS, Alhawamdeh R, Qaraghuli S. Effects of COVID-19 on children with autism. World J Virol. 2022;11:411-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 29. | Fattorusso A, Di Genova L, Dell'Isola GB, Mencaroni E, Esposito S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (1)] |
| 30. | Robinson-Agramonte MLA, Noris García E, Fraga Guerra J, Vega Hurtado Y, Antonucci N, Semprún-Hernández N, Schultz S, Siniscalco D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 31. | Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals (Basel). 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 32. | Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 914] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 33. | Khalil R, Tindle R, Boraud T, Moustafa AA, Karim AA. Social decision making in autism: On the impact of mirror neurons, motor control, and imitative behaviors. CNS Neurosci Ther. 2018;24:669-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Geurts HM, de Wit S. Goal-directed action control in children with autism spectrum disorders. Autism. 2014;18:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Chiola S, Edgar NU, Shcheglovitov A. iPSC toolbox for understanding and repairing disrupted brain circuits in autism. Mol Psychiatry. 2022;27:249-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877-1885. [PubMed] |
| 37. | Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951-961. [PubMed] |
| 39. | Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 369] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Klin A. Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J Autism Dev Disord. 1993;23:15-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Owens AP, Mathias CJ, Iodice V. Autonomic Dysfunction in Autism Spectrum Disorder. Front Integr Neurosci. 2021;15:787037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Ming X, Patel R, Kang V, Chokroverty S, Julu PO. Respiratory and autonomic dysfunction in children with autism spectrum disorders. Brain Dev. 2016;38:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Meltzer A, Van de Water J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology. 2017;42:284-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 44. | Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1521] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 45. | Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 518] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 46. | Cordeiro CN, Tsimis M, Burd I. Infections and Brain Development. Obstet Gynecol Surv. 2015;70:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 48. | Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 49. | Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry. 2007;61:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 51. | Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16:469-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 52. | Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146:R151-R162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 53. | Arad M, Piontkewitz Y, Albelda N, Shaashua L, Weiner I. Immune activation in lactating dams alters sucklings' brain cytokines and produces non-overlapping behavioral deficits in adult female and male offspring: A novel neurodevelopmental model of sex-specific psychopathology. Brain Behav Immun. 2017;63:35-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. J Pediatr. 1978;93:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 115] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Deykin EY, MacMahon B. Viral exposure and autism. Am J Epidemiol. 1979;109:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Lintas C, Altieri L, Lombardi F, Sacco R, Persico AM. Association of autism with polyomavirus infection in postmortem brains. J Neurovirol. 2010;16:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 708] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 58. | Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. J Autism Dev Disord. 2003;33:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Moreno JL, Kurita M, Holloway T, López J, Cadagan R, Martínez-Sobrido L, García-Sastre A, González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT₂A and mGlu₂ receptors in the adult offspring. J Neurosci. 2011;31:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 895] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 61. | Getahun D, Fassett MJ, Peltier MR, Wing DA, Xiang AH, Chiu V, Jacobsen SJ. Association of Perinatal Risk Factors with Autism Spectrum Disorder. Am J Perinatol. 2017;34:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, Daniele Fallin M, Levy S, Schieve LA, Yeargin-Allsopp M, Sabourin KR, Ames JL. Infection and Fever in Pregnancy and Autism Spectrum Disorders: Findings from the Study to Explore Early Development. Autism Res. 2019;12:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | Fang SY, Wang S, Huang N, Yeh HH, Chen CY. Prenatal Infection and Autism Spectrum Disorders in Childhood: A Population-Based Case-Control Study in Taiwan. Paediatr Perinat Epidemiol. 2015;29:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45:4015-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 65. | Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17:564-579. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 66. | Cissé YM, Chan JC, Nugent BM, Banducci C, Bale TL. Brain and placental transcriptional responses as a readout of maternal and paternal preconception stress are fetal sex specific. Placenta. 2020;100:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Lee BK, Gross R, Francis RW, Karlsson H, Schendel DE, Sourander A, Reichenberg A, Parner ET, Hornig M, Yaniv A, Leonard H, Sandin S. Birth seasonality and risk of autism spectrum disorder. Eur J Epidemiol. 2019;34:785-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Getahun D, Fassett MJ, Xiang AH, Chiu VY, Takhar HS, Shaw SF, Peltier MR. The Effect of Neonatal Sepsis on Risk of Autism Diagnosis. Am J Perinatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Abdallah MW, Hougaard DM, Nørgaard-Pedersen B, Grove J, Bonefeld-Jørgensen EC, Mortensen EL. Infections during pregnancy and after birth, and the risk of autism spectrum disorders: a register-based study utilizing a Danish historic birth cohort. Turk Psikiyatri Derg. 2012;23:229-235. [PubMed] |
| 70. | Rosen NJ, Yoshida CK, Croen LA. Infection in the first 2 years of life and autism spectrum disorders. Pediatrics. 2007;119:e61-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Anlar B, Oktem F, Török T. Human parvovirus B19 antibodies in infantile autism. J Child Neurol. 1994;9:104-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Sylvester Jørgensen O, Vejlsgaard Goldschmidt V, Faber Vestergaard B. Herpes simplex virus (HSV) antibodies in child psychiatric patients and normal children. Acta Psychiatr Scand. 1982;66:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Hutton J. Does Rubella Cause Autism: A 2015 Reappraisal? Front Hum Neurosci. 2016;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Mawson AR, Croft AM. Rubella Virus Infection, the Congenital Rubella Syndrome, and the Link to Autism. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Hwang SJ, Chen YS. Congenital rubella syndrome with autistic disorder. J Chin Med Assoc. 2010;73:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Hofmann J, Pletz MW, Liebert UG. Rubella virus-induced cytopathic effect in vitro is caused by apoptosis. J Gen Virol. 1999;80 (Pt 7):1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Maenner MJ, Shaw KA, Baio J; EdS1, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee LC, Harrington RA, Huston M, Hewitt A; PhD-7, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS, Dietz PM. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1651] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 78. | Brecht KF, Goelz R, Bevot A, Krägeloh-Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long-term neuropsychological sequelae. J Pediatr. 2015;166:834-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Lee SM, Mitchell R, Knight JA, Mazzulli T, Relton C, Khodayari Moez E, Hung RJ. Early-childhood cytomegalovirus infection and children's neurocognitive development. Int J Epidemiol. 2021;50:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Engman ML, Sundin M, Miniscalco C, Westerlund J, Lewensohn-Fuchs I, Gillberg C, Fernell E. Prenatal acquired cytomegalovirus infection should be considered in children with autism. Acta Paediatr. 2015;104:792-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Schottstedt V, Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, Hildebrandt M, Jansen B, Montag-Lessing T, Offergeld R, Pauli G, Seitz R, Schlenkrich U, Strobel J, Willkommen H, von König CH. Human Cytomegalovirus (HCMV) - Revised. Transfus Med Hemother. 2010;37:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Zammarchi L, Lazzarotto T, Andreoni M, Campolmi I, Pasquini L, Di Tommaso M, Simonazzi G, Tomasoni LR, Castelli F, Galli L, Borchi B, Clerici P, Bartoloni A, Tavio M, Trotta M. Management of cytomegalovirus infection in pregnancy: is it time for valacyclovir? Clin Microbiol Infect. 2020;26:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Zhang XY, Fang F. Congenital human cytomegalovirus infection and neurologic diseases in newborns. Chin Med J (Engl). 2019;132: 2109-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Aronoff DM, Correa H, Rogers LM, Arav-Boger R, Alcendor DJ. Placental pericytes and cytomegalovirus infectivity: Implications for HCMV placental pathology and congenital disease. Am J Reprod Immunol. 2017;78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Liu T, Zheng X, Li Q, Chen J, Yin Z, Xiao J, Zhang D, Li W, Qiao Y, Chen S. Role of human cytomegalovirus in the proliferation and invasion of extravillous cytotrophoblasts isolated from early placentae. Int J Clin Exp Med. 2015;8:17248-17260. [PubMed] |
| 86. | Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. J Autism Dev Disord. 2004;34:583-586. [PubMed] |
| 87. | Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, DeLorenze GN, Kharrazi M, Yolken R, Ashwood P, Van de Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2017;22:273-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 88. | Slawinski BL, Talge N, Ingersoll B, Smith A, Glazier A, Kerver J, Paneth N, Racicot K. Maternal cytomegalovirus sero-positivity and autism symptoms in children. Am J Reprod Immunol. 2018;79:e12840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Pass RF, Arav-Boger R. Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Res. 2018;7:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | Endo T, Goto K, Ito K, Sugiura T, Terabe K, Cho S, Nishiyama M, Sugiyama K, Togari H. Detection of congenital cytomegalovirus infection using umbilical cord blood samples in a screening survey. J Med Virol. 2009;81:1773-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Dogan Y, Yuksel A, Kalelioglu IH, Has R, Tatli B, Yildirim A. Intracranial ultrasound abnormalities and fetal cytomegalovirus infection: report of 8 cases and review of the literature. Fetal Diagn Ther. 2011;30:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Rochat MJ, Distefano G, Maffei M, Toni F, Posar A, Scaduto MC, Resca F, Cameli C, Bacchelli E, Maestrini E, Visconti P. Brain Magnetic Resonance Findings in 117 Children with Autism Spectrum Disorder under 5 Years Old. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Natsume T, Inaba Y, Osawa Y, Fukuyama T. High Incidence of Hippocampal Abnormalities in Pediatric Patients with Congenital Cytomegalovirus Infection. Neuropediatrics. 2022;53:239-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Kawatani M, Nakai A, Okuno T, Kobata R, Moriuchi M, Moriuchi H, Tsukahara H, Mayumi M. Detection of cytomegalovirus in preserved umbilical cord from a boy with autistic disorder. Pediatr Int. 2010;52:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Lin CH, Chou IC, Lee IC, Hong SY. Cytomegalovirus Infection in Infancy May Increase the Risk of Subsequent Epilepsy and Autism Spectrum Disorder in Childhood. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Yang XY, Wang YY, Zhou YP, He J, Mei MJ, Zhang MN, Wang B, Zhou WJ, Luo MH, Wang QH, Li ZY, Xu Y, Lu Q, Zou LP. Postnatal Cytomegalovirus Infection May Increase the Susceptibility of Tuberous Sclerosis Complex to Autism Spectrum Disorders. Microbiol Spectr. 2022;10:e0186421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 97. | Al-Beltagi M. Autism medical comorbidities. World J Clin Pediatr. 2021;10:15-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 151] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (14)] |
| 98. | Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10:e114989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 99. | Ganguli S, Chavali PL. Intrauterine Viral Infections: Impact of Inflammation on Fetal Neurodevelopment. Front Neurosci. 2021;15:771557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Mahic M, Mjaaland S, Bøvelstad HM, Gunnes N, Susser E, Bresnahan M, Øyen AS, Levin B, Che X, Hirtz D, Reichborn-Kjennerud T, Schjølberg S, Roth C, Magnus P, Stoltenberg C, Surén P, Hornig M, Lipkin WI. Maternal Immunoreactivity to Herpes Simplex Virus 2 and Risk of Autism Spectrum Disorder in Male Offspring. mSphere. 2017;2:e00016-17. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Gentile I, Zappulo E, Bonavolta R, Maresca R, Riccio MP, Buonomo AR, Portella G, Vallefuoco L, Settimi A, Pascotto A, Borgia G, Bravaccio C. Prevalence of herpes simplex virus 1 and 2 antibodies in patients with autism spectrum disorders. In Vivo. 2014;28:667-671. [PubMed] |
| 102. | Zerboni L, Reichelt M, Arvin A. Varicella-zoster virus neurotropism in SCID mouse-human dorsal root ganglia xenografts. Curr Top Microbiol Immunol. 2010;342:255-276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Gentile I, Zappulo E, Bonavolta R, Maresca R, Riccio MP, Buonomo AR, Portella G, Settimi A, Pascotto A, Borgia G, Bravaccio C. Exposure to Varicella Zoster Virus is higher in children with autism spectrum disorder than in healthy controls. Results from a case-control study. In Vivo. 2014;28:627-631. [PubMed] |
| 104. | Koch E, Demontis D. Drug repurposing candidates to treat core symptoms in autism spectrum disorder. Front Pharmacol. 2022;13:995439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 105. | Doyon-Plourde P, Fakih I, Tadount F, Fortin É, Quach C. Impact of influenza vaccination on healthcare utilization - A systematic review. Vaccine. 2019;37:3179-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Abdullahi H, Elnahas A, Konje JC. Seasonal influenza during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2021;258:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Kępińska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and Influenza at the Centenary of the 1918-1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front Psychiatry. 2020;11:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 108. | Mahic M, Che X, Susser E, Levin B, Reichborn-Kjennerud T, Magnus P, Stoltenberg C, Chauhan L, Briese T, Bresnahan M, Surén P, Hornig M, Mjaaland S, Lipkin WI. Epidemiological and Serological Investigation into the Role of Gestational Maternal Influenza Virus Infection and Autism Spectrum Disorders. mSphere. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 528] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 110. | Zerbo O, Qian Y, Yoshida C, Fireman BH, Klein NP, Croen LA. Association Between Influenza Infection and Vaccination During Pregnancy and Risk of Autism Spectrum Disorder. JAMA Pediatr. 2017;171:e163609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 111. | Becerra-Culqui TA, Getahun D, Chiu V, Sy LS, Tseng HF. Prenatal Influenza Vaccination or Influenza Infection and Autism Spectrum Disorder in Offspring. Clin Infect Dis. 2022;75:1140-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 112. | Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604 (7907):697-707. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 1033] [Article Influence: 258.3] [Reference Citation Analysis (0)] |
| 113. | Vianna FSL, Fraga LR, Abeche AM, Silva AAD, Sanseverino MTV, Schuler-Faccini L. COVID-19 during pregnancy and adverse outcomes: Concerns and recommendations from The Brazilian Teratology Information Service. Genet Mol Biol. 2021;44:e20200224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, Ferdosian F, Bahrami R. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr Pathol. 2020;39:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 115. | Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49:207-228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 116. | Steinman G. COVID-19 and autism. Med Hypotheses. 2020;142: 109797. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Riikonen R, Makkonen I, Vanhala R, Turpeinen U, Kuikka J, Kokki H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev Med Child Neurol. 2006;48:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Theoharides TC. Ways to Address Perinatal Mast Cell Activation and Focal Brain Inflammation, including Response to SARS-CoV-2, in Autism Spectrum Disorder. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Edlow AG, Castro VM, Shook LL, Kaimal AJ, Perlis RH. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw Open. 2022;5:e2215787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 120. | Brynge M, Sjöqvist H, Gardner RM, Lee BK, Dalman C, Karlsson H. Maternal infection during pregnancy and likelihood of autism and intellectual disability in children in Sweden: a negative control and sibling comparison cohort study. Lancet Psychiatry. 2022;9:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 121. | Dutheil F, Bourdel N, Comptour A. The Coronavirus Might be Paradoxically Beneficial on the Risk of Autism. J Autism Dev Disord. 2021;51:1805-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 122. | Hoffman MC, Freedman R, Law AJ, Clark AM, Hunter SK. Maternal nutrients and effects of gestational COVID-19 infection on fetal brain development. Clin Nutr ESPEN. 2021;43:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Jupille H, Seixas G, Mousson L, Sousa CA, Failloux AB. Zika Virus, a New Threat for Europe? PLoS Negl Trop Dis. 2016;10:e0004901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandão WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrão-Braga PC. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 920] [Cited by in RCA: 1023] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 125. | Freitas DA, Souza-Santos R, Carvalho LMA, Barros WB, Neves LM, Brasil P, Wakimoto MD. Congenital Zika syndrome: A systematic review. PLoS One. 2020;15:e0242367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 126. | Javed F, Manzoor KN, Ali M, Haq IU, Khan AA, Zaib A, Manzoor S. Zika virus: what we need to know? J Basic Microbiol. 2018;58:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 127. | Grant R, Fléchelles O, Tressières B, Dialo M, Elenga N, Mediamolle N, Mallard A, Hebert JC, Lachaume N, Couchy E, Hoen B, Fontanet A. In utero Zika virus exposure and neurodevelopment at 24 months in toddlers normocephalic at birth: a cohort study. BMC Med. 2021;19:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 128. | Vianna P, Gomes JDA, Boquett JA, Fraga LR, Schuch JB, Vianna FSL, Schuler-Faccini L. Zika Virus as a Possible Risk Factor for Autism Spectrum Disorder: Neuroimmunological Aspects. Neuroimmunomodulation. 2018;25:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, Pone M, Abreu de Carvalho LM, Pone SM, Zin AA, Tsui I, Salles TRS, da Cunha DC, Costa RP, Malacarne J, Reis AB, Hasue RH, Aizawa CYP, Genovesi FF, Einspieler C, Marschik PB, Pereira JP, Gaw SL, Adachi K, Cherry JD, Xu Z, Cheng G, Moreira ME. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med. 2019;25:1213-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 130. | Abtibol-Bernardino MR, de Almeida Peixoto LFA, de Oliveira GA, de Almeida TF, Rodrigues GRI, Otani RH, Soares Chaves BC, de Souza Rodrigues C, de Andrade ABCA, de Fatima Redivo E, Fernandes SS, da Costa Castilho M, Gomes Benzecry S, Bôtto-Menezes C, Martinez-Espinosa FE, Costa Alecrim MDG. Neurological Findings in Children without Congenital Microcephaly Exposed to Zika Virus in Utero: A Case Series Study. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 131. | Henderson T. Is Valacyclovir a Mood Stabilizer? Autism-Open Access. 2013;3:118. [DOI] [Full Text] |
| 132. | Kimberlin DW, Whitley RJ, Wan W, Powell DA, Storch G, Ahmed A, Palmer A, Sánchez PJ, Jacobs RF, Bradley JS, Robinson JL, Shelton M, Dennehy PH, Leach C, Rathore M, Abughali N, Wright P, Frenkel LM, Brady RC, Van Dyke R, Weiner LB, Guzman-Cottrill J, McCarthy CA, Griffin J, Jester P, Parker M, Lakeman FD, Kuo H, Lee CH, Cloud GA; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011;365:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 133. | Elchaar GM, Maisch NM, Augusto LM, Wehring HJ. Efficacy and safety of naltrexone use in pediatric patients with autistic disorder. Ann Pharmacother. 2006;40:1086-1095. [PubMed] |
| 134. | Lee KM, Chiu KB, Didier PJ, Baker KC, MacLean AG. Naltrexone treatment reverses astrocyte atrophy and immune dysfunction in self-harming macaques. Brain Behav Immun. 2015;50:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Mouthon L, Lortholary O. Intravenous immunoglobulins in infectious diseases: where do we stand? Clin Microbiol Infect. 2003;9:333-338. [PubMed] [DOI] [Full Text] |
| 136. | Rossignol DA, Frye RE. A Systematic Review and Meta-Analysis of Immunoglobulin G Abnormalities and the Therapeutic Use of Intravenous Immunoglobulins (IVIG) in Autism Spectrum Disorder. J Pers Med. 2021;11: 488. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Connery K, Tippett M, Delhey LM, Rose S, Slattery JC, Kahler SG, Hahn J, Kruger U, Cunningham MW, Shimasaki C, Frye RE. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl Psychiatry. 2018;8:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 138. | Lee TM, Lee KM, Lee CY, Lee HC, Tam KW, Loh EW. Effectiveness of N-acetylcysteine in autism spectrum disorders: A meta-analysis of randomized controlled trials. Aust N Z J Psychiatry. 2021;55:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 139. | Chen YW, Lin HC, Ng MC, Hsiao YH, Wang CC, Gean PW, Chen PS. Activation of mGluR2/3 underlies the effects of N-acetylcystein on amygdala-associated autism-like phenotypes in a valproate-induced rat model of autism. Front Behav Neurosci. 2014;8:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Boris M, Kaiser CC, Goldblatt A, Elice MW, Edelson SM, Adams JB, Feinstein DL. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J Neuroinflammation. 2007;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 141. | Capano L, Dupuis A, Brian J, Mankad D, Genore L, Hastie Adams R, Smile S, Lui T, Odrobina D, Foster JA, Anagnostou E. A pilot dose finding study of pioglitazone in autistic children. Mol Autism. 2018;9:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 142. | Sabourin KR, Reynolds A, Schendel D, Rosenberg S, Croen LA, Pinto-Martin JA, Schieve LA, Newschaffer C, Lee LC, DiGuiseppi C. Infections in children with autism spectrum disorder: Study to Explore Early Development (SEED). Autism Res. 2019;12:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Bauer R, Timothy KW, Golden A. Update on the Molecular Genetics of Timothy Syndrome. Front Pediatr. 2021;9:668546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 144. | Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 145. | Napoli E, Wong S, Hertz-Picciotto I, Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014;133:e1405-e1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 146. | Babaknejad N, Sayehmiri F, Sayehmiri K, Mohamadkhani A, Bahrami S. The Relationship between Zinc Levels and Autism: A Systematic Review and Meta-analysis. Iran J Child Neurol. 2016;10:1-9. [PubMed] |
| 147. | Maggini S, Wenzlaff S, Hornig D. Essential role of vitamin C and zinc in child immunity and health. J Int Med Res. 2010;38:386-414. [PubMed] |
| 148. | Saavedra MJ, Aziz J, Cacchiarelli San Román N. Scurvy due to restrictive diet in a child with autism spectrum disorder: case report. Arch Argent Pediatr. 2018;116:e684-e687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 149. | Liuzzo Scorpo M, Corsello G, Maggio MC. Scurvy as an Alarm Bell of Autistic Spectrum Disorder in the First World: A Case Report of a 3-Year-Old Girl. Am J Case Rep. 2021;22:e930583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Liu Z, Wang J, Xu Q, Hong Q, Zhu J, Chi X. Research Progress in Vitamin A and Autism Spectrum Disorder. Behav Neurol. 2021;2021:5417497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 151. | Roche FC, Harris-Tryon TA. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Cantorna MT, Snyder L, Arora J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit Rev Biochem Mol Biol. 2019;54:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 153. | Krajmalnik-Brown R, Lozupone C, Kang DW, Adams JB. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 154. | Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (7)] |
| 155. | Davidson M. Vaccination as a cause of autism-myths and controversies. Dialogues Clin Neurosci. 2017;19: 403-407. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 156. | Godlee F, Smith J, Marcovitch H. Wakefield's article linking MMR vaccine and autism was fraudulent. BMJ. 2011;342:c7452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 157. | Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 158. | Zerbo O, Modaressi S, Goddard K, Lewis E, Fireman BH, Daley MF, Irving SA, Jackson LA, Donahue JG, Qian L, Getahun D, DeStefano F, McNeil MM, Klein NP. Vaccination Patterns in Children After Autism Spectrum Disorder Diagnosis and in Their Younger Siblings. JAMA Pediatr. 2018;172:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaru T, Hungary; Masyeni S, Indonesia S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH